Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Endpoints
- Favorable (reverse) LV remodeling, classified as a reduction in EDV by more than 10%.
- Functional LV improvement (classified as a more than 10% increase of EF).
- A combined endpoint of “favorable LV remodeling” associated with “functional LV improvement” intended as a reduction of EDV by more than 10% associated to simultaneous improvement in EF of more than 10%.
- TAPSE improvement (>10% of basal value).
- Reduction in NT-proBNP (>10% of basal value).
- Improvement of the NYHA functional class.
2.3. Statistical Analysis
3. Results
3.1. Differences of Relevant Clinical Parameters from Baseline
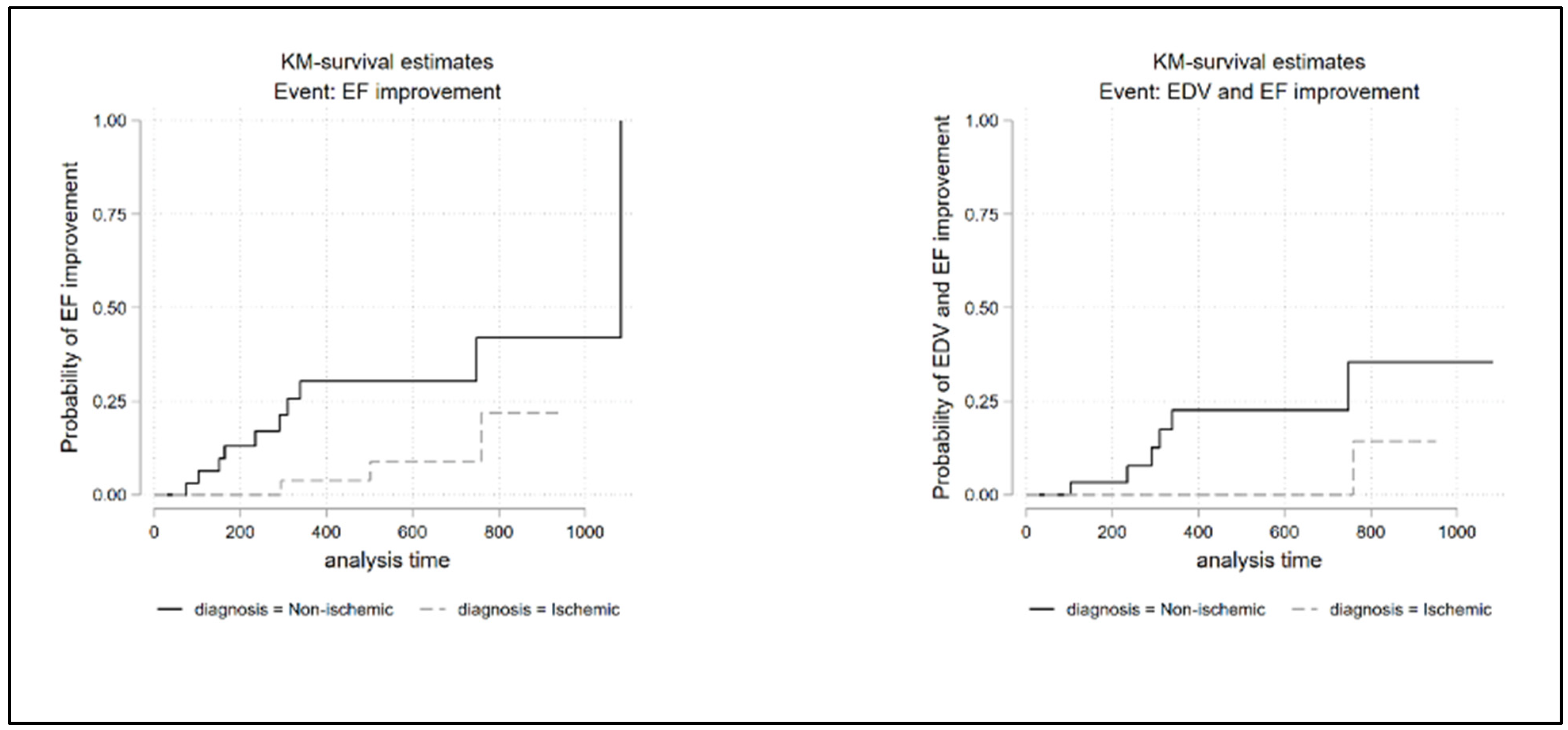
3.2. Survival Analysis
3.3. Factors Predictive of Reverse Remodeling
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 42, 3599–3726. [Google Scholar]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. ACE Inhibitors—A cornerstone of the treatment of heart failure. N. Engl. J. Med. 1991, 325, 351–353. [Google Scholar] [CrossRef]
- Zannad, F. Pharmacotherapy in heart failure with reduced ejection fraction during the last 20 years, and the way ahead for precision medicine. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 10–12. [Google Scholar] [CrossRef]
- Bao, J.; Kan, R.; Chen, J.; Xuan, H.; Wang, C.; Li, D.; Xu, T. Combination pharmacotherapies for cardiac reverse remodeling in heart failure patients with reduced ejection fraction: A systematic review and network meta-analysis of randomized clinical trials. Pharmacol. Res. 2021, 169, 105573. [Google Scholar] [CrossRef]
- Mcmurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Castrichini, M.; Manca, P.; Nuzzi, V.; Barbati, G.; De Luca, A.; Korcova, R.; Stolfo, D.; Di Lenarda, A.; Merlo, M.; Sinagra, G. Sacubitril/Valsartan Induces Global Cardiac Reverse Remodeling in Long-Lasting Heart Failure with Reduced Ejection Fraction: Standard and Advanced Echocardiographic Evidences. J. Clin. Med. 2020, 9, 906. [Google Scholar] [CrossRef]
- Gronda, E.; Iacoviello, M.; Napoli, C. The PARAGON-HF Trial: Toward Extension to Patients with HF Middle Range Ejection Fraction. JACC Heart Fail. 2020, 8, 697–698. [Google Scholar] [CrossRef]
- Solomon, S.D.; Rizkala, A.R.; Lefkowitz, M.P.; Shi, V.C.; Gong, J.; Anavekar, N.; Anker, S.D.; Arango, J.L.; Arenas, J.L.; Atar, D.; et al. Baseline Characteristics of Patients with Heart Failure and Preserved Ejection Fraction in the PARAGON-HF Trial. Circ. Hearth Fail. 2018, 11, e004962. [Google Scholar] [CrossRef]
- McMurray, J.J.; Jackson, A.M.; Lam, C.S.; Redfield, M.M.; Anand, I.S.; Ge, J.; Lefkowitz, M.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared with Men with Heart Failure and Preserved Ejection Fraction. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Jhund, P.S.; Solomon, S.D.; McMurray, J.J.V. Response by Jackson et al. to Letter Regarding Article, ‘Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared with Men with Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF’. Circulation 2020, 142, E5–E6. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Piña, I.L.; Rocha, R.A.; Shah, A.M.; et al. Association of Change in N-Terminal Pro–B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment with Cardiac Structure and Function in Patients with Heart Failure with Reduced Ejection Fraction. JAMA 2019, 322, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Camacho, A.; Piña, I.L.; Rocha, R.; Williamson, K.M.; Maisel, A.S.; Felker, G.M.; Prescott, M.F.; Butler, J.; Solomon, S.D.; et al. Reverse Cardiac Remodeling and Outcome After Initiation of Sacubitril/Valsartan. Circ. Hearth Fail. 2020, 13, e006946. [Google Scholar] [CrossRef] [PubMed]
- Balmforth, C.; Simpson, J.; Shen, L.; Jhund, P.S.; Lefkowitz, M.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.; Solomon, S.D.; Swedberg, K.; et al. Outcomes and Effect of Treatment According to Etiology in HFrEF: An Analysis of PARADIGM-HF. JACC. Heart Fail. 2019, 7, 457–465. [Google Scholar] [CrossRef]
- Ioannou, A.; Metaxa, S.; Simon, S.; Mandal AK, J.; Missouris, C.G. Comparison of the Effect of Sacubitril/Valsartan on Left Ventricular Systolic Function in Patients with Non-ischaemic and Ischaemic Cardiomyopathy. Cardiovasc. Drugs Ther. 2020, 34, 755–762. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chiou, W.-R.; Hsu, C.-Y.; Lin, P.-L.; Liang, H.-W.; Chung, F.-P.; Liao, C.-T.; Lin, W.-Y.; Chang, H.-Y. Different left ventricular remodelling patterns and clinical outcomes between non-ischaemic and ischaemic aetiologies in heart failure patients receiving sacubitril/valsartan treatment. Eur. Hearth J. Cardiovasc. Pharmacother. 2020, 8, 118–129. [Google Scholar] [CrossRef]
- Abumayyaleh, M.; Pilsinger, C.; El-Battrawy, I.; Kummer, M.; Kuschyk, J.; Borggrefe, M.; Mügge, A.; Aweimer, A.; Akin, I. Clinical Outcomes in Patients with Ischemic versus Non-Ischemic Cardiomyopathy after Angiotensin-Neprilysin Inhibition Therapy. J. Clin. Med. 2021, 10, 4989. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Popović, Z.B.; Thomas, J.D. Assessing observer variability: A user’s guide. Cardiovasc. Diagn. Ther. 2017, 7, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Emdin, M.; Maisel, A.S. Sacubitril/Valsartan, Cardiac Fibrosis, and Remodeling in Heart Failure. J. Am. Coll. Cardiol. 2019, 73, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Giallauria, F.; Vitale, G.; Pacileo, M.; Di Lorenzo, A.; Oliviero, A.; Passaro, F.; Calce, R.; Parlato, A.; Testa, C.; D’Ambrosio, G.; et al. Sacubitril/Valsartan Improves Autonomic Function and Cardiopulmonary Parameters in Patients with Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2020, 9, 1897. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.G.; Schuurman, R.; Barison, A.; Ripoli, A.; Coceani, M.; Chiappino, S.; Todiere, G.; Srebot, V.; Passino, C.; Aquaro, G.D.; et al. Response to Letters Regarding Article, “Myocardial Fibrosis as a Key Determinant of Left Ventricular Remodeling in Idiopathic Dilated Cardiomyopathy: A Contrast-Enhanced Cardiovascular Magnetic Study”. Circ. Cardiovasc. Imaging 2013, 6, e79. [Google Scholar] [CrossRef] [PubMed]
- Tayal, U.; Wage, R.; Newsome, S.; Manivarmane, R.; Izgi, C.; Muthumala, A.; Dungu, J.N.; Assomull, R.; Hatipoglu, S.; Halliday, B.P.; et al. Predictors of left ventricular remodelling in patients with dilated cardiomyopathy—A cardiovascular magnetic resonance study. Eur. J. Hearth Fail. 2020, 22, 1160–1170. [Google Scholar] [CrossRef]
- Wilcox, J.E.; Fonarow, G.; Yancy, C.W.; Albert, N.M.; Curtis, A.B.; Heywood, J.T.; Inge, P.J.; McBride, M.L.; Mehra, M.; O’Connor, C.M.; et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: Findings from IMPROVE HF. Am. Hearth J. 2012, 163, 49–56. [Google Scholar] [CrossRef]
- Liu, L.-W.; Wu, P.-C.; Chiu, M.-Y.; Tu, P.-F.; Fang, C.-C. Sacubitril/Valsartan Improves Left Ventricular Ejection Fraction and Reverses Cardiac Remodeling in Taiwanese Patients with Heart Failure and Reduced Ejection Fraction. Acta Cardiol. Sin. 2020, 36, 125–132. [Google Scholar]
- Dini, F.L.; Carluccio, E.; Simioniuc, A.; Biagioli, P.; Reboldi, G.; Galeotti, G.G.; Raineri, C.; Gargani, L.; Scelsi, L.; Mandoli, G.E.; et al. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur. J. Hearth Fail. 2016, 18, 1462–1471. [Google Scholar] [CrossRef]
- Ghio, S.; Recusani, F.; Klersy, C.; Sebastiani, R.; Laudisa, M.L.; Campana, C.; Gavazzi, A.; Tavazzi, L. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am. J. Cardiol. 2000, 85, 837–842. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Akkan, D.; Iversen, K.; Køber, L.; Torp-Pedersen, C.; Hassager, C. Right ventricular dysfunction as an independent predictor of short- and long-term mortality in patients with heart failure. Eur. J. Hearth Fail. 2007, 9, 610–616. [Google Scholar] [CrossRef]
- Correale, M.; Mallardi, A.; Mazzeo, P.; Tricarico, L.; Diella, C.; Romano, V.; Ferraretti, A.; Leopizzi, A.; Merolla, G.; Di Biase, M.; et al. Sacubitril/valsartan improves right ventricular function in a real-life population of patients with chronic heart failure: The Daunia Heart Failure Registry. IJC Hearth Vasc. 2020, 27, 100486. [Google Scholar] [CrossRef] [PubMed]

| Non-Ischemic | Ischemic | All | p Value | |
|---|---|---|---|---|
| (n = 37) | (n = 42) | (n = 79) | ||
| Age (years) | 67 (12) | 69 (12) | 68 (12) | 0.422 |
| Male sex, no. (%) | 25 (68) | 39 (93) | 64 (81) | 0.01 |
| BMI (Kg/m2) | 25 (5) | 27 (4) | 26 (4) | 0.222 |
| Heart rate (bpm) | 72 (65–82) | 70 (59–78) | 70 (64–80) | 0.183 |
| Systolic pressure (mmHg) | 115 (110–130) | 110 (100–120) | 115 (105–120) | 0.193 |
| Diastolic pressure (mmHg) | 75 (65–80) | 70 (60–75) | 70 (60–75) | 0.096 |
| Serum creatinine (mg/dL) | 1.04 (0.95–1.3) | 1.19 (1.06–1.47) | 1.11 (0.99–1.4) | 0.011 |
| GFR (mL/min/1,73 m2) | 61 (50–83) | 59 (44–76) | 60 (46–77) | 0.178 |
| Kalium (mmol/L) | 4.4 (4.2–4.8) | 4.4 (4.2–4.7) | 4.4 (4.2–4.7) | 0.711 |
| NT-proBNP (pg/mL) | 4040 (1279–5929) | 2445 (1548–5366) | 3265 (1548–5493) | 0.432 |
| Time from diagnosis > 5 years, no. (%) | 23 (62%) | 27 (64%) | 50 (63%) | 0.446 |
| NYHA class, no. (%) | ||||
| 2 | 17 (46) | 9 (21) | 26 (33) | 0.03 |
| 3 | 20 (54) | 33 (79) | 53 (67) | |
| Diastolic dysfunction | 12 (32) | 11 (26) | 23 (29) | 0.019 |
| 1 | 18 (49) | 11 (26) | 29 (37) | |
| 2 | 7 (19) | 20 (48) | 27 (34) | |
| 3 | ||||
| Rhythm at enrollment, no. (%) | ||||
| Sinus | 13 (35) | 25 (59) | 38 (48) | 0.05 |
| Atrial fibrillation | 10 (27) | 4 (10) | 14 (18) | |
| Biventricular Pacing | 14 (38) | 13 (31) | 27 (34) | |
| Peak SV dosage, no. (%) | ||||
| 24/26 mg | 26 (70) | 29 (69) | 55 (70) | 1 |
| 49/51 mg | 11 (30) | 13 (31) | 24 (30) | |
| Hypertension, no. (%) | 19 (51) | 28 (67) | 47 (60) | 0.178 |
| Diabetes, no. (%) | 5 (14) | 11 (26) | 16 (20) | 0.262 |
| History of Atrial fibrillation, no. (%) | 17 (46) | 20 (48) | 37 (47) | 1 |
| Previous hospitalization for HF, no. (%) | 29 (78) | 34 (81) | 63 (80) | 0.787 |
| Previous myocardial infarction, no. (%) | 0 (0) | 29 (69) | 29 (37) | <0.001 |
| TIA/Stroke, no. (%) | 3 (8) | 2 (5) | 5 (6) | 0.661 |
| ACEi no. (%) | 32 (86) | 35 (83) | 67 (85) | 0.762 |
| ARB, no. (%) | 3 (8) | 5 (12) | 8 (10) | 0.717 |
| Digitalis, no. (%) | 2 (5) | 0 (0) | 2 (3) | 0.216 |
| Beta blocker, no. (%) | 35 (95) | 38 (90) | 73 (92) | 0.679 |
| MRA, no. (%) | 21 (57) | 26 (62) | 47 (59) | 0.654 |
| ICD, no. (%) | 19 (51) | 23 (55) | 42 (53) | 0.823 |
| CRT, no. (%) | 14 (38) | 14 (33) | 28 (35) | 0.814 |
| EDVi (mL/m2) | 110 (100–136) | 124 (104–152) | 121 (102–148) | 0.12 |
| ESVi (mL/m2) | 78 (70–96) | 88 (73–115) | 82 (70–111) | 0.168 |
| EF (%) | 31 (24–33) | 31 (23–34) | 31 (23–34) | 0.949 |
| LAVi (mL/m2) | 52 (41–73) | 53 (43–68) | 53 (41–69) | 0.94 |
| TAPSE (mm) | 18 (16–20) | 18 (16–21) | 18 (16–20) | 0.711 |
| E/A | 1.1 (0.6–2.0) | 1.2(0.7–2.6) | 1.2(0.6–2.4) | 0.206 |
| E/E’ | 12.4 (10.3–18.7) | 14 (10–19) | 13.8 (10.3–18.6) | 0.743 |
| PAPs (mmHg) | 33 (28–44) | 37 (29–49) | 34 (28–47) | 0.385 |
| Follow-Up—Baseline | Non-Ischemic (n = 37) | Ischemic (n = 42) | All (n = 79) | p Value Non-Ischemic (n = 37) | p Value Ischemic (n = 42) | p Value All (n = 79) |
|---|---|---|---|---|---|---|
| Systolic pressure (mmHg) | −6 (15) | −9 (14) | −7 (15) | 0.0059 | 0.0005 | 9.23 × 10−6 |
| GFR (mL/min/1.73 m2) | −1 (12) | −4 (13) | −3 (12) | 0.6196 | 0.0189 | 0.0439 |
| NT-pro-BNP (pg/mL) | −571 (−3922; 64) | −251 (−2180; 489) | −538 (−3156; 279) | 0.0236 | 0.2129 | 0.0159 |
| Furosemide dosage (mg) | −3 (29) | −15 (46) | −10 (39) | 0.4108 | 0.018 | 0.0221 |
| EDV (mL) | −20 (30) | −11 (41) | −15 (37) | 0.0004 | 0.0794 | 0.0002 |
| EDVi (mL/m2) | −10.87 (16.09) | −5.91 (21.87) | −8.23 (19.42) | 0.0002 | 0.0833 | 0.0002 |
| ESV (mL) | −23 (27) | −10 (36) | −16 (33) | 4.00 × 10−6 | 0.0515 | 5.64 × 10−6 |
| ESVi (mL/m2) | −12.58 (14.59) | −5.18 (19.35) | −8.65 (17.57) | 3.19 × 10−6 | 0.0527 | 4.20 × 10−6 |
| EF (%) | 6 (8) | 1 (6) | 3 (7) | 3.26 × 10−5 | 0.2555 | 0.0002 |
| TAPSE (mm) | 1.19 (1.96) | −0.33 (2.3) | 0 (2) | 0.0009 | 0.4863 | 0.0736 |
| E/E’ | 1.39 (5.2) | −0.13 (8.2) | 0.55 (7.02) | 0.0497 | 0.5156 | 0.0763 |
| Follow-Up—Baseline | Beta (SE) | 95% CI | p |
|---|---|---|---|
| Endpoints | |||
| SBP (mmHg) | −0.73 (3.2) | −7.2; 5.7 | 0.842 |
| GFR (mL/min) | −3.22 (2.9) | −9.1; 2.6 | 0.276 |
| NT-proBNP (pg/mL) | 1134 (1674) | −2227; 4496 | 0.501 |
| Furosemide dosage (mg) | −11 (8.7) | −28.5; 6.3 | 0.208 |
| NYHA | −0.2 (0.14) | −0.5; 0.05 | 0.108 |
| Diastolic dysfunction | 0.005 (0.14) | −0.28; 0.27 | 0.974 |
| E/E’ | −1.27 (1.55) | −4.4; 1.8 | 0.415 |
| Other parameters | |||
| EDV (mL) | 8 (0.7) | −9; 25.8 | 0.34 |
| ESV (mL) | 14 (7.5) | −1.08; 29 | 0.068 |
| EF (%) | −6 (1.7) | −9; −2.4 | 0.001 |
| TAPSE (mm) | −1.7 (1.6) | −2.75; −0.72 | 0.001 |
| Endpoints | HR (95% CI) | p |
|---|---|---|
| Favorable reverse remodeling (EDV reduction >10% baseline) | 0.77 (0.36–1.63) | 0.496 |
| Functional LV improvement (EF increase > 10% baseline) | 3.96 (1.03–15.24) | 0.045 |
| Combined endpoint of EDV and EF improvement | 0.17 (0.02–1.49) | 0.109 |
| TAPSE improvement | 0.15 (0.04–0.53) | 0.003 |
| Decrease in NT-proBNP > 326 pg/mL | 0.76 (0.34–1.65) | 0.483 |
| NYHA improvement | 1.45 (0.73–2.88) | 0.289 |
| Death or hospitalization for HF | 0.63 (0.23–1.73) | 0.368 |
| Furosemide dosage reduction >10% baseline | 1.04 (0.44–2.48) | 0.921 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cemin, R.; Casablanca, S.; Foco, L.; Schoepf, E.; Erlicher, A.; Di Gaetano, R.; Ermacora, D. Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology. J. Clin. Med. 2023, 12, 621. https://doi.org/10.3390/jcm12020621
Cemin R, Casablanca S, Foco L, Schoepf E, Erlicher A, Di Gaetano R, Ermacora D. Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology. Journal of Clinical Medicine. 2023; 12(2):621. https://doi.org/10.3390/jcm12020621
Chicago/Turabian StyleCemin, Roberto, Simona Casablanca, Luisa Foco, Elisabeth Schoepf, Andrea Erlicher, Renato Di Gaetano, and Davide Ermacora. 2023. "Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology" Journal of Clinical Medicine 12, no. 2: 621. https://doi.org/10.3390/jcm12020621
APA StyleCemin, R., Casablanca, S., Foco, L., Schoepf, E., Erlicher, A., Di Gaetano, R., & Ermacora, D. (2023). Reverse Remodeling and Functional Improvement of Left Ventricle in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan: Comparison between Non-Ischemic and Ischemic Etiology. Journal of Clinical Medicine, 12(2), 621. https://doi.org/10.3390/jcm12020621





