Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
- Moderate and/or severe obesity (body mass index (BMI) of between 30 and 39.99);
- Waist circumference >94 cm;
- Severe erectile dysfunction ((ED)—International Index of Erectile Function 5 Items (IIEF-5) score below 7) that is unresponsive to conventional treatment with PDE5i during the last 3 months, according to a previously published procedure [10];
- TT < 12 nmol/L according to the Italian Society of Endocrinology guidelines [11];
- Normal–low gonadotropin levels (lowest quartiles of the reference range);
- Levels of sex hormone-binding globulin (SHBG) that are reduced compared to the reference range (confirmed data);
- Homeostasis Model Assessment Index (HOMA) > 2.5 [12].
- Group A (n = 35): Patients with an active desire for fatherhood. They were prescribed conventional treatment for fertility using urofollitropin 150 IU subcutaneously (three times a week) associated with hCG 2000 IU subcutaneously (twice a week) for 4 months;
- Group B (n = 35): Patients with no active desire for fatherhood. They were prescribed liraglutide 0.6 mg subcutaneously every day for the first week, then 1.2 mg (second week), 1.8 mg (third week), 2.4 mg (fourth week), and 3.0 mg (from the fifth week for another three months);
- Group C (n = 40): Patients who had already fathered a child and were not seeking fertility. They were prescribed transdermal testosterone gel (2%) at 60 mg every day for 4 months.
- Patients with primary testicular disease: cryptorchidism, testicular tumor, varicocele (all grades) or previous varicocelectomy, previous orchitis, or previous testicular torsion;
- Patients with increased levels of prolactin or TSH;
- Patients with hypothalamic-pituitary lesions described via magnetic resonance imaging;
- Patients with arterial hypertension, dyslipidemia, impaired fasting blood glucose, or diabetes mellitus.
- Weight, height, BMI, and waist circumference;
- HOMA index, TT, LH, FSH, SHBG, total PSA, and hematocrit;
- Semen analysis (except for group C patients);
- IIEF-5 and the frequency of PDE5i use, as needed, for sexual activity.
2.1. Hormone Measurements
2.2. Semen Analysis
2.3. Statistical Analysis
2.4. Ethical Approval
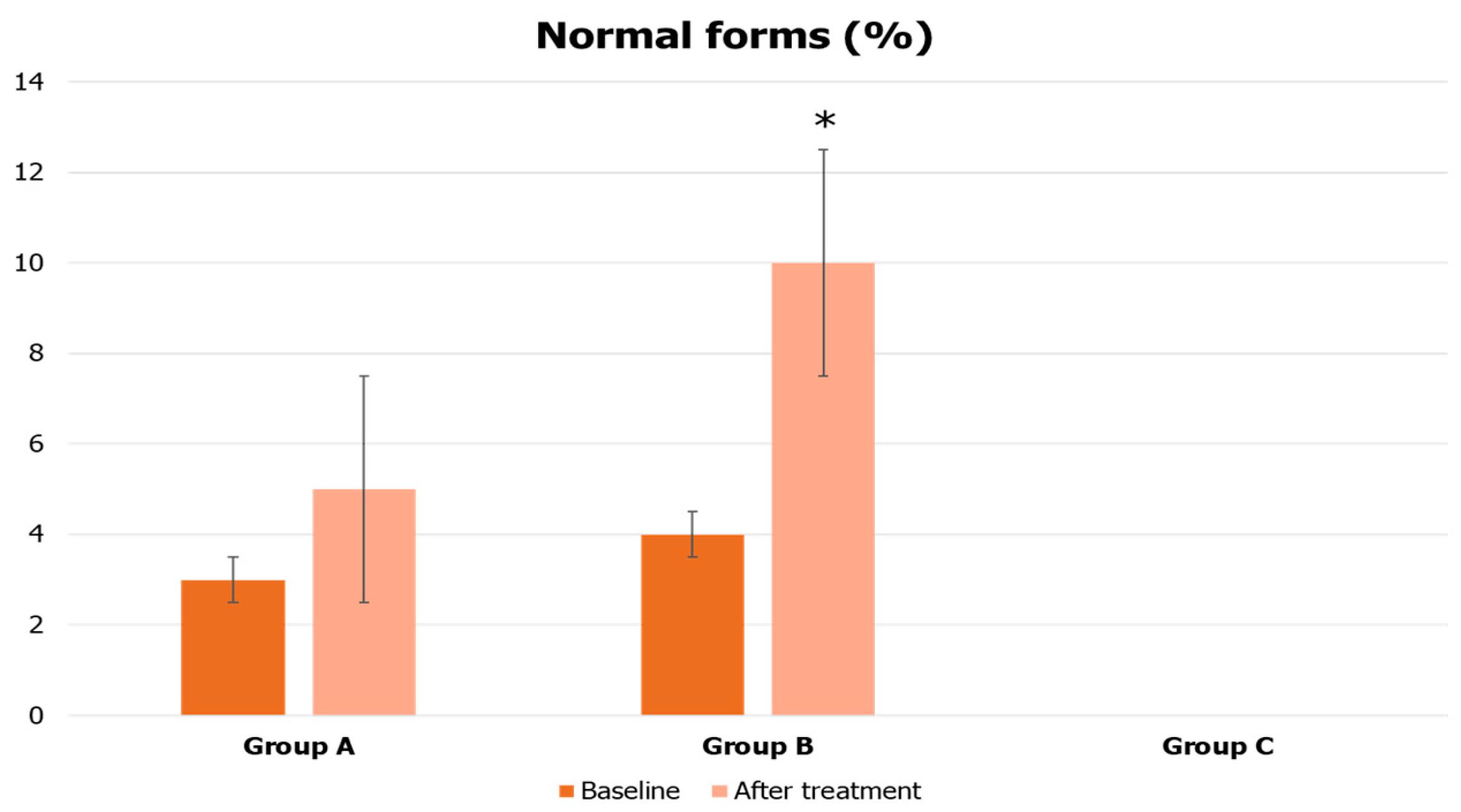
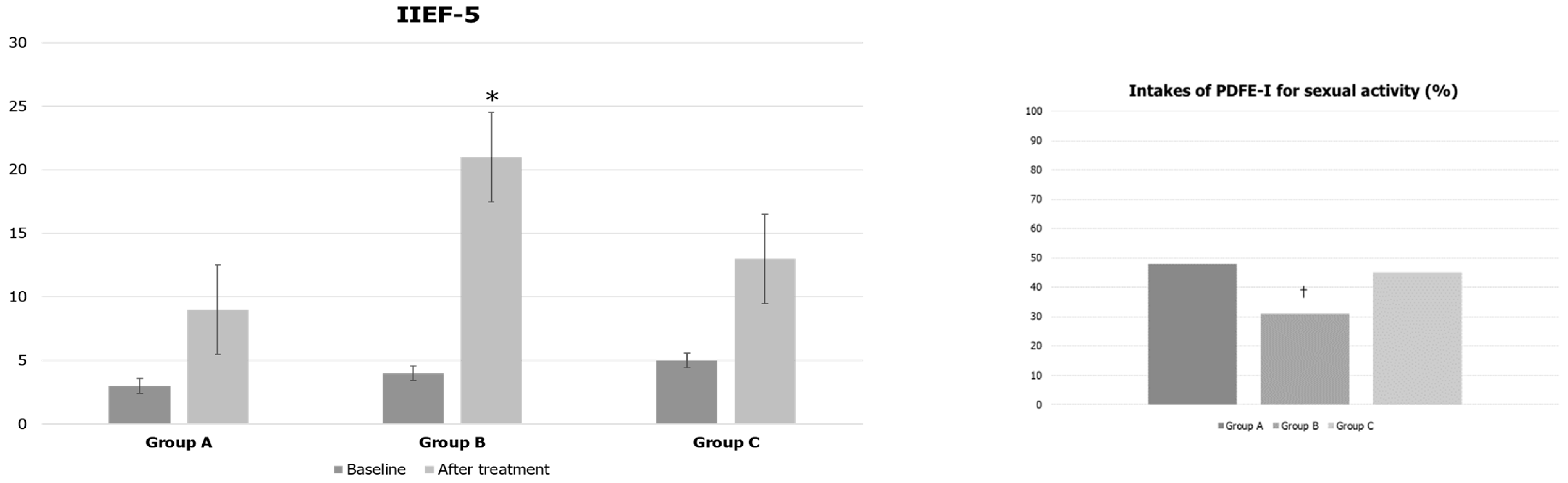
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and Metabolic Syndrome: A Meta-Analysis Study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi, L.; Favilla, V.; Morgia, G.; Cimino, S.; Russo, G.; La Vignera, S. The gonadal function in obese adolescents: Review. J. Endocrinol. Investig. 2014, 37, 1133–1142. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A.; Bruzziches, R.; Pili, M.; Spera, G. Phosphodiesterase 5 Inhibitors in the Treatment of Erectile Dysfunction. Curr. Pharm. Des. 2006, 12, 3467–3484. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A.; Francomano, D.; Lenzi, A. Does testosterone supplementation increase PDE5-inhibitor responses in difficult-to-treat erectile dysfunction patients? Expert Opin. Pharmacother. 2015, 16, 625–628. [Google Scholar] [CrossRef]
- Aversa, A.; Duca, Y.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Androgen Deficiency and Phosphodiesterase Type 5 Expression Changes in Aging Male: Therapeutic Implications. Front. Endocrinol. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Cimino, L.; Russo, G.I.; Morgia, G.; Calogero, A.E. Late-onset hypogonadism: The advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male 2016, 19, 34–39. [Google Scholar] [CrossRef]
- Song, S.-H.; Sung, S.; Her, Y.S.; Oh, M.; Shin, D.H.; Lee, J.; Baek, J.; Lee, W.S.; Kim, D.S. Misuse of testosterone replacement therapy in men in infertile couples and its influence on infertility treatment. Clin. Exp. Reprod. Med. 2019, 46, 173–177. [Google Scholar] [CrossRef]
- Aversa, A.; Letizia, C.; Francomano, D.; Bruzziches, R.; Natali, M.; Lenzi, A. A spontaneous, double-blind, double-dummy cross-over study on the effects of daily vardenafil on arterial stiffness in patients with vasculogenic erectile dysfunction. Int. J. Cardiol. 2012, 160, 187–191. [Google Scholar] [CrossRef]
- Isidori, A.M.; Aversa, A.; Calogero, A.; Ferlin, A.; Francavilla, S.; Lanfranco, F.; Pivonello, R.; Rochira, V.; Corona, G.; Maggi, M. Adult- and late-onset male hypogonadism: The clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2022, 45, 2385–2403. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Thorens, B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA 1992, 89, 8641–8645. [Google Scholar] [CrossRef]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The Gut Hormones PYY3-36 and GLP-17-36 amide Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef]
- Elias, C.F.; Kelly, J.F.; Lee, C.E.; Ahima, R.S.; Drucker, D.J.; Saper, C.B.; Elmquist, J.K. Chemical characterization of leptin-activated neurons in the rat brain. J. Comp. Neurol. 2000, 423, 261–281. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 2006, 55, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Fabbrini, E.; Isidori, A.; Aversa, A.; Fabbri, A. Leptin in reproduction. Trends Endocrinol. Metab. 2001, 12, 65–72. [Google Scholar] [CrossRef]
- Pelusi, C. The Effects of the New Therapeutic Treatments for Diabetes Mellitus on the Male Reproductive Axis. Front. Endocrinol. 2022, 13, 821113. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, P.; Emerenziani, G.P.; Antinozzi, C.; Sacchetti, M.; Di Luigi, L. Exercise as a drug for glucose management and prevention in type 2 diabetes mellitus. Curr. Opin. Pharmacol. 2021, 59, 95–102. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Podbregar, A.; Goricar, K.; Gregoric, N.; Janez, A. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr. Connect. 2019, 8, 195–202. [Google Scholar] [CrossRef]
- Izzi-Engbeaya, C.; Jones, S.; Crustna, Y.; Machenahalli, P.C.; Papadopoulou, D.; Modi, M.; Panayi, C.; Starikova, J.; Eng, P.C.; Phylactou, M.; et al. Effects of Glucagon-like Peptide-1 on the Reproductive Axis in Healthy Men. J. Clin. Endocrinol. Metab. 2020, 105, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Gaspari, T.; Liu, H.; Welungoda, I.; Hu, Y.; Widdop, R.E.; Knudsen, L.B.; Simpson, R.W.; Dear, A.E. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/- mouse model. Diabetes Vasc. Dis. Res. 2011, 8, 117–124, Erratum in: Diab. Vasc. Dis. Res. 2012, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Araujo, A.B.; Kupelian, V.; O’Donnell, A.B.; McKinlay, J.B. The Relative Contributions of Aging, Health, and Lifestyle Factors to Serum Testosterone Decline in Men. J. Clin. Endocrinol. Metab. 2007, 92, 549–555. [Google Scholar] [CrossRef]
- Aversa, A. Drugs targeted to improve endothelial function: Clinical correlates between sexual and internal medicine. Curr. Pharm. Des. 2008, 14, 3698–3699. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233 R249. [Google Scholar] [CrossRef] [PubMed]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Natali, M.; Lenzi, A. Testosterone and phosphodiesterase type-5 inhibitors: New strategy for preventing endothelial damage in internal and sexual medicine? Ther. Adv. Urol. 2009, 1, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health—2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef]
- Saad, F.; Aversa, A.; Isidori, A.M.; Zafalon, L.; Zitzmann, M.; Gooren, L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur. J. Endocrinol. 2011, 165, 675–685. [Google Scholar] [CrossRef]
- Grossmann, M.; Wierman, M.E.; Angus, P.; Handelsman, D.J. Reproductive Endocrinology of Nonalcoholic Fatty Liver Disease. Endocr. Rev. 2019, 40, 417–446. [Google Scholar] [CrossRef] [PubMed]
- Kumarendran, B.; O’Reilly, M.W.; Manolopoulos, K.N.; Toulis, K.A.; Gokhale, K.M.; Sitch, A.J.; Wijeyaratne, C.N.; Coomarasamy, A.; Arlt, W.; Nirantharakumar, K. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018, 15, e1002542. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, V.; Maseroli, E.; Rastrelli, G.; Scavello, I.; Cipriani, S.; Todisco, T.; Marchiani, S.; Sorbi, F.; Fambrini, M.; Petraglia, F.; et al. SHBG as a Marker of NAFLD and Metabolic Impairments in Women Referred for Oligomenorrhea and/or Hirsutism and in Women with Sexual Dysfunction. Front. Endocrinol. 2021, 12, 641446. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mousiolis, A.; Mintziori, G.; Goulis, D.G. Nonalcoholic fatty liver disease in males with low testosterone concentrations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological Role of ROS in Sperm Function. In Male Infertility: Contemporary Clini¬cal Approaches, Andrology, ART and Antooxidants; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–345. [Google Scholar]
- Fan, W.; Xu, Y.; Liu, Y.; Zhang, Z.; Lu, L.; Ding, Z. Obesity or Overweight, a Chronic Inflammatory Status in Male Reproductive System, Leads to Mice and Human Subfertility. Front. Physiol. 2018, 8, 1117. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.; Bossio, S.; Rago, V.; Greco, E.A.; Lofaro, D.; LA Russa, A.; DI Luigi, L.; LA Vignera, S.; Aversa, A. NLRP3-inflammasome activation in male reproductive system diseases. Minerva Endocrinol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
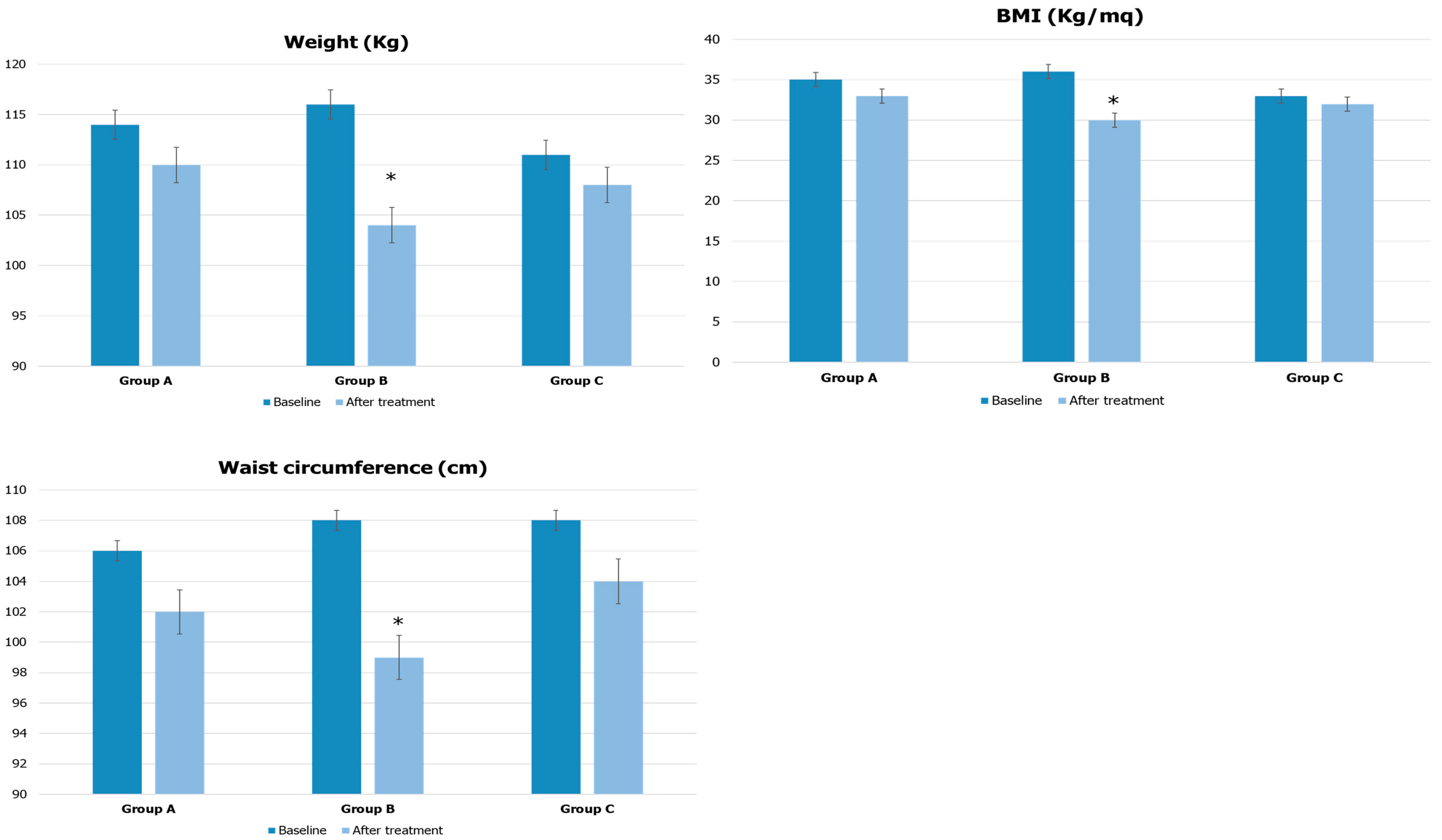
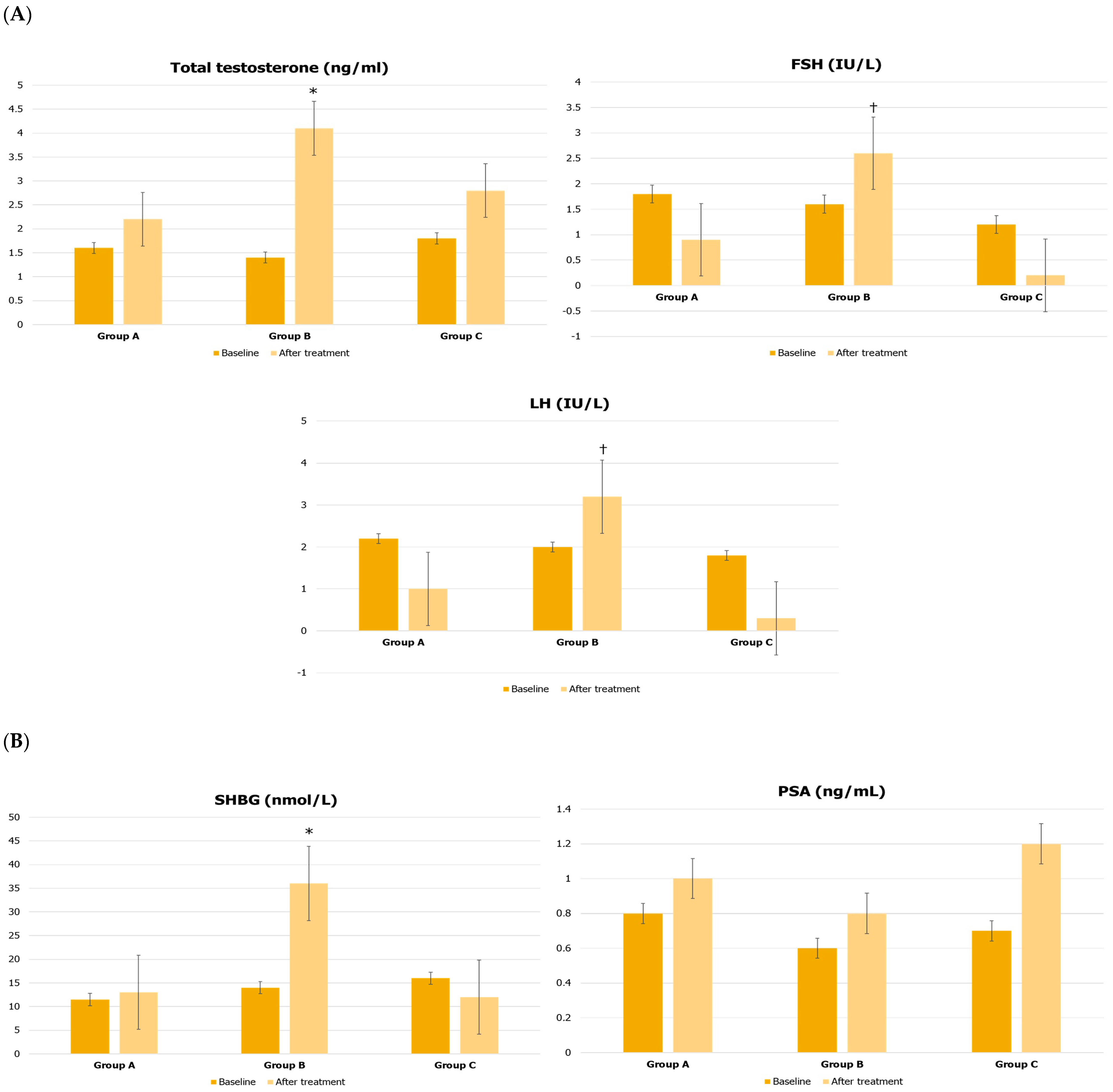
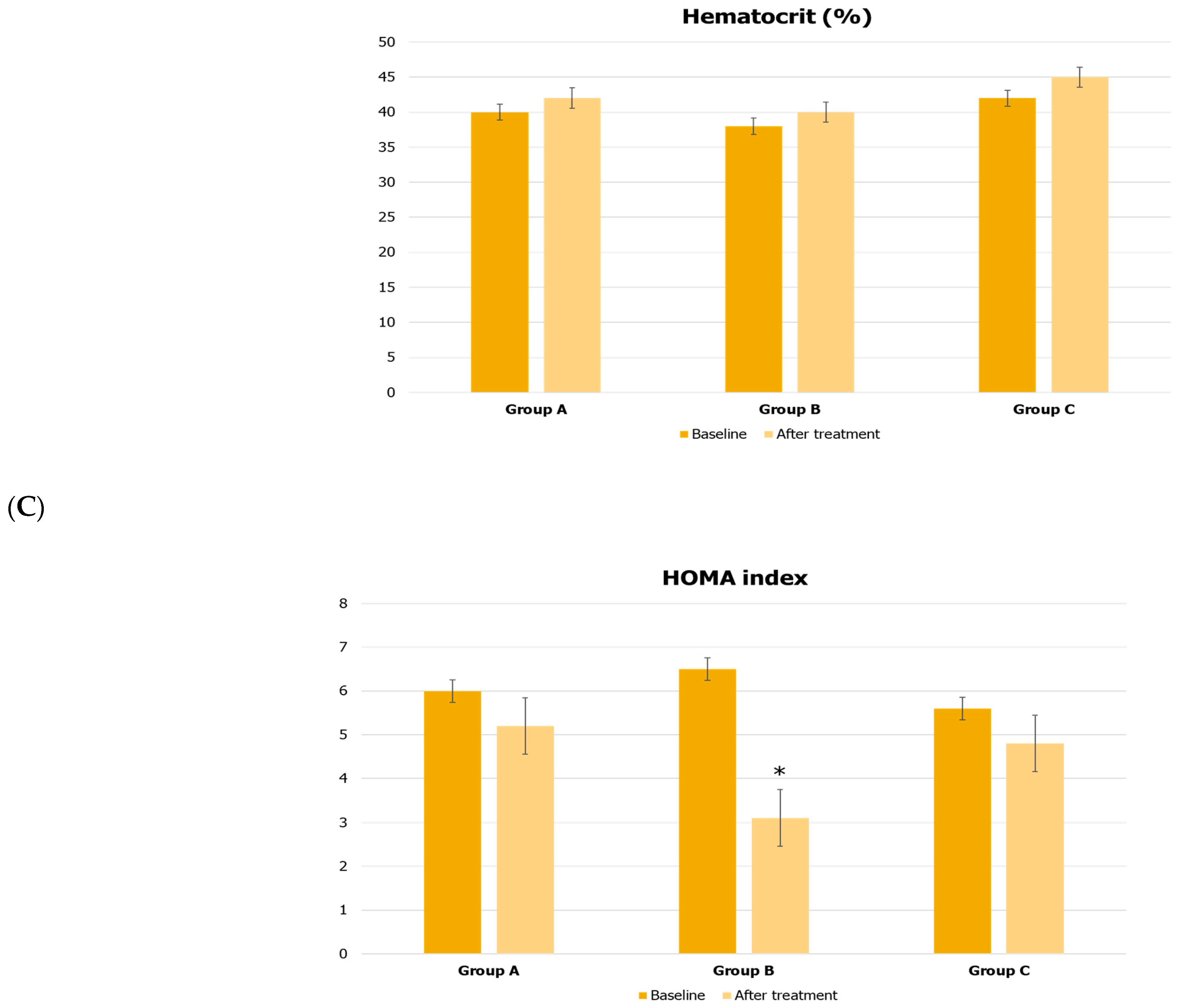
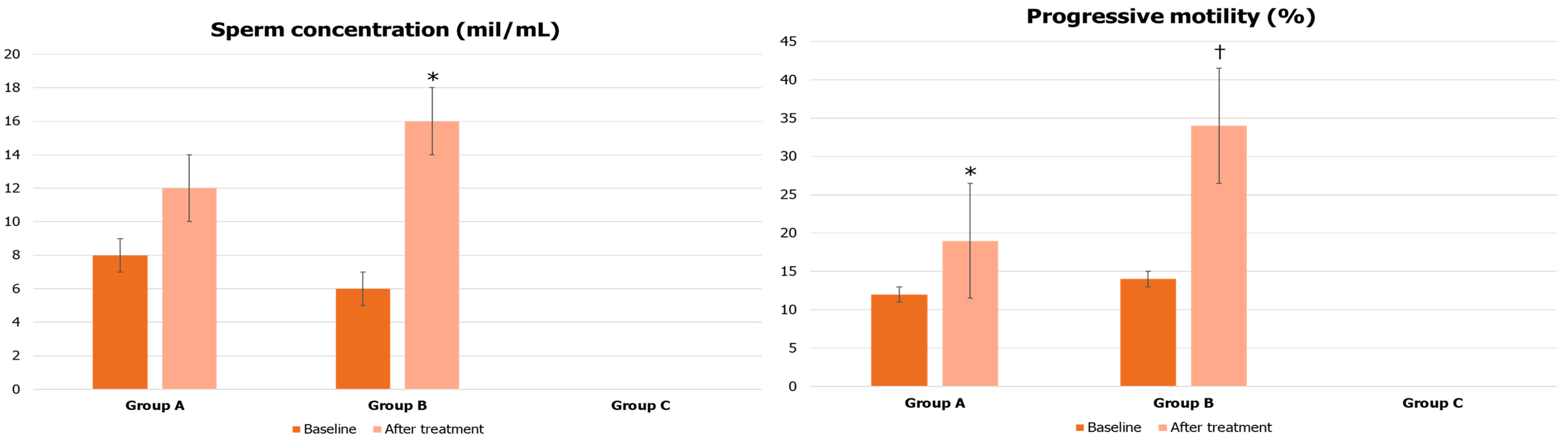
| Parameters | Group A | Group B | Group C |
|---|---|---|---|
| Weight (kg) | 114 ± 6 | 116 ± 10 | 111 ± 5 |
| BMI (kg/m2) | 35 ± 3 | 36 ± 3 | 33 ± 2 |
| Waist circumference (cm) | 106 ± 5 | 108 ± 6 | 108 ± 4 |
| Total testosterone (ng/mL) | 1.6 ± 0.2 | 1.4 ± 0.6 | 1.8 ± 0.4 |
| Follicle-stimulating hormone (IU/L) | 1.8 ± 0.2 | 1.6 ± 0.3 | 1.2 ± 0.4 |
| Luteinizing hormone (IU/L) | 2.2 ± 0.2 | 2.0 ± 0.3 | 1.8 ± 0.4 |
| Sex hormone-binding globulin (nmol/L) | 11.5 ± 3.0 | 14.0 ± 3.0 | 16 ± 6.0 |
| Prostate-specific antigen (ng/mL) | 0.8 ± 0.2 | 0.6 ± 0.3 | 0.7 ± 0.1 |
| Hematocrit (%) | 40 ± 4 | 38 ± 6 | 42 ± 4 |
| HOMA index | 6.0 ± 1.2 | 6.5 ± 1.9 | 5.6 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Vignera, S.; Condorelli, R.A.; Calogero, A.E.; Cannarella, R.; Aversa, A. Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results. J. Clin. Med. 2023, 12, 672. https://doi.org/10.3390/jcm12020672
La Vignera S, Condorelli RA, Calogero AE, Cannarella R, Aversa A. Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results. Journal of Clinical Medicine. 2023; 12(2):672. https://doi.org/10.3390/jcm12020672
Chicago/Turabian StyleLa Vignera, Sandro, Rosita A. Condorelli, Aldo E. Calogero, Rossella Cannarella, and Antonio Aversa. 2023. "Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results" Journal of Clinical Medicine 12, no. 2: 672. https://doi.org/10.3390/jcm12020672
APA StyleLa Vignera, S., Condorelli, R. A., Calogero, A. E., Cannarella, R., & Aversa, A. (2023). Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results. Journal of Clinical Medicine, 12(2), 672. https://doi.org/10.3390/jcm12020672










