Day -1 CD34+ Cells and Platelet Count Predict the Number of Apheresis in Poor-Mobilizer Patients Rescued by Plerixafor
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Mobilization Protocol and Apheresis Procedures
2.3. Collected Data and Definitions
2.4. Study Objectives and Definitions
2.5. Statistical Analysis
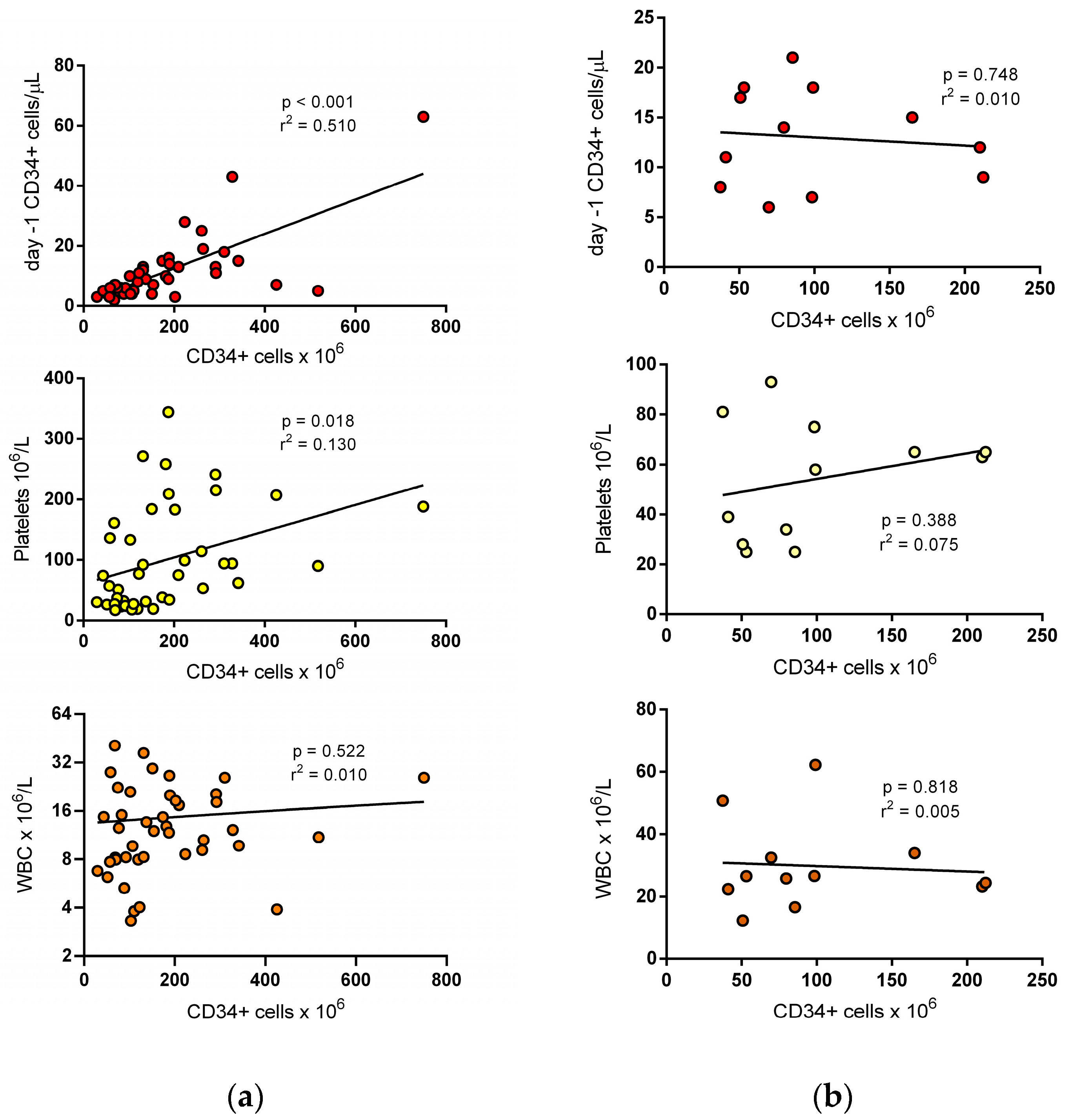
3. Results
3.1. Variables Predicting the Completion of the Target Dose at First Apheresis
3.2. Variables Predicting the Completion of the Target Dose at Second Apheresis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. Hematopoietic Cell Transplantation and Cellular Therapy Survey of the EBMT: Monitoring of Activities and Trends over 30 Years. Bone Marrow Transplant. 2021, 56, 1651–1664. [Google Scholar] [CrossRef]
- D’Souza, A.; Fretham, C.; Lee, S.J.; Arora, M.; Brunner, J.; Chhabra, S.; Devine, S.; Eapen, M.; Hamadani, M.; Hari, P.; et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol. Blood Marrow Transplant. 2020, 26, e177–e182. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R. Update on the POEMS Syndrome. Blood Res. 2022, 57, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Vaxman, I.; Dispenzieri, A. The Role of Autologous Stem Cell Transplantation in Amyloidosis. Oncology 2021, 35, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Haghiri, S.; Fayech, C.; Mansouri, I.; Dufour, C.; Pasqualini, C.; Bolle, S.; Rivollet, S.; Dumas, A.; Boumaraf, A.; Belhout, A.; et al. Long-Term Follow-up of High-Risk Neuroblastoma Survivors Treated with High-Dose Chemotherapy and Stem Cell Transplantation Rescue. Bone Marrow Transplant. 2021, 56, 1984–1997. [Google Scholar] [CrossRef]
- Pierantoni, F.; Maruzzo, M.; Bimbatti, D.; Finotto, S.; Marino, D.; Galiano, A.; Basso, U.; Zagonel, V. High Dose Chemotherapy Followed by Autologous Hematopoietic Stem Cell Transplantation for Advanced Germ Cell Tumors: State of the Art and a Single-Center Experience. Crit. Rev. Oncol. Hematol. 2022, 169, 103568. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Shah, A. Stem Cell Therapy as a Treatment for Autoimmune Disease-Updates in Lupus, Scleroderma, and Multiple Sclerosis. Curr. Allergy Asthma Rep. 2021, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Costa, L.; Schriber, J.; DiPersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines and Recommendations. Biol. Blood Marrow Transplant. 2014, 20, 295–308. [Google Scholar] [CrossRef]
- Duong, H.K.; Savani, B.N.; Copelan, E.; Devine, S.; Costa, L.J.; Wingard, J.R.; Shaughnessy, P.; Majhail, N.; Perales, M.A.; Cutler, C.S.; et al. Peripheral Blood Progenitor Cell Mobilization for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1262–1273. [Google Scholar] [CrossRef]
- To, L.B.; Dyson, P.G.; Juttner, C.A. Cell-Dose Effect in Circulating Stem-Cell Autografting. Lancet 1986, 2, 404–405. [Google Scholar] [CrossRef]
- Weaver, C.H.; Hazelton, B.; Birch, R.; Palmer, P.; Allen, C.; Schwartzberg, L.; West, W. An Analysis of Engraftment Kinetics as a Function of the CD34 Content of Peripheral Blood Progenitor Cell Collections in 692 Patients after the Administration of Myeloablative Chemotherapy. Blood 1995, 86, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, W.; Appelbaum, F.; Rowley, S.; Storb, R.; Sanders, J.; Lilleby, K.; Gooley, T.; Demirer, T.; Schiffman, K.; Weaver, C.; et al. Factors That Influence Collection and Engraftment of Autologous Peripheral-Blood Stem Cells. J. Clin. Oncol. 1995, 13, 2547–2555. [Google Scholar] [CrossRef]
- Pérez-Simón, J.A.; Martín, A.; Caballero, D.; Corral, M.; Nieto, M.J.; Gonzalez, M.; Vazquez, L.; López-Berges, C.; Cañizo, M.C.; Mateos, M.V.; et al. Clinical Significance of CD34+ Cell Dose in Long-Term Engraftment Following Autologous Peripheral Blood Stem Cell Transplantation. Bone Marrow Transplant. 1999, 24, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.; Bredeson, C.; Allan, D.; Tay, J. Systematic Review of Randomized Controlled Trials of Hematopoietic Stem Cell Mobilization Strategies for Autologous Transplantation for Hematologic Malignancies. Biol. Blood Marrow Transplant. 2012, 18, 1191–1203. [Google Scholar] [CrossRef]
- Namdaroglu, S.; Korkmaz, S.; Altuntas, F. Management of Mobilization Failure in 2017. Transfus. Apher. Sci. 2017, 56, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.; Silva, M. Plerixafor: A Chemokine Receptor-4 Antagonist for Mobilization of Hematopoietic Stem Cells for Transplantation after High-Dose Chemotherapy for Non-Hodgkin’s Lymphoma or Multiple Myeloma. Clin. Ther. 2010, 32, 821–843. [Google Scholar] [CrossRef] [PubMed]
- Pusic, I.; Jiang, S.Y.; Landua, S.; Uy, G.L.; Rettig, M.P.; Cashen, A.F.; Westervelt, P.; Vij, R.; Abboud, C.N.; Stockerl-Goldstein, K.E.; et al. Impact of Mobilization and Remobilization Strategies on Achieving Sufficient Stem Cell Yields for Autologous Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 1045–1056. [Google Scholar] [CrossRef]
- Chen, J.; Lazarus, H.M.; Dahi, P.B.; Avecilla, S.; Giralt, S.A. Getting Blood out of a Stone: Identification and Management of Patients with Poor Hematopoietic Cell Mobilization. Blood Rev. 2021, 47, 100771. [Google Scholar] [CrossRef]
- Wuchter, P.; Ran, D.; Bruckner, T.; Schmitt, T.; Witzens-Harig, M.; Neben, K.; Goldschmidt, H.; Ho, A.D. Poor Mobilization of Hematopoietic Stem Cells-Definitions, Incidence, Risk Factors, and Impact on Outcome of Autologous Transplantation. Biol. Blood Marrow Transplant. 2010, 16, 490–499. [Google Scholar] [CrossRef]
- Olivieri, A.; Marchetti, M.; Lemoli, R.; Tarella, C.; Iacone, A.; Lanza, F.; Rambaldi, A.; Bosi, A. Proposed Definition of “poor Mobilizer” in Lymphoma and Multiple Myeloma: An Analytic Hierarchy Process by Ad Hoc Working Group Gruppo ItalianoTrapianto Di Midollo Osseo. Bone Marrow Transplant. 2012, 47, 342–351. [Google Scholar] [CrossRef]
- Olivieri, J.; Attolico, I.; Nuccorini, R.; Pascale, S.P.; Chiarucci, M.; Poiani, M.; Corradini, P.; Farina, L.; Gaidano, G.; Nassi, L.; et al. Predicting Failure of Hematopoietic Stem Cell Mobilization before It Starts: The Predicted Poor Mobilizer (PPM) Score. Bone Marrow Transplant. 2018, 53, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Rettig, M.P.; Cashen, A.F. Plerixafor, a CXCR4 Antagonist for the Mobilization of Hematopoietic Stem Cells. Expert Opin. Biol. Ther. 2008, 8, 1797–1804. [Google Scholar] [CrossRef]
- Bilgin, Y.M. Use of Plerixafor for Stem Cell Mobilization in the Setting of Autologous and Allogeneic Stem Cell Transplantations: An Update. J. Blood Med. 2021, 12, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Romon, I.; Castillo, C.; Cid, J.; Lozano, M. Use of Plerixafor to Mobilize Haematopoietic Progenitor Cells in Healthy Donors. Vox Sang. 2022, 117, 6–16. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Stadtmauer, E.A.; Nademanee, A.; Micallef, I.N.M.; Stiff, P.J.; Kaufman, J.L.; Maziarz, R.T.; Hosing, C.; Früehauf, S.; Horwitz, M.; et al. Plerixafor and G-CSF versus Placebo and G-CSF to Mobilize Hematopoietic Stem Cells for Autologous Stem Cell Transplantation in Patients with Multiple Myeloma. Blood 2009, 113, 5720–5726. [Google Scholar] [CrossRef]
- DiPersio, J.F.; Micallef, I.N.; Stiff, P.J.; Bolwell, B.J.; Maziarz, R.T.; Jacobsen, E.; Nademanee, A.; McCarty, J.; Bridger, G.; Calandra, G. Phase III Prospective Randomized Double-Blind Placebo-Controlled Trial of Plerixafor plus Granulocyte Colony-Stimulating Factor Compared with Placebo plus Granulocyte Colony-Stimulating Factor for Autologous Stem-Cell Mobilization and Transplantation for Patients with Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2009, 27, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
- Lor, K.W.; Helmons, P.J.; Belew, H.; Lane, J.R.; Ball, E.D. Plerixafor as First- and Second-Line Strategies for Autologous Stem Cell Mobilization in Patients with Non-Hodgkin’s Lymphoma or Multiple Myeloma. Pharmacotherapy 2012, 32, 596–603. [Google Scholar] [CrossRef]
- Hübel, K.; Fresen, M.M.; Salwender, H.; Basara, N.; Beier, R.; Theurich, S.; Christopeit, M.; Bogner, C.; Galm, O.; Hartwig, R.; et al. Plerixafor with and without Chemotherapy in Poor Mobilizers: Results from the German Compassionate Use Program. Bone Marrow Transplant. 2011, 46, 1045–1052. [Google Scholar] [CrossRef]
- Hübel, K.; Fresen, M.M.; Apperley, J.F.; Basak, G.W.; Douglas, K.W.; Gabriel, I.H.; Geraldes, C.; Jaksic, O.; Koristek, Z.; Kröger, N.; et al. European Data on Stem Cell Mobilization with Plerixafor in Non-Hodgkin’s Lymphoma, Hodgkin’s Lymphoma and Multiple Myeloma Patients. A Subgroup Analysis of the European Consortium of Stem Cell Mobilization. Bone Marrow Transplant. 2012, 47, 1046–1050. [Google Scholar] [CrossRef]
- Milone, G.; Conticello, C.; Leotta, S.; Michieli, M.G.; Martino, M.; Marco, A.L.; Di Spadaro, A.; Cupri, A.; Condorelli, A.; Milone, G.A.; et al. Plerixafor On-Demand in Association with Low-Dose Cyclophosphamide and G-CSF in the Mobilization of Patients with Multiple Myeloma: High Effectiveness, Low Toxicity, and Affordable Cost. Leuk. Res. Rep. 2020, 14, 100227. [Google Scholar] [CrossRef]
- Vaxman, I.; Muchtar, E.; Jacob, E.; Kapoor, P.; Kumar, S.; Dispenzieri, A.; Buadi, F.; Dingli, D.; Gonsalves, W.; Kourelis, T.; et al. The Efficacy and Safety of Chemotherapy-Based Stem Cell Mobilization in Multiple Myeloma Patients Who Are Poor Responders to Induction: The Mayo Clinic Experience. Transplant. Cell. Ther. 2021, 27, 770.e1. [Google Scholar] [CrossRef]
- Ishii, A.; Jo, T.; Arai, Y.; Oshima, S.; Kanda, J.; Kitawaki, T.; Matsui, K.; Niwa, N.; Nakagawa, Y.; Takaori-Kondo, A.; et al. Development of a Quantitative Prediction Model for Peripheral Blood Stem Cell Collection Yield in the Plerixafor Era. Cytotherapy 2022, 24, 49–58. [Google Scholar] [CrossRef]
- Mohty, M.; Hübel, K.; Kröger, N.; Aljurf, M.; Apperley, J.; Basak, G.W.; Bazarbachi, A.; Douglas, K.; Gabriel, I.; Garderet, L.; et al. Autologous Haematopoietic Stem Cell Mobilisation in Multiple Myeloma and Lymphoma Patients: A Position Statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014, 49, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ortu La Barbera, E.; Chiusolo, P.; Laurenti, L.; Menichella, G.; Di Febo, A.L.; Piccirillo, N.; Sora, F.; Marra, R.; Teofili, L.; Leone, G.; et al. MiCMA: An Alternative Treatment for Refractory or Recurrent Hodgkin’s Disease. Ann. Oncol. 2000, 11, 867–871. [Google Scholar] [CrossRef]
- Teofili, L.; Chiusolo, P.; Valentini, C.G.; Metafuni, E.; Bellesi, S.; Orlando, N.; Bianchi, M.; Giammarco, S.; Sica, S.; Bacigalupo, A. Bone Marrow Haploidentical Transplant with Post-Transplantation Cyclophosphamide: Does Graft Cell Content Have an Impact on Main Clinical Outcomes? Cytotherapy 2020, 22, 158–165. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised Response Criteria for Malignant Lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Corbingi, A.; Metafuni, E.; Di Salvatore, M.; Putzulu, R.; Chiusolo, P.; Schinzari, G.; Massini, G.; Rossi, E.; Zini, G.; Cassano, A.; et al. Successful “on-Demand” Plerixafor for Autologous Peripheral Blood Stem-Cells Transplantation for Relapsed/Refractory Germ Cell Tumors. J. Clin. Apher. 2022, 37, 65–69. [Google Scholar] [CrossRef]
- Lee, K.H.; Jung, S.K.; Kim, S.J.; Jang, J.H.; Kim, K.; Kim, W.S.; Jung, C.W.; Kim, D.W.; Kang, E.S. Incidence and Risk Factors of Poor Mobilization in Adult Autologous Peripheral Blood Stem Cell Transplantation: A Single-Centre Experience. Vox Sang. 2014, 107, 407–415. [Google Scholar] [CrossRef]
- Armitage, S.; Hargreaves, R.; Samson, D.; Brennan, M.; Kanfer, E.; Navarrete, C. CD34 Counts to Predict the Adequate Collection of Peripheral Blood Progenitor Cells. Bone Marrow Transplant. 1997, 20, 587–591. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/mozobil-epar-product-information_en.pdf (accessed on 1 January 2023).
- Lanza, F.; Lemoli, R.M.; Olivieri, A.; Laszlo, D.; Martino, M.; Specchia, G.; Pavone, V.; Imola, M.; Pasini, A.; Milone, G.; et al. Factors Affecting Successful Mobilization with Plerixafor: An Italian Prospective Survey in 215 Patients with Multiple Myeloma and Lymphoma. Transfusion 2014, 54, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bakeer, M.; Zubair, A.C.; Roy, V. Low Baseline Platelet Count Predicts Poor Response to Plerixafor in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Mobilization. Cytotherapy 2020, 22, 16–20. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C.A.; Kauppi, M.; Baldwin, T.; Hyland, C.D.; Metcalf, D.; Willson, T.A.; Carpinelli, M.R.; Smyth, G.K.; Alexander, W.S.; Hilton, D.J. Regulation of Hematopoietic Stem Cells by Their Mature Progeny. Proc. Natl. Acad. Sci. USA 2010, 107, 21689–21694. [Google Scholar] [CrossRef] [PubMed]
| Basal demographics | Age at apheresis (years) | 58.9 (49.7–63.3) |
| Males (%) | 31 (74) | |
| Body weight (kg) | 73.5 (61.5–85) | |
| Diagnosis | Hodgkin/non-Hodgkin Lymphoma | 3/15 (7/36) |
| Multiple Myeloma/Plasma Cell Leukemia/POEMS | 16/2/1 (38/5/2) | |
| Others a | 5 (12) | |
| Disease status | Any response | 37 (88) |
| Stable disease/progressive disease | 5 (12) | |
| Prior therapies | 1 chemotherapy line | 20 (48) |
| 2 chemotherapy lines | 16 (38) | |
| ≥3 chemotherapy lines | 6 (14) | |
| Lenalidomide-containing regimen | 7 (17) | |
| Radiation therapy | 5 (12) | |
| Patients needing transfusions b | RBC | 6 (14) |
| Platelets | 11 (26) | |
| Procedures | Day 1 apheresis | 42 (100) |
| Day 2 apheresis | 12 (29) | |
| CD34+ cell yield (106/kg) | Day 1 apheresis | 2.8 (1.4–4.3) |
| Day 2 apheresis | 2.6 (1.5–4.0) | |
| Total blood volume processed c | Day 1 apheresis | 2.75 (2.47–2.94) |
| Day 2 apheresis | 2.79 (2.40–2.98) |
| CD34+ Cells Yield ≥ 2 × 106/kg | Yes (n = 29) | No (n = 13) |
|---|---|---|
| Age at apheresis, years | 58 (49–63) | 60 (50–64) |
| Sex (male) (%) | 21 (72) | 10 (77) |
| Lymphoma (%) | 9 (31) | 9 (69) |
| Multiple myeloma (%) | 14 (48) | 2 (15) |
| Responsive disease (%) | 26 (90) | 11 (85) |
| Prior chemotherapy regimens ≥ 3 (%) | 4 (14) | 2 (15) |
| Previous lenalidomide (%) | 6 (21) | 1 (8) |
| Prior radiation (%) | 5 (17) | 0 |
| Day -1 CD34+ cells/µL | 11.0 (6.5–12.5) | 5.0 (3.5–6.0) |
| Day -1 WBC count (109/L) | 12.1 (8.8–19.2) | 8.2 (7.2–21.6) |
| Day -1 hemoglobin (g/dL) | 11.2 (9.1–12.1) | 10.3 (9.3–11.2) |
| Day -1 platelet count (109/L) | 94 (36–197) | 32 (25–65) |
| Previous RBC transfusions (%) | 4 (14) | 2 (15) |
| Previous PLT transfusions (%) | 5 (17) | 6 (46) |
| Total blood volume processed | 2.7 (2.4–3.0) | 2.7 (2.5–2.9) |
| CD34+ cell yield (×106/kg) at first apheresis | 3.3 (2.6–5.6) | 1.2 (1.0–1.4) |
| CD34+ Cells Yield ≥ 2 × 106/kg | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age at apheresis, years | 0.98 (0.94–1.03) | 0.460 | NS | |
| Sex, female versus male | 0.79 (0.17–3.62) | 0.759 | NS | |
| Body weight, kg | 0.98 (0.95–1.02) | 0.349 | NS | |
| Lymphoma diagnosis | 0.20 (0.05–0.82) | 0.026 | NS | |
| Responsive disease | 0.63 (0.09–4.34) | 0.64 | NS | |
| Number of prior chemotherapy regimens ≥ 3 | 1.14 (0.18–7.15) | 0.892 | NS | |
| Previous lenalidomide | 3.13 (0.34–29.09) | 0.316 | NS | |
| Day -1 CD34+ cells/µL | 1.47 (1.10–1.95) | 0.009 | NS | |
| Day -1 WBC count (109/L) | 1.00 (1.00–1.00) | 0.978 | 2.06 (1.06–4.08) | 0.037 |
| Day -1 hemoglobin (g/dL) | 1.19 (0.79–1.76) | 0.399 | NS | |
| Day -1 platelet count (109/L) | 1.01 (1.01–1.02) | 0.033 | 1.04 (1.00–1.07) | 0.049 |
| Previous RBC transfusions | 0.88 (0.14–5.53) | 0.892 | NS | |
| Previous PLT transfusions | 0.24 (0.57–1.04) | 0.57 | NS | |
| Total blood volume processed | 1.37 (0.22–8.41) | 0.733 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, C.G.; Pellegrino, C.; Putzulu, R.; Bonanni, M.; Massini, G.; Orlando, N.; Forni, F.; Bianchi, M.; Piccirillo, N.; Teofili, L. Day -1 CD34+ Cells and Platelet Count Predict the Number of Apheresis in Poor-Mobilizer Patients Rescued by Plerixafor. J. Clin. Med. 2023, 12, 618. https://doi.org/10.3390/jcm12020618
Valentini CG, Pellegrino C, Putzulu R, Bonanni M, Massini G, Orlando N, Forni F, Bianchi M, Piccirillo N, Teofili L. Day -1 CD34+ Cells and Platelet Count Predict the Number of Apheresis in Poor-Mobilizer Patients Rescued by Plerixafor. Journal of Clinical Medicine. 2023; 12(2):618. https://doi.org/10.3390/jcm12020618
Chicago/Turabian StyleValentini, Caterina Giovanna, Claudio Pellegrino, Rossana Putzulu, Matteo Bonanni, Giuseppina Massini, Nicoletta Orlando, Franca Forni, Maria Bianchi, Nicola Piccirillo, and Luciana Teofili. 2023. "Day -1 CD34+ Cells and Platelet Count Predict the Number of Apheresis in Poor-Mobilizer Patients Rescued by Plerixafor" Journal of Clinical Medicine 12, no. 2: 618. https://doi.org/10.3390/jcm12020618
APA StyleValentini, C. G., Pellegrino, C., Putzulu, R., Bonanni, M., Massini, G., Orlando, N., Forni, F., Bianchi, M., Piccirillo, N., & Teofili, L. (2023). Day -1 CD34+ Cells and Platelet Count Predict the Number of Apheresis in Poor-Mobilizer Patients Rescued by Plerixafor. Journal of Clinical Medicine, 12(2), 618. https://doi.org/10.3390/jcm12020618





