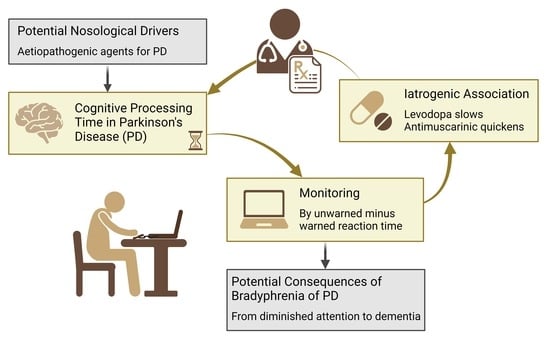
Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background
Abstract
1. Introduction
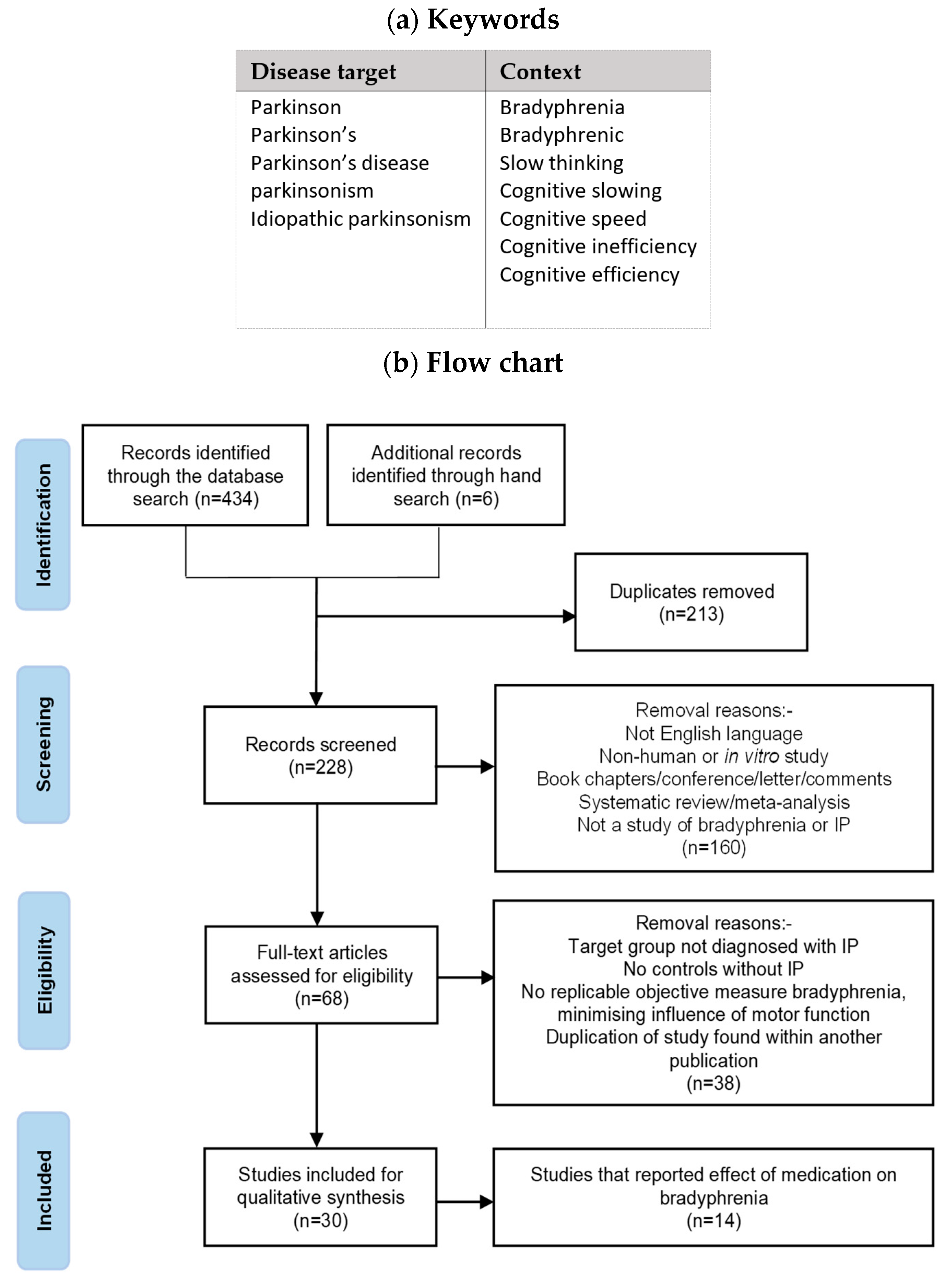
2. Materials and Methods
2.1. Participants
2.2. Reaction Time Testing
2.3. Efficiency of Response to Warning Signal
2.4. Systematic Review on Bradyphrenia
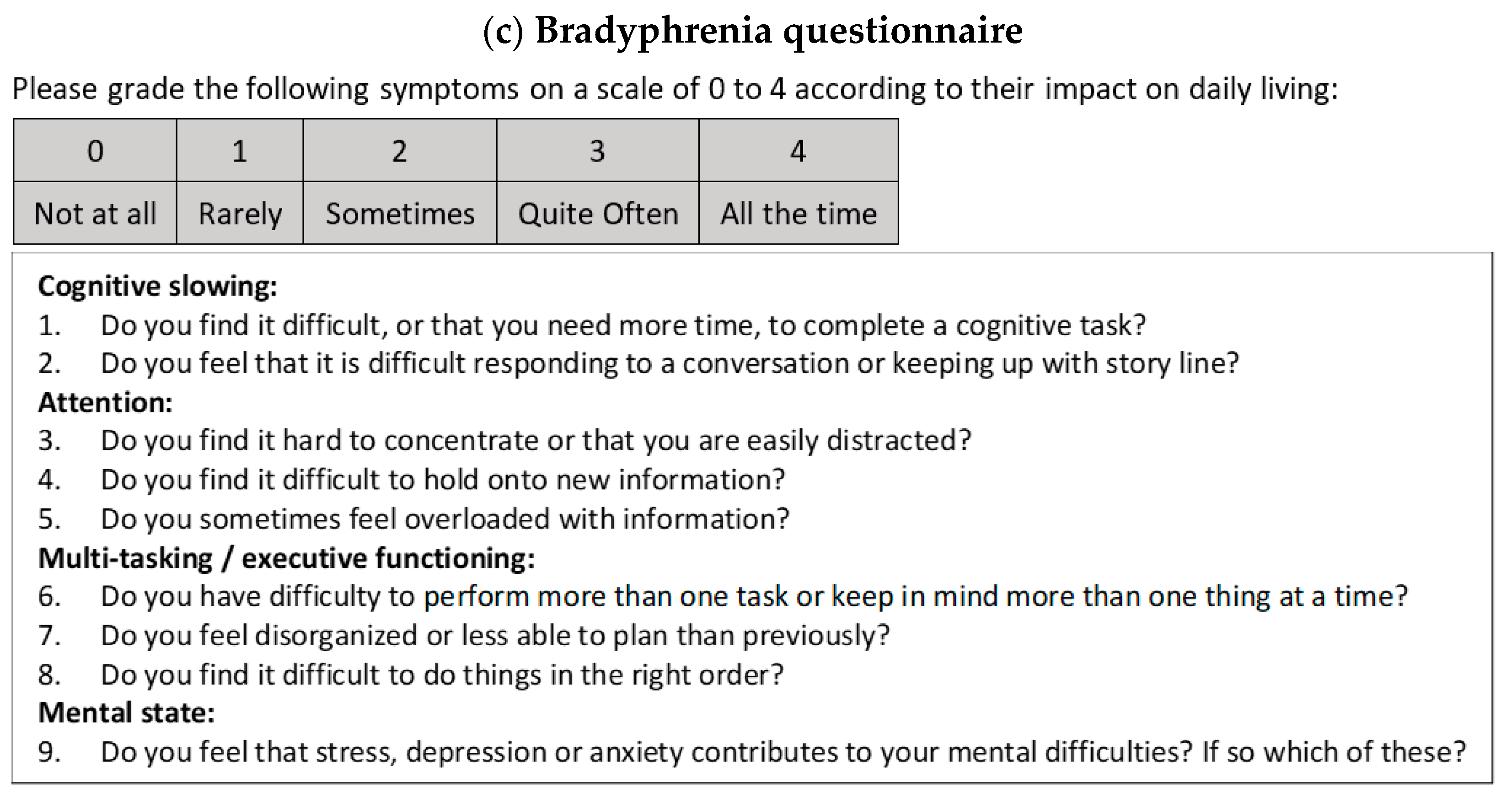
2.4.1. Development of Questionnaire
2.4.2. Assessment of Iatrogenicity
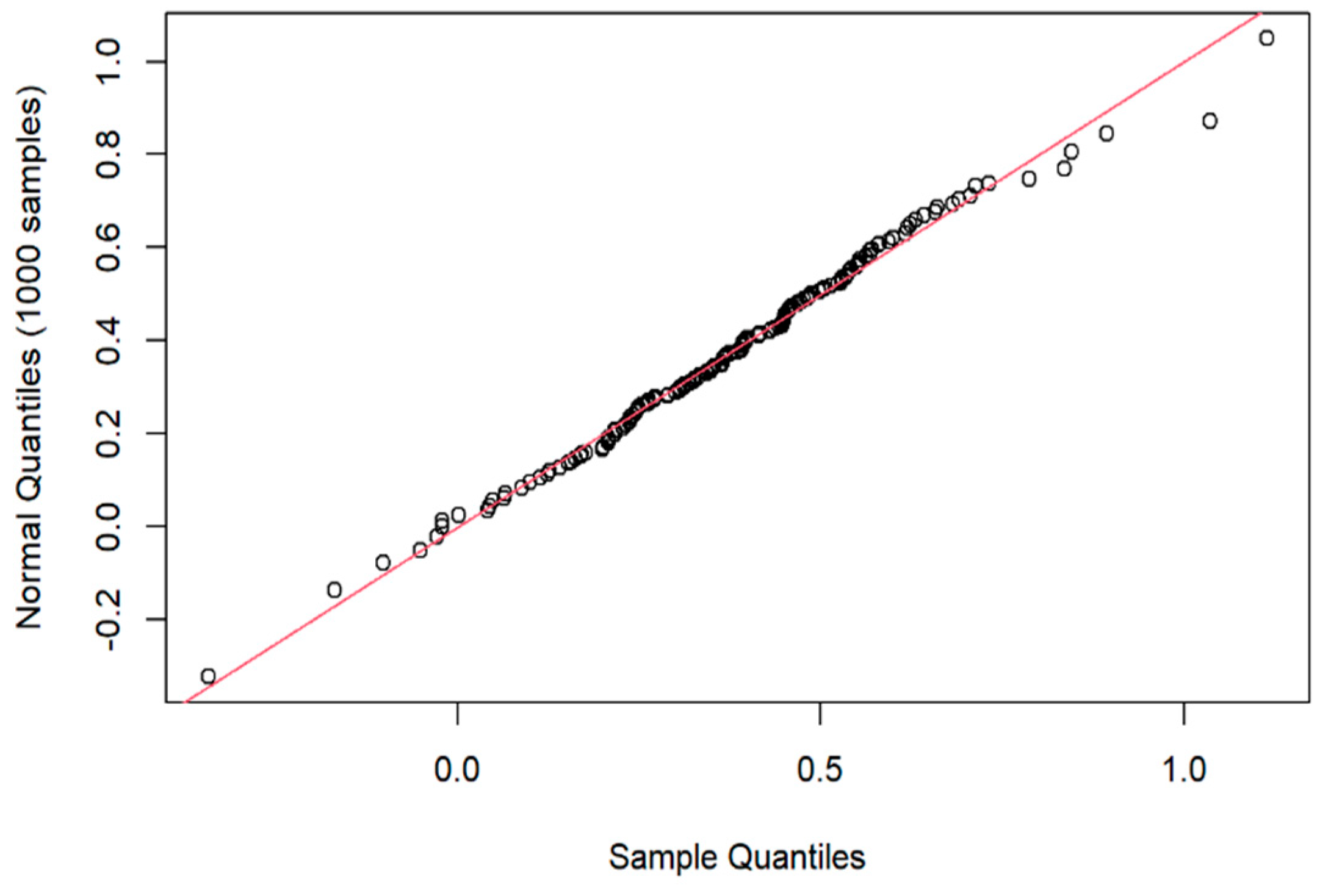
2.5. Statistical Analysis
3. Results
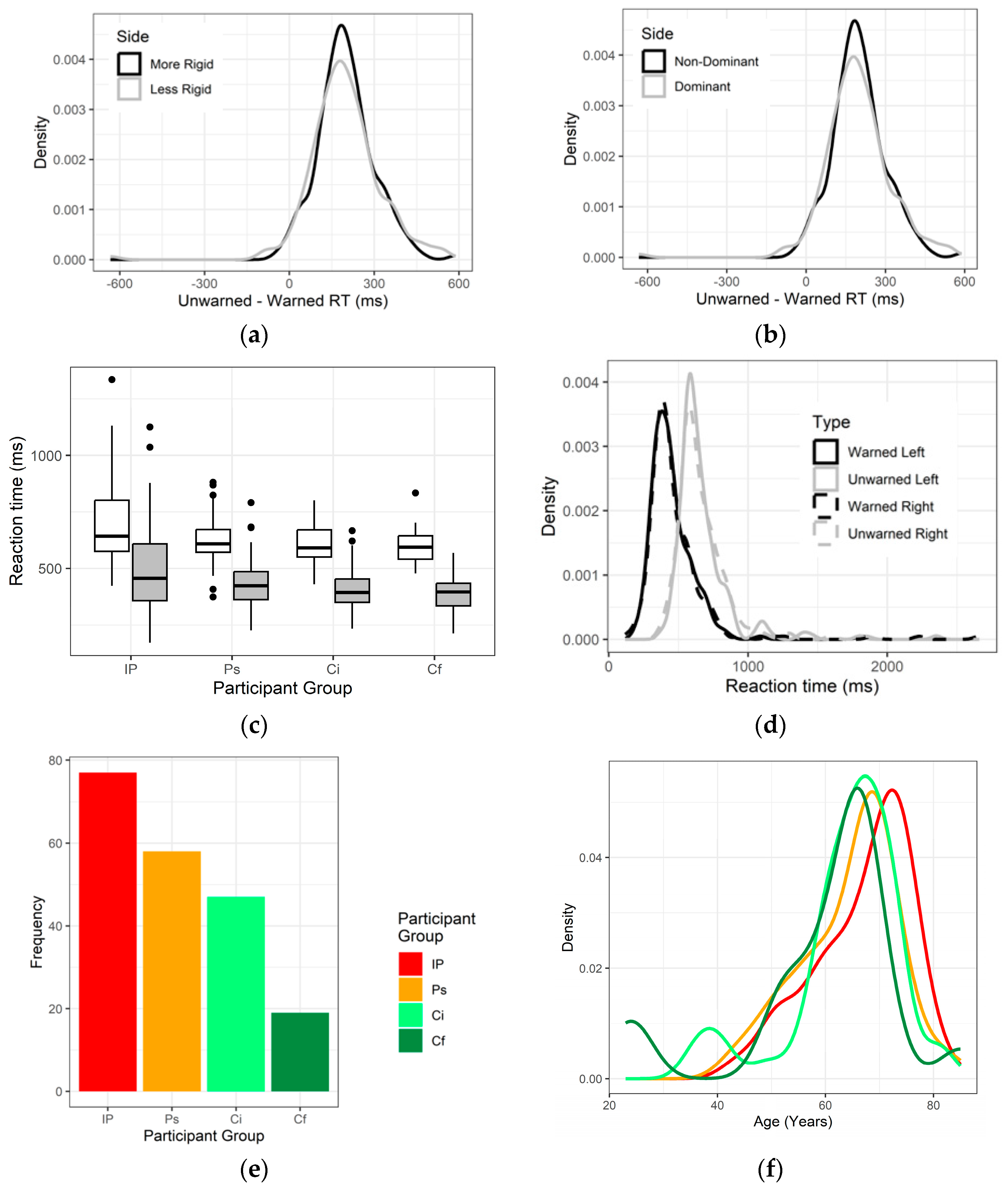
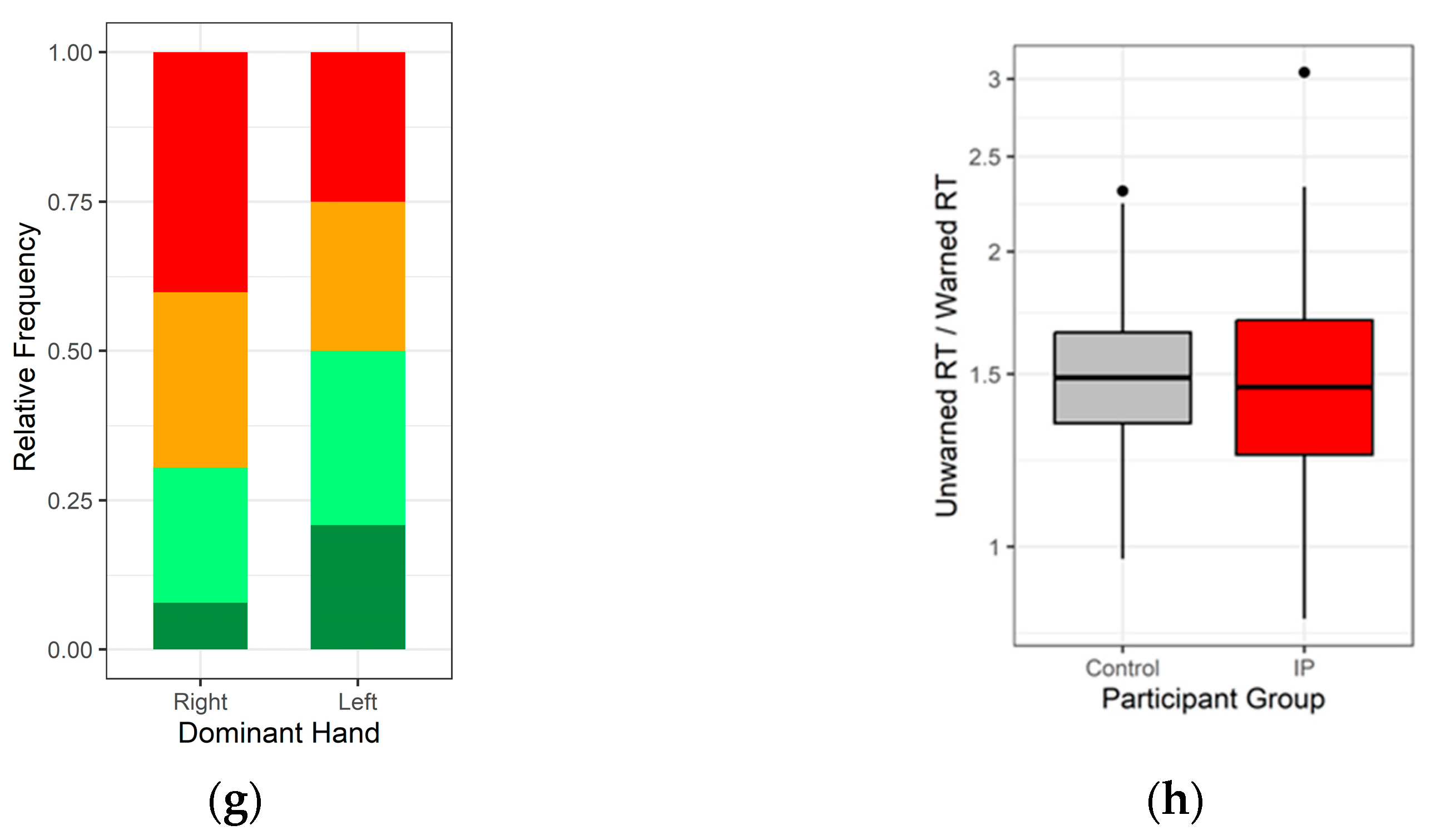
3.1. Demographics
3.2. Is Cognitive Efficiency Different between Those with and without Diagnosed IP?
3.3. Effect of Medicines on Cognitive Efficiency
3.4. Effect of Medicines on Reaction Time
3.5. Longitudinal Follow-Up on Cognitive Efficiency
3.6. Bradyphrenia Questionnaire
3.7. Systematic Review of Medicinal Effects on Bradyphrenia in IP
4. Discussion
4.1. Set in the Context of Systematic Review of Medicinal Effects on Cognitive Processing Time
4.2. Potential for influencing Research, Practice and Policy
4.3. Wider Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naville, F. Etudes sur les complications et les sequelles mentales de l’encéphalite épidémique. Encéphale 1922, 17, 369–375. [Google Scholar]
- Dobbs, R.J.; Bowes, S.G.; Charlett, A.; Henley, M.M.; Frith, C.; Dickins, J.; Dobbs, S.M. Hypothesis: The bradyphrenia of parkinsonism is a nosological entity. Acta Neurol. Scand. 1993, 87, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.J.; Bowes, S.G.; Henley, M.; Charlett, A.; O’Neill, C.J.; Dickins, J.; Nicholson, P.W.; Dobbs, S.M. Assessment of the bradyphrenia of parkinsonism: A novel use of delayed auditory feedback. Acta Neurol. Scand. 1993, 87, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Koerts, J.; Van Beilen, M.; Tucha, O.; Leenders, K.L.; Brouwer, W.H. Executive Functioning in Daily Life in Parkinson’s Disease: Initiative; Planning and Multi-Task Performance. PLoS ONE 2011, 6, e29254. [Google Scholar] [CrossRef]
- Zhang, C.; Reeves, S.; David, A.S.; Costello, H.; Rogers, J. Neuropsychiatric features of Parkinson’s disease in the era prior to the use of dopaminergic therapies. Cogn. Neuropsychiatry 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Chapman, K.R.; Bing-Canar, H.; Alosco, M.L.; Steinberg, E.G.; Martin, B.; Chaisson, C.; Kowall, N.; Tripodis, Y.; Stern, R.A. Mini Mental State Examination and Logical Memory scores for entry into Alzheimer’s disease trials. Alzheimer Res. Ther. 2016, 8, 9. [Google Scholar] [CrossRef]
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Poewe, W.; Berger, W.; Benke, T.; Schelosky, L. High-speed memory scanning in Parkinson’s disease: Adverse effects of levodopa. Ann. Neurol. 1991, 29, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cereb. Cortex 2001, 11, 1136–1143. [Google Scholar] [CrossRef]
- Press, D.; Mechanic, D.; Tarsy, D. Cognitive slowing in Parkinson’s disease resolves after practice. J. Neurol. Neurosurg. Psychiatry 2002, 73, 524–528. [Google Scholar] [CrossRef][Green Version]
- Poston, K.L.; Williams, S.Y.; Zhang, K. Compensatory Neural Mechanism. A Cognitively Unimpaired Parkinson Disease. Ann. Neurol. 2016, 79, 448–463. [Google Scholar] [CrossRef]
- Rogers, D.; Lees, A.; Smith, E.; Trimble, M.; Stern, G.M. Bradyphrenia in parkinson’s disease and psychomotor retardation in depressive illness: An experimental study. Brain 1987, 110, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Grande, L.J.; Crosson, B.; Heilman, K.M.; Bauer, R.M.; Kilduff, P. Visual Selective Attention in Parkinson’s Disease: Dissociation of Exogenous and Endogenous Inhibition. Neuropsychology 2006, 20, 370–382. [Google Scholar] [CrossRef]
- Russ, M.; Seger, L. The effect of task complexity on reaction times in memory scanning and visual discrimination in Parkinson’s disease. Neuropsychologia 1995, 33, 561–575. [Google Scholar] [CrossRef]
- Wilson, R.S.; Kaszniak, A.W.; Klawans, H.L.; Garron, D.C. High speed memory scanning in Parkinsonism. Cortex 1980, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Periáñez, J.A.; Ríos-Lago, M. Components determining the slowness of information processing in parkinson’s disease. Brain Behav. 2021, 11, e02031. [Google Scholar] [CrossRef]
- Zimmermann, P.; Sprengelmeyer, R.; Fimm, B.; Wallesch, C.W. Cognitive slowing in decision tasks in early and advanced Parkinson’s disease. Brain Cogn. 1992, 18, 60–69. [Google Scholar] [CrossRef]
- Cooper, J.; Sagar, H.; Tidswell, P.; Jordan, N. Slowed central processing in simple and go/no-go reaction time tasks in Parkinson’s disease. Brain 1994, 117, 517–529. [Google Scholar] [CrossRef]
- Tachibana, H.; Aragane, K.; Miyata, Y.; Sugita, M. Electrophysiological analysis of cognitive slowing in Parkinson’s disease. J. Neurol. Sci. 1997, 149, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, K.; Denève, C.; Ronval, M. Is the Paced Auditory Serial Addition Test (PASTA) a Valid Means of Assessing Executive Function in Parkinson’s Disease? Cortex 2007, 43, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Aragane, K.; Kawabata, K.; Sugita, M. P3 Latency Change in Aging and Parkinson Disease. Arch. Neurol. 1997, 54, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.M.; Ryan, S.; Hayee, B.H.; Bjarnason, I.; Augustin, A.D.; Umamahesan, C.; Taylor, D.; Weller, C.; Dobbs, S.M.; Dobbs, R.J. Distinctive Pathophysiology Underlying Constipation in Parkinson’s Disease: Implications for Cognitive Inefficiency. J. Clin. Med. 2020, 9, 1916. [Google Scholar] [CrossRef]
- Hill, A.B. The environment and disease: Association or causation? Proc. Roy. Soc. Med. 1965, 58, 295–330. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Sampaio, C.; Costa, J.; Lees, A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst. Rev. 2003, 2002, CD003735. [Google Scholar] [PubMed]
- Ostadkarampour, M.; Putnins, E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021, 12, 676239. [Google Scholar] [CrossRef]
- Dobbs, R.J.; Dobbs, S.M.; Weller, C.; Charlett, A.; Taylor, D. Time-Lag between Establishing Clinical Pharmacology Principles and Advances in Practice: The Case of Tolerance to Levodopa. J. Pharmacol. Clin. Toxicol. 2017, 5, 1084. [Google Scholar]
- Bloem, B.R.; Grimbergen, Y.A.; van Dijk, J.G.; Munneke, M. The “posture second” strategy: A review of wrong priorities in Parkinson’s disease. J. Neurol. Sci. 2006, 248, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.J.; Stevenson, J.M. Anticholinergic drugs and dementia: Time for transparency in the face of uncertainty. Cochrane Database Syst. Rev. 2021, 2021, ED000154. [Google Scholar] [CrossRef] [PubMed]
- Mur, J.; Cox, S.; Marioni, R.; Russ, T.; Muniz-Terrera, G. Association between anticholinergic burden and dementia in UK Biobank. Innov. Aging 2021, 5 (Suppl. S1), 928–929. [Google Scholar] [CrossRef]
- Richardson, K.; Fox, C.; Maidment, I.; Steel, N.; Loke, Y.K.; Arthur, A.; Myint, P.K.; Grossi, C.M.; Mattishent, K.; Bennett, K.; et al. Anticholinergic drugs and risk of dementia: Case-control study. BMJ 2018, 361, k1315. [Google Scholar] [CrossRef]
- Coupland, C.A.C.; Hill, T.; Dening, T.; Morriss, R.; Moore, M.; Hippisley-Cox, J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA Intern. Med. 2019, 179, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Median (Lower, Upper Quartile) * at First Assessment | |
|---|---|---|
| IP (N = 77) | Remainder (N = 124) | |
| Demographic | ||
| Age (years) | 69 (61, 74) | 66 (59, 70) |
| Sex (male) | 57% | 40% |
| Height (cm) | 170 (160, 177) | 169 (162, 178) |
| Weight (kg) | 72 (63, 84) | 71 (63, 82) |
| Body mass index (kg/m2) | 24.4 (20.5, 28.9) | 22.3 (19.6, 26.9) |
| Dominant hand (right) | 92% | 85% |
| Time since diagnosis (years) | 5 (2, 10) | - |
| Medication: (total daily dose): % receiving | ||
| Anti-parkinsonian medication | 74% | - |
| Levodopa dosage (mg) | 300 (250, 413): 52% | - |
| Monoamine oxidase-B inhibitor | 48% | - |
| Catechol-O-methyl-transferase inhibitor | 13% | - |
| Dopaminergic agonist dosage (mg) † | 1.19 (0.71, 2.10): 47% | - |
| Amantadine dosage (mg) | 100 (100, 100): 6% | - |
| Anti-muscarinic (trihexyphenidyl) dosage (mg) | 4 (4, 6): 12% | - |
| Laxatives | 52% | 12% |
| Non-steroidal anti-inflammatory drug (NSAID) ⱡ | 9% | 9% |
| Anti-depressant | 4% | 8% |
| Anti-psychotic | 9% | 0% |
| Psychomotor and psychometric | ||
| Mini-mental state examination (maximum 30) | 30 (29, 30) | 30 (30, 30) |
| Depression score (63) | 10 (6, 15) | 4 (1, 10) |
| Anxiety score (63) | 12 (6, 17) | 5 (2, 8) |
| Average unwarned reaction time (ms) § | 643 (576, 801) | 604 (555, 669) |
| Average warned reaction time (ms) § | 456 (358, 609) | 411 (351, 465) |
| Bradyphrenia questionnaire score # (maximum 36) | 13 (6, 19) | 6 (3, 11) |
| Binary Predictor | Effect of Presence on Cognitive Efficiency (%) (N = 77) | |
|---|---|---|
| Mean (95% CI) | p-Value | |
| Levodopa | −8.6 (−15.6, −2.0) | 0.01 |
| Anti-muscarinic | 24.6 (7.3, 44.8) | 0.005 |
| Monoamine oxidase-B inhibitor | 8.3 (0.0, 17.4) | 0.065 |
| Binary Predictor | Effect of Presence on Reaction Time (ms) * (n = 77) | |||
|---|---|---|---|---|
| Mean Unwarned (95% CI) | p-Value | Mean Warned (95% CI) | p-Value | |
| Levodopa | 108 (60,157) | 0.001 | 133 (85, 182) | 0.001 |
| Dopaminergic agonist | 109 (37, 182) | 0.003 | 81 (6, 155) | 0.03 |
| Anti-psychotic † | 193 (49, 339) | 0.009 | ||
| MAOBI ⱡ | −130 (−199, −61) | 0.001 | −137 (−210, −63) | 0.001 |
| Amantadine † | −118 (−235, −1.3) | 0.05 | ||
| Predictor Question * | Effect on Cognitive Efficiency Mean (95% CI) | p-Value |
|---|---|---|
| Q1 | 0.065 (0.015, 0.116) | 0.01 |
| Q2 | −0.017 (−0.074, 0.041) | 0.6 |
| Q3 | −0.004 (−0.061, 0.053) | 0.9 |
| Q4 | −0.005 (−0.051, 0.042) | 0.9 |
| Q5 | −0.001 (−0.51, 0.049) | 0.96 |
| Q6 | −0.004 (−0.050, 0.041) | 0.9 |
| Q7 | −0.033 (−0.079, 0.012) | 0.2 |
| Q8 | 0.025 (−0.024, 0.073) | 0.3 |
| Q9 | −0.003 (−0.052, 0.047) | 0.9 |
| Total Daily Dose Levodopa * or Levodopa Equivalent † [and Other Medication] | Number Subjects | Primary Relevant Outcome Measures | Findings | |
|---|---|---|---|---|
| Within-subject comparisons | ||||
| on/off effect | ||||
| Poewe et al. (1991) [12] | Mean levodopa 1211 (SD 395) mg [none other] | 12 | Memory scanning speed § (‘off-state’ measured first) | Speed slower in ‘on-state’ compared with off-state |
| Cools et al. (2001) [13] | [levodopa, dopaminergic agonist or selegiline] | 15 | Task-set switching | At a lower cognitive load, dopaminergic medication remedied impairment in switching between two tasks |
| Press et al. (2002) [14] | [9 receiving levodopa, 3 dopaminergic agonist, 3 anticholinergic, 1 amantadine, 1 tolcapone] | 10 | Memory scanning speed § (on- and off-states order counterbalanced) | No change in speed or accuracy with dopaminergic state |
| Poston et al. (2016) [15] | Mean ‘levodopa equivalent’ † 659 (SD 397) mg | 24 | Memory scanning speed § during functional MRI scanning (on- and off-states order counterbalance) | Speed slower in on-state, but accuracy unaffected. Putamen hyperactivation in ‘off-state’ (cf 23 controls), lost in on-state. Loss correlated with slower memory scanning. |
| before and during de novo dopaminergic treatment | ||||
| Rogers et al. (1987) [16] | [10 levodopa, 2 dopaminergic agonist, with anticholinergic stoppage in 1] | 12 | Digit symbol test, with correction for motor response time using test with lower cognitive load | No change after introduction |
| off and on medication | ||||
| Grande et al. (2006) [17] | [11 levodopa, 3 dopaminergic agonist] | 14 | Negative priming with slower response latency to cued than non-cued tests in IP (inadequate counterbalancing by treatment order) | No difference according to medication status |
| Between-subject comparisons | ||||
| dose–response | ||||
| Dobbs et al. (1993) [2] | Median 500 (interquartile range 300, 600) [all on levodopa] | 81 | Ratio unwarned to warned RT ⱡ | No effect on efficiency (dose, duration of therapy, plasma concentration during ‘therapeutic window’ or of long t½ metabolite 3-O-methyldopa) |
| Russ and Seger (1995) [18] | Grand mean 550 mg | 58 | Memory scanning § (28 all on levodopa) and visual discrimination (30, 21 of whom on levodopa) speed | Difference in speed between most and least complex test unrelated to levodopa dose |
| presence/absence-specified medication (univariate analysis) | ||||
| Wilson et al. (1980) [19] | [divided into taking dopaminergic or cholinergic medication, plus 1 untreated] | 20 | Memory scanning speed § | No difference by taking dopaminergic or cholinergic medication |
| Dobbs et al. (1993) [2] | Median 500 (interquartile range 300, 600) [81 levodopa, 10 dopaminergic agonist, 21 selegiline, 5 amantadine, 21 anti-cholinergic] | 103 | Ratio unwarned to warned RT ⱡ | No effect on cognitive efficiency of levodopa, but improved with anticholinergic and with amantadine |
| Arroyo et al. (2021) [20] | Mean ‘levodopa equivalent’ † 697 (SD 425) mg | 48 | Choice reaction time adjusted for simple reaction time | Dopaminergic medication dosage not correlated with cognitive processing time |
| untreated and treated groups | ||||
| Zimmermann et al. (1992) [21] | Maximum 500 mg [9 levodopa or dopaminergic agonist, 3 selegiline, 1 anti- cholinergic, 1 amantadine] | 19 | Choice RT ⱡ (with uncoded or coded imperative) minus simple RT ⱡ | 10 untreated recently diagnosed IP were impaired by coded imperative, but not by uncoded (compared with 17 controls): 9 treated impaired by both |
| Cooper et al. (1994) [22] | [levodopa, dopaminergic agonist, or anticholinergic monotherapy] | 100 | Choice reaction time corrected by subtraction of simple RT ⱡ | 37 untreated newly diagnosed IP, 26 on recently started monotherapy, and 37 chronically treated not differentially affected by medicinal treatment |
| Tachibana et al. (1997) [23] # | [21 levodopa, 16 trihexyphenidyl, 7 dopaminergic agonist, 5 amantadine plus 6 untreated] | 29 | Latencies in EEG waveforms (elicited during a semantic discrimination task) known to be longer in PD than healthy controls | No significant effect of levodopa or trihexyphenidyl dosage |
| Dujardin et al. (2007) [24] | [not specified] | 27 | Paced auditory serial addition test | No difference between 13 treated and 14 early untreated. |
| Maintenance and medication withdrawal groups | ||||
| Cools et al. (2001) [13] | 417 (SD 227) and 482 (337) mg, respectively [all levodopa, dopaminergic agonist and/or selegiline, 3 anticholinergics] | 29 | Task-set switching | Dopaminergic medication reduced impairment in switching between two tasks in 14 where medication ‘as normal’ compared with 15 where ≥18 h abstinence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Baker, K.; Umamahesan, C.; Gilmour, S.; Charlett, A.; Taylor, D.; Young, A.H.; Dobbs, R.J.; Dobbs, S.M. Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background. J. Clin. Med. 2023, 12, 6499. https://doi.org/10.3390/jcm12206499
Wang W, Baker K, Umamahesan C, Gilmour S, Charlett A, Taylor D, Young AH, Dobbs RJ, Dobbs SM. Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background. Journal of Clinical Medicine. 2023; 12(20):6499. https://doi.org/10.3390/jcm12206499
Chicago/Turabian StyleWang, Wenjing, Kieran Baker, Chianna Umamahesan, Steven Gilmour, André Charlett, David Taylor, Allan H. Young, R. John Dobbs, and Sylvia M. Dobbs. 2023. "Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background" Journal of Clinical Medicine 12, no. 20: 6499. https://doi.org/10.3390/jcm12206499
APA StyleWang, W., Baker, K., Umamahesan, C., Gilmour, S., Charlett, A., Taylor, D., Young, A. H., Dobbs, R. J., & Dobbs, S. M. (2023). Bradyphrenia and Tachyphrenia in Idiopathic Parkinsonism Appear, in Part, Iatrogenic: An Observational Study with Systematic Review Background. Journal of Clinical Medicine, 12(20), 6499. https://doi.org/10.3390/jcm12206499