Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016
Abstract
1. Introduction
2. Materials and Methods
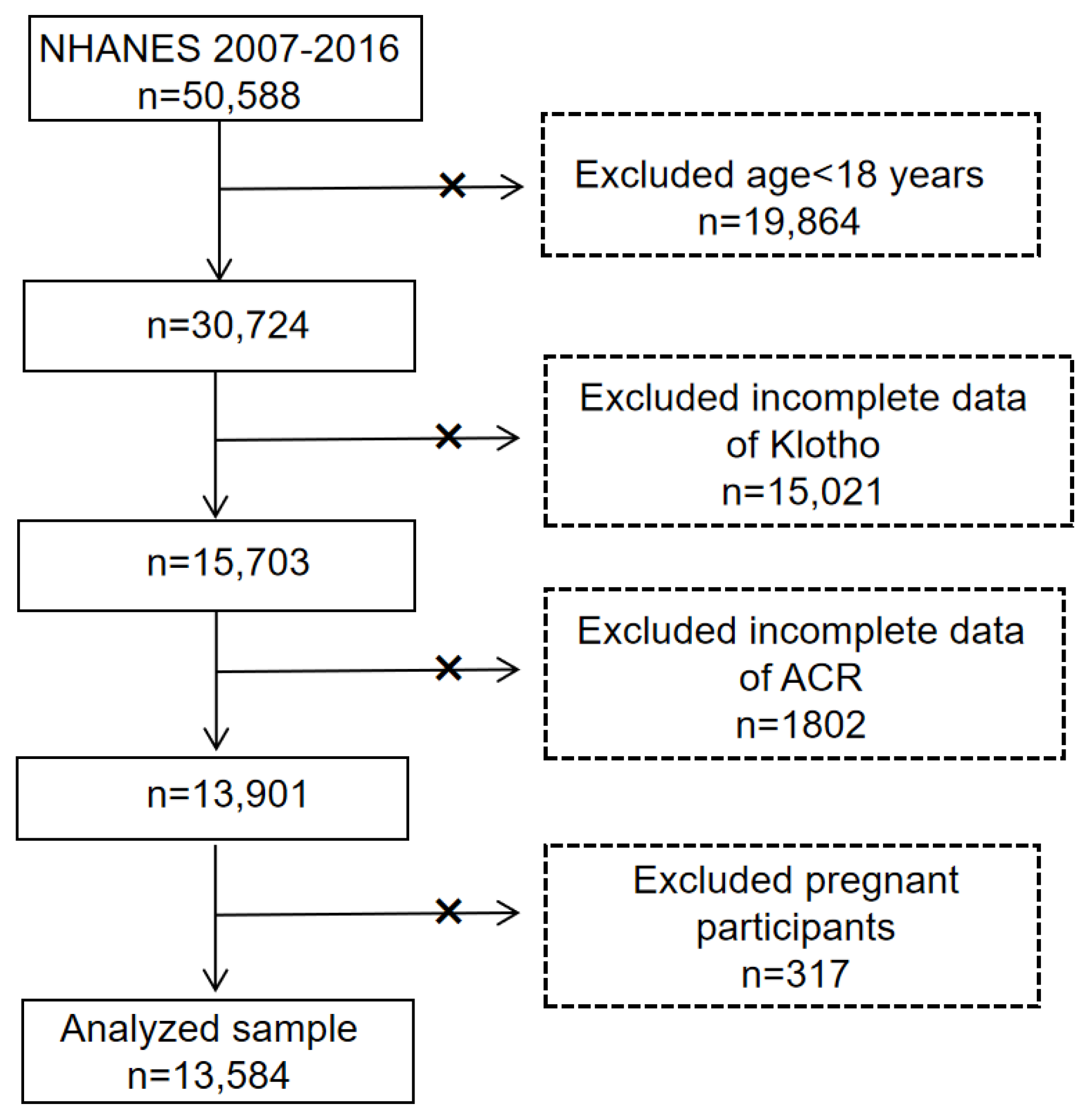
2.1. Sample Population
2.2. Assessment of Increased UAE
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Features
3.2. The Association between α-Klotho and ACR
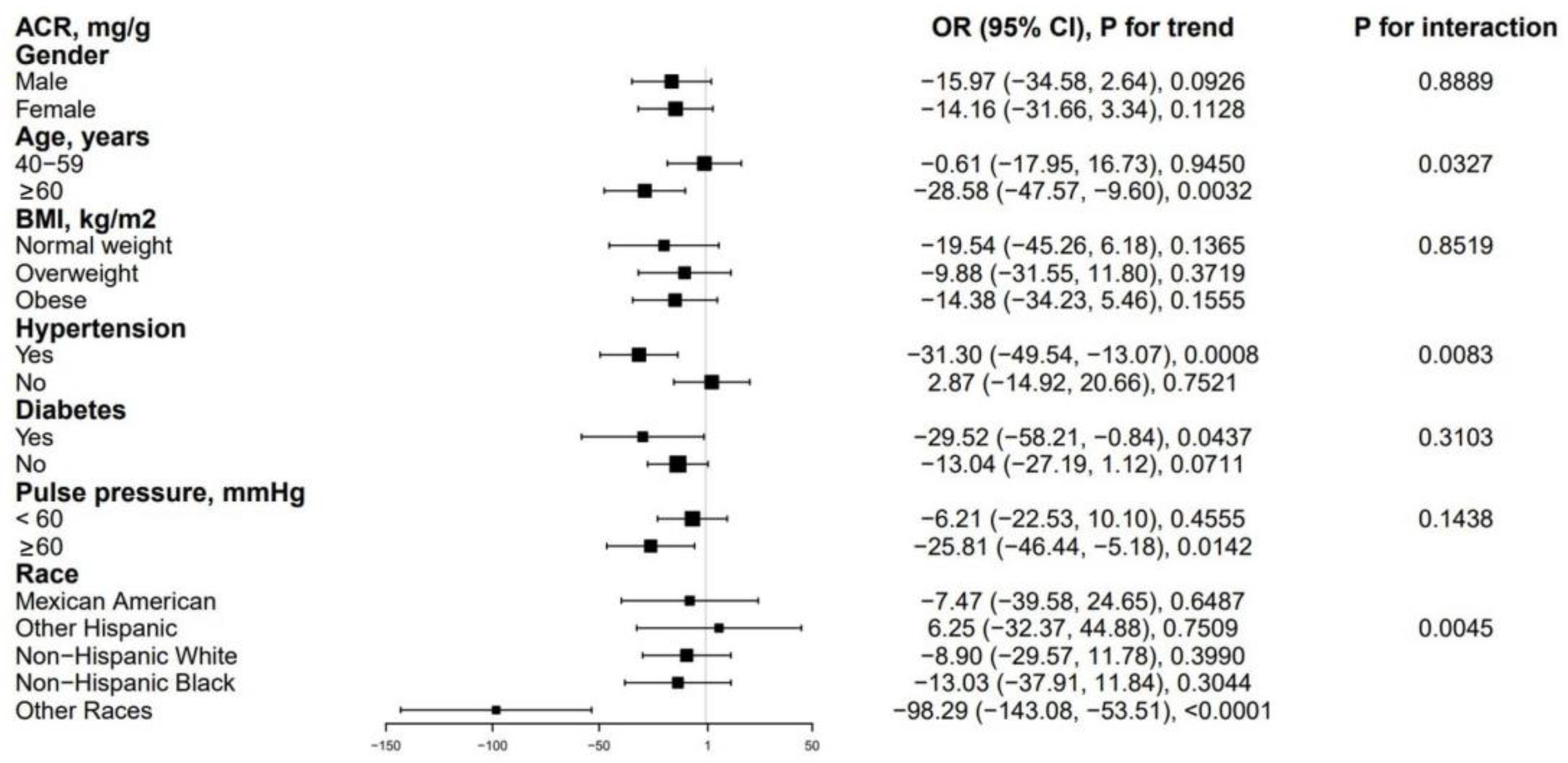
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olauson, H.; Mencke, R.; Hillebrands, J.L.; Larsson, T.E. Tissue expression and source of circulating αKlotho. Bone 2017, 100, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kinoshita, S.; Shiraishi, N.; Nakagawa, S.; Sekine, S.; Fujimori, T.; Nabeshima, Y.I. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech. Dev. 2000, 98, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Miyake, M.; Hori, S.; Onishi, S.; Iida, K.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. γ-Klotho is correlated with resistance to docetaxel in castration-resistant prostate cancer. Oncol. Lett. 2020, 19, 2306–2316. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Yanucil, C.; Kentrup, D.; Campos, I.; Czaya, B.; Heitman, K.; Westbrook, D.; Osis, G.; Grabner, A.; Wende, A.R.; Vallejo, J.; et al. Soluble α-klotho and heparin modulate the pathologic cardiac actions of fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2022, 102, 261–279. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Soluble α-Klotho levels, glycemic control and renal function in US adults with type 2 diabetes. Acta Diabetol. 2022, 59, 803–809. [Google Scholar] [CrossRef]
- Yu, L.; Kang, L.; Ren, X.Z.; Diao, Z.L.; Liu, W.H. Circulating α-Klotho Levels in Hemodialysis Patients and Their Relationship to Atherosclerosis. Kidney Blood Press. Res. 2018, 43, 1174–1182. [Google Scholar] [CrossRef]
- Ligumsky, H.; Merenbakh-Lamin, K.; Keren-Khadmy, N.; Wolf, I.; Rubinek, T. The role of α-klotho in human cancer: Molecular and clinical aspects. Oncogene 2022, 41, 4487–4497. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Bulka, C.M. Low Serum Klotho Associated With All-cause Mortality Among a Nationally Representative Sample of American Adults. J. Gerontol A Biol. Sci. Med. Sci. 2022, 77, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Koyama, D.; Sato, Y.; Aizawa, M.; Maki, T.; Kurosawa, M.; Kuro-o, M.; Furukawa, Y. Soluble αKlotho as a candidate for the biomarker of aging. Biochem. Biophys. Res. Commun. 2015, 467, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Davidsohn, N.; Pezone, M.; Vernet, A.; Graveline, A.; Oliver, D.; Slomovic, S.; Punthambaker, S.; Sun, X.; Liao, R.; Bonventre, J.V.; et al. A single combination gene therapy treats multiple age-related diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 23505–23511. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Kuro, O.M.; Chen, C.H.; Sue, Y.M.; Chen, Y.C.; Wu, H.H.; Cheng, C.Y. The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur. J. Pharm. 2013, 698, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, X.; Zhao, Y.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological ageing and the risks of all-cause and cause-specific mortality among people with diabetes: A prospective cohort study. J. Epidemiol. Community Health 2022, 76, 771–778. [Google Scholar] [CrossRef]
- Ortiz, A.; Mattace-Raso, F.; Soler, M.J.; Fouque, D. Ageing meets kidney disease. Age Ageing 2022, 51, afac157. [Google Scholar] [CrossRef]
- Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef]
- Kim, H.R.; Nam, B.Y.; Kim, D.W.; Kang, M.W.; Han, J.H.; Lee, M.J.; Shin, D.H.; Doh, F.M.; Koo, H.M.; Ko, K.I.; et al. Circulating α-klotho levels in CKD and relationship to progression. Am. J. Kidney Dis. 2013, 61, 899–909. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. The emerging role of Klotho in clinical nephrology. Nephrol. Dial. Transplant. 2012, 27, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat. Rev. Nephrol 2013, 9, 650–660. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; James, M.T.; Klarenbach, S.; Quinn, R.R.; Wiebe, N.; Hemmelgarn, B.R. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: A cohort study. Ann. Intern. Med. 2011, 154, 12–21. [Google Scholar] [CrossRef]
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; Jong, P.E.; Coresh, J.; Astor, B.C.; Matsushita, K.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef]
- Fernández-Fernández, B.; Valiño-Rivas, L.; Sánchez-Niño, M.D.; Ortiz, A. Albuminuria Downregulation of the Anti-Aging Factor Klotho: The Missing Link Potentially Explaining the Association of Pathological Albuminuria with Premature Death. Adv. Ther. 2020, 37, 62–72. [Google Scholar] [CrossRef]
- Rabelink, T.J.; de Zeeuw, D. The glycocalyx--linking albuminuria with renal and cardiovascular disease. Nat. Rev. Nephrol. 2015, 11, 667–676. [Google Scholar] [CrossRef]
- Ortiz, A.; Fernandez-Fernandez, B. Humble kidneys predict mighty heart troubles. Lancet Diabetes Endocrinol. 2015, 3, 489–491. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008, 31 (Suppl. S1), S12–S54. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2007, 28, 1462–1536. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Katz, R.; Kritchevsky, S.; Ix, J.; Shlipak, M.; Gutiérrez, O.M.; Newman, A.; Hoofnagle, A.; Fried, L.; Semba, R.D.; et al. Association between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J. Am. Soc. Nephrol. 2017, 28, 1859–1866. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Deng, L.; Jin, K.; Xiong, X.; Su, X.; Qiu, S.; Yang, L. The association between serum soluble Klotho and chronic kidney disease among us adults ages 40 to 79 years: Cross-sectional study. Front. Public Health 2022, 10, 995314. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Technical Report Series 894. WHO. 2000. Available online: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 5 September 2016).
- Landry, T.; Shookster, D.; Huang, H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism 2021, 121, 154819. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015, 314, 1021–1029. [Google Scholar] [CrossRef]
- Ushigome, E.; Fukui, M.; Hamaguchi, M.; Matsumoto, S.; Mineoka, Y.; Nakanishi, N.; Senmaru, T.; Yamazaki, M.; Hasegawa, G.; Nakamura, N. Morning pulse pressure is associated more strongly with elevated albuminuria than systolic blood pressure in patients with type 2 diabetes mellitus: Post hoc analysis of a cross-sectional multicenter study. Diabetes Res. Clin. Pract. 2013, 101, 270–277. [Google Scholar] [CrossRef]
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief. 2017, 289, 1–8. [Google Scholar]
- US Department of Health and Human Services, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm (accessed on 6 January 2023).
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, M.; Prabhu, G.; Lagbo, J.; Qayyum, R. Serum Klotho and pulse pressure; insight from NHANES. Int J. Cardiol. 2022, 355, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, F.; Peng, Y.; Wang, P.; Ma, B.; Li, L.; Si, C.; Wang, X.; Zhang, M.; Song, F. Association of serum Klotho levels with cancer and cancer mortality: Evidence from National Health and Nutrition Examination Survey. Cancer Med. 2022. [CrossRef] [PubMed]
- Maltese, G.; Fountoulakis, N.; Siow, R.C.; Gnudi, L.; Karalliedde, J. Perturbations of the anti-ageing hormone Klotho in patients with type 1 diabetes and microalbuminuria. Diabetologia 2017, 60, 911–914. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Ferri, C.; Pérez-Delgado, N.; Górriz, J.L.; Martínez-Castelao, A.; Ortiz, A.; Mora-Fernández, C. Effects of Pentoxifylline on Soluble Klotho Concentrations and Renal Tubular Cell Expression in Diabetic Kidney Disease. Diabetes Care 2018, 41, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating αKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef]
- Yildirim, M.; Acikgoz, S.B.; Genc, A.B.; Yaylaci, S.; Dheir, H.; Sipahi, S.S. The levels of inflammatory biomarkers in hemodialysis and peritoneal dialysis patients. Rev. Assoc. Med. Bras. (1992) 2021, 67, 718–723. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Storoniak, H.; Soroczyńska-Cybula, M.; Maziewski, M.; Dębska-Ślizień, A. Serum Levels of α-Klotho, Inflammation-Related Cytokines, and Mortality in Hemodialysis Patients. J. Clin. Med. 2022, 11, 6518. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Haruna, Y.; Sasaki, T.; Kashihara, N. Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin. Exp. Nephrol. 2016, 20, 671–678. [Google Scholar] [CrossRef]
- Kim, J.H.; Xie, J.; Hwang, K.H.; Wu, Y.L.; Oliver, N.; Eom, M.; Park, K.S.; Barrezueta, N.; Kong, I.D.; Fracasso, R.P.; et al. Klotho May Ameliorate Proteinuria by Targeting TRPC6 Channels in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 140–151. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Lin, Q.; Shao, X.; Wu, J.; Zhang, W.; Cai, H.; Zhou, W.; Jiang, N.; Zhang, Z.; et al. αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol. Res. 2021, 167, 105531. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.S.; Chang, Y.S.; Park, C.W.; et al. Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. 2019, 225, e13190. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Oh, K.W.; Lee, W.Y.; Kim, S.Y.; Jung, C.H.; Kim, B.J.; Sung, K.C.; Kim, B.S.; Kang, J.H.; Lee, M.H.; et al. The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism 2006, 55, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Sanchez-Delgado, G.; García-Lario, J.V.; Castillo, M.J.; Ruiz, J.R. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging 2020, 12, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Żelaźniewicz, A.; Nowak-Kornicka, J.; Pawłowski, B. S-Klotho level and physiological markers of cardiometabolic risk in healthy adult men. Aging 2022, 14, 708–727. [Google Scholar] [CrossRef]
- Marino, F.; Scalise, M.; Salerno, N.; Salerno, L.; Molinaro, C.; Cappetta, D.; Torella, M.; Greco, M.; Foti, D.; Sasso, F.C.; et al. Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age. Diabetes 2022, 71, 1081–1098. [Google Scholar] [CrossRef]
- Xiao, L.; Zan, G.; Liu, C.; Xu, X.; Li, L.; Chen, X.; Zhang, Z.; Yang, X. Associations Between Blood Pressure and Accelerated DNA Methylation Aging. J. Am. Heart Assoc. 2022, 11, e022257. [Google Scholar] [CrossRef]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.; Imaizumi, T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef]
- Citterio, L.; Delli Carpini, S.; Lupoli, S.; Brioni, E.; Simonini, M.; Fontana, S.; Zagato, L.; Messaggio, E.; Barlassina, C.; Cusi, D.; et al. Klotho Gene in Human Salt-Sensitive Hypertension. Clin. J. Am. Soc. Nephrol. 2020, 15, 375–383. [Google Scholar] [CrossRef]
- Luo, L.; Hao, Q.; Dong, B.; Yang, M. The Klotho gene G-395A polymorphism and metabolic syndrome in very elderly people. BMC Geriatr. 2016, 16, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolf, I.; Stein, D.; Shahmoon, S.; Ziv, S.I.; Hemi, R.; Kanety, H.; Rubinek, T.; Modan-Moses, D. Alteration in serum klotho levels in anorexia nervosa patients. Clin. Nutr. 2016, 35, 958–962. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Takashi, Y.; Maeda, Y.; Toyokawa, K.; Oda, N.; Yoshioka, R.; Sekiguchi, D.; Minami, M.; Kawanami, D. Fibroblast growth factor 23 and kidney function in patients with type 1 diabetes. PLoS ONE 2022, 17, e0274182. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, J.; Wolf, M. Regulation and Effects of FGF23 in Chronic Kidney Disease. Annu. Rev. Physiol. 2020, 82, 365–390. [Google Scholar] [CrossRef]
- Silva, A.P.; Mendes, F.; Pereira, L.; Fragoso, A.; Gonçalves, R.B.; Santos, N.; Rato, F.; Neves, P.L. Klotho levels: Association with insulin resistance and albumin-to-creatinine ratio in type 2 diabetic patients. Int. Urol. Nephrol. 2017, 49, 1809–1814. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Kang, M.; Kim, S.; Park, S.K.; Sung, S.; Hyun, Y.Y.; Jung, J.Y.; Ahn, C.; Oh, K.H. Low Klotho/Fibroblast Growth Factor 23 Ratio Is an Independent Risk Factor for Renal Progression in Chronic Kidney Disease: Finding From KNOW-CKD. Front. Med. 2022, 9, 904963. [Google Scholar] [CrossRef]
| Characteristic | α-Klotho Levels Tertiles, pg/mL | p-Value for Trend | |||
|---|---|---|---|---|---|
| Overall | <704.00 | 704.00–918.90 | ≥918.90 | ||
| No. of participants | 13,584 | 4528 | 4526 | 4530 | |
| α-Klotho (pg/mL), median (IQR) | 803.10 (655.50–994.23) | 594.10 (520.58–655.50) | 803.05 (752.40–856.30) | 1100.20 (994.20–1269.85) | <0.001 |
| Age (year), mean (SD) | 57.65 ± 10.83 | 58.86 ± 11.01 | 57.49 ± 10.77 | 56.59 ± 10.59 | <0.001 |
| 40–59 years, % | 7415, 54.59% | 49.71 | 55.52 | 58.52 | |
| 60–79 years, % | 6169, 45.41% | 50.29 | 44.48 | 41.48 | |
| Gender, % | <0.001 | ||||
| Male | 48.43 | 51.46 | 49.60 | 44.24 | |
| Female | 51.57 | 48.54 | 50.40 | 55.76 | |
| Race, % | <0.001 | ||||
| Mexican American | 15.92 | 15.90 | 16.59 | 15.25 | |
| Other Hispanic | 11.52 | 10.29 | 11.73 | 12.54 | |
| Non-Hispanic White | 42.99 | 45.85 | 44.56 | 38.57 | |
| Non-Hispanic Black | 19.71 | 19.21 | 16.28 | 23.62 | |
| Other Races | 9.86 | 8.75 | 10.83 | 10.02 | |
| Education level, % | <0.001 | ||||
| below high school | 28.14 | 29.09 | 27.57 | 27.75 | |
| High school or GED | 22.11 | 23.37 | 22.29 | 20.68 | |
| Above high school | 49.75 | 47.55 | 50.13 | 51.57 | |
| BMI (kg/m2), mean (SD) | 29.71 ± 6.66 | 29.81 ± 6.38 | 29.75 ± 6.64 | 29.58 ± 6.94 | 0.006 |
| Normal weight <25, % | 23.88 | 22.11 | 23.49 | 26.02 | |
| Overweight 25–29.9, % | 34.66 | 35.83 | 34.80 | 33.36 | |
| Obese ≥ 30, % | 41.46 | 42.06 | 41.71 | 40.62 | |
| HDL-C (mmol/L) | 1.37 ± 0.43 | 1.37 ± 0.44 | 1.36 ± 0.42 | 1.39 ± 0.43 | <0.001 |
| Triglycerides (mmol/L) | 1.90 ± 1.60 | 1.99 ± 1.92 | 1.89 ± 1.38 | 1.81 ± 1.46 | <0.001 |
| ALT (IU/L) | 25.56 ± 18.99 | 24.68 ± 15.60 | 25.06 ± 19.58 | 26.94 ± 21.28 | <0.001 |
| AST (IU/L) | 26.42 ± 16.68 | 25.62 ± 13.71 | 25.66 ± 13.74 | 27.98 ± 21.30 | <0.001 |
| Diabetes, % | 0.018 | ||||
| Yes | 17.71 | 18.73 | 16.48 | 17.92 | |
| no | 82.29 | 81.27 | 83.52 | 82.08 | |
| Hypertension, % | <0.001 | ||||
| Yes | 46.25 | 49.76 | 43.70 | 45.30 | |
| no | 53.75 | 50.24 | 56.30 | 54.70 | |
| Pulse pressure, % | <0.001 | ||||
| <60 | 63.86 | 60.04 | 65.21 | 66.32 | |
| ≥60 | 36.14 | 39.96 | 34.79 | 33.68 | |
| Urine albumin, % | <0.001 | ||||
| Normal (<30) | 86.01 | 83.92 | 87.27 | 86.82 | |
| Microalbuminuria (30–299) | 11.35 | 12.52 | 10.41 | 11.13 | |
| Macroalbuminuria (≥300) | 2.64 | 3.56 | 2.32 | 2.05 | |
| Outcome | β (95%CI) 1, p-Value | ||
|---|---|---|---|
| Crude Model 2 | Model 1 3 | Model 2 4 | |
| α-Klotho (continuous) | −18.07 (−32.95, −3.19), 0.0197 | −3.24 (−6.39, −0.09), 0.0476 | −12.22 (−23.91, −0.53), 0.0448 |
| α-Klotho (tertiles) | |||
| T1 | Reference | Reference | Reference |
| T2 | −20.26 (−32.85, −7.67), 0.0023 | −3.04 (−5.93, −0.16), 0.0422 | −13.92 (−24.68, −3.16), 0.0139 |
| T3 | −19.31 (−32.19, −6.44), 0.0043 | −3.34 (−6.30, −0.38), 0.0300 | −12.67 (−22.86, −2.47), 0.0179 |
| p for trend | 0.0041 | 0.0294 | 0.0176 |
| Variables | β (95%CI) | p-Value |
|---|---|---|
| Log 2 α-Klotho | ||
| per one-unit increase | −12.22 (−23.91, −0.53) | 0.0448 |
| Age (year) | −0.53 (−1.04, −0.01) | 0.0503 |
| Female (versus male) | −13.37 (−25.51, −1.23) | 0.0348 |
| Race (versus Mexican American) | ||
| Other Hispanic | 3.40 (−32.63, 39.44) | 0.8538 |
| Non-Hispanic white | −26.45 (−47.52, −5.39) | 0.0168 |
| Non-Hispanic black | −17.47 (−38.89, 3.95) | 0.1151 |
| Other race/ethnicity | 4.93 (−36.32, 46.19) | 0.8155 |
| Education level (versus less than high school) | ||
| High school or GED | −0.68 (−17.15, 15.80) | 0.9363 |
| Above high school | −7.03 (−20.69, 6.62) | 0.3170 |
| BMI (kg/m2) (versus normal weight < 25) | ||
| Overweight 25–29.9 | −10.68 (−17.04, −4.31) | 0.0018 |
| Obese ≥ 30 | −3.85 (−0.68, −14.99) | 0.5014 |
| HDL-C (mmol/L) | 18.58 (−0.26, 37.41) | 0.0579 |
| Triglycerides (mmol/L) | 7.86 (1.04, 14.67) | 0.0277 |
| ALT (IU/L) | −0.58 (−0.99, −0.17) | 0.0069 |
| AST (IU/L) | 0.34 (−0.10, 0.77) | 0.1360 |
| Diabetes (no versus yes) | −85.73 (−114.11, −57.35) | <0.0001 |
| Hypertension (no versus yes) | −21.24 (−29.30, −13.18) | <0.0001 |
| Pulse pressure (elevated versus normal) | 34.65 (21.78, 47.53) | <0.0001 |
| Models | ACR | |
|---|---|---|
| β (95%CI) | p-Value | |
| Model I | ||
| One line slope | −14.31 (−27.13, −1.50) | 0.0286 |
| Model II | ||
| Turning point (K) | 9.91 | |
| <9.91 slope 1 | −39.95 (−58.82, −21.07) | <0.0001 |
| >9.91 slope 2 | 41.02 (8.47, 73.57) | 0.0135 |
| slope 2-slope 1 | 80.97 (37.18, 124.76) | 0.0003 |
| Predicted at 9.91 | 28.15 (17.98, 38.33) | |
| Log likelihood ratio test | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.; Li, Y.; Qin, Z.; Zhang, Z.; Wang, L.; Yang, Q.; Su, B. Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. J. Clin. Med. 2023, 12, 637. https://doi.org/10.3390/jcm12020637
Chang K, Li Y, Qin Z, Zhang Z, Wang L, Yang Q, Su B. Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. Journal of Clinical Medicine. 2023; 12(2):637. https://doi.org/10.3390/jcm12020637
Chicago/Turabian StyleChang, Kaixi, Yupei Li, Zheng Qin, Zhuyun Zhang, Liya Wang, Qinbo Yang, and Baihai Su. 2023. "Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016" Journal of Clinical Medicine 12, no. 2: 637. https://doi.org/10.3390/jcm12020637
APA StyleChang, K., Li, Y., Qin, Z., Zhang, Z., Wang, L., Yang, Q., & Su, B. (2023). Association between Serum Soluble α-Klotho and Urinary Albumin Excretion in Middle-Aged and Older US Adults: NHANES 2007–2016. Journal of Clinical Medicine, 12(2), 637. https://doi.org/10.3390/jcm12020637





