Correlation of ENT Symptoms with Age, Sex, and Anti-SARS-CoV-2 Antibody Titer in Plasma
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Available online: https://covid19.who.int/ (accessed on 28 September 2022).
- El-Anwar, M.W.; Elzayat, S.; Fouad, Y.A. ENT manifestation in COVID-19 patients. Auris Nasus Larynx 2020, 47, 559–564. [Google Scholar] [CrossRef]
- El-Anwar, M.W.; Eesa, M.; Mansour, W.; Zake, L.G.; Hendawy, E. Analysis of Ear, Nose and Throat Manifestations in COVID-19 Patients. Int. Arch. Otorhinolaryngol. 2021, 25, e343–e348. [Google Scholar] [CrossRef]
- Zięba, N.; Lisowska, G.; Dadok, A.; Kaczmarek, J.; Stryjewska-Makuch, G.; Misiołek, M. Frequency and Severity of Ear-Nose-Throat (ENT) Symptoms during COVID-19 Infection. Medicina 2022, 58, 623. [Google Scholar] [CrossRef]
- Özçelik Korkmaz, M.; Eğilmez, O.K.; Özçelik, M.A.; Güven, M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, D.M.; Lee, H.; Ham, S.Y.; Oh, S.M.; Jeong, H.; Jung, J.; Kang, C.K.; Park, J.Y.; Kang, Y.M.; et al. Dynamics of viral load and anti-SARS-CoV-2 antibodies in patients with positive RT-PCR results after recovery from COVID-19. Korean J. Intern. Med. 2021, 36, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemi, H.; Eshghi, P.; Cheraghali, A.M.; Imani Fooladi, A.A.; Bolouki Moghaddam, F.; Imanizadeh, S.; Moeini Maleki, M.; Ranjkesh, M.; Rezapour, M.; Bahramifar, A.; et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2020, 59, 102875. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Pekosz, A.; Park, H.S.; Ursin, R.L.; Shapiro, J.R.; Benner, S.E.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.J.; et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef]
- Skorek, A.; Jaźwińska-Curyłło, A.; Romanowicz, A.; Kwaśniewski, K.; Narożny, W.; Tretiakow, D. Assessment of anti-SARS-CoV-2 antibodies level in convalescents plasma. J. Med. Virol. 2022, 94, 1130–1137. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, A.; Gabellon, S.; Pagani, J.L.; Monti, M.; Bochud, P.Y.; Manuel, O.; Coste, A.; Greub, G.; Perreau, M.; Pantaleo, G.; et al. Anti-SARS-CoV-2 Titers Predict the Severity of COVID-19. Viruses 2021, 14, 1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef]
- Elibol, E. Otolaryngological symptoms in COVID-19. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1233–1236. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Heras, M.; Fernández-Reyes, M.J. Serum potassium concentrations: Importance of normokalaemia. Concentraciones séricas de potasio: Importancia de la normopotasemia. Med. Clin. 2017, 148, 562–565. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, C.; Shen, Y.; Xia, L.; Xiao, L.; Sun, Y.; Wang, H.; Chen, Z.; Wu, Y.; Shi, H.; et al. Serum Albumin Levels as a Potential Marker for the Predictive and Prognostic Factor in Sudden Sensorineural Hearing Loss: A Prospective Cohort Study. Front. Neurol. 2021, 12, 747561. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Cunningham, A.C.; Goh, H.P.; Koh, D. Treatment of COVID-19: Old tricks for new challenges. Crit. Care 2020, 24, 91. [Google Scholar] [CrossRef]
- Johannesen, C.K.; St Martin, G.; Lendorf, M.E.; Gerred, P.; Fyfe, A.; Paton, R.S.; Thompson, C.; Molsted, S.; Kann, C.E.; Jensen, C.A.; et al. Prevalence and duration of anti-SARS-CoV-2 antibodies in healthcare workers. Dan. Med. J. 2022, 69, A11210843. [Google Scholar] [PubMed]
- Soo, Y.O.; Cheng, Y.; Wong, R.; Hui, D.S.; Lee, C.K.; Tsang, K.K.; Ng, M.H.; Chan, P.; Cheng, G.; Sung, J.J. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin. Microbiol. Infect. 2004, 10, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Chung, C.R.; Ha, Y.E.; Han, T.H.; Ki, C.S.; Kang, E.S.; Park, J.K.; Peck, K.R.; Cho, D. Possible Transfusion-Related Acute Lung Injury Following Convalescent Plasma Transfusion in a Patient With Middle East Respiratory Syndrome. Ann. Lab. Med. 2016, 36, 393–395. [Google Scholar] [CrossRef]
- Tsuchida, T.; Hirose, M.; Inoue, Y.; Kunishima, H.; Otsubo, T.; Matsuda, T. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J. Med. Virol. 2022, 94, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
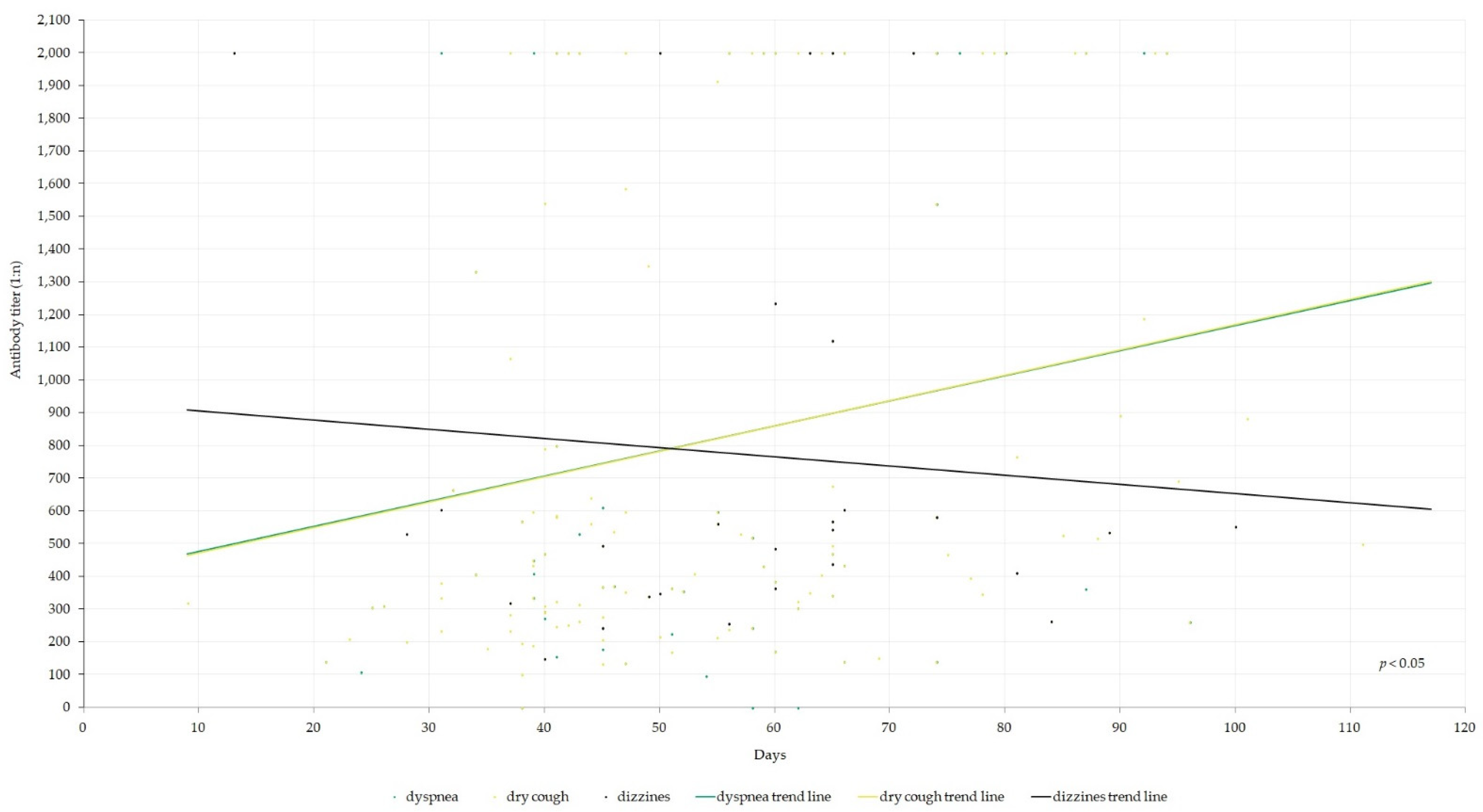
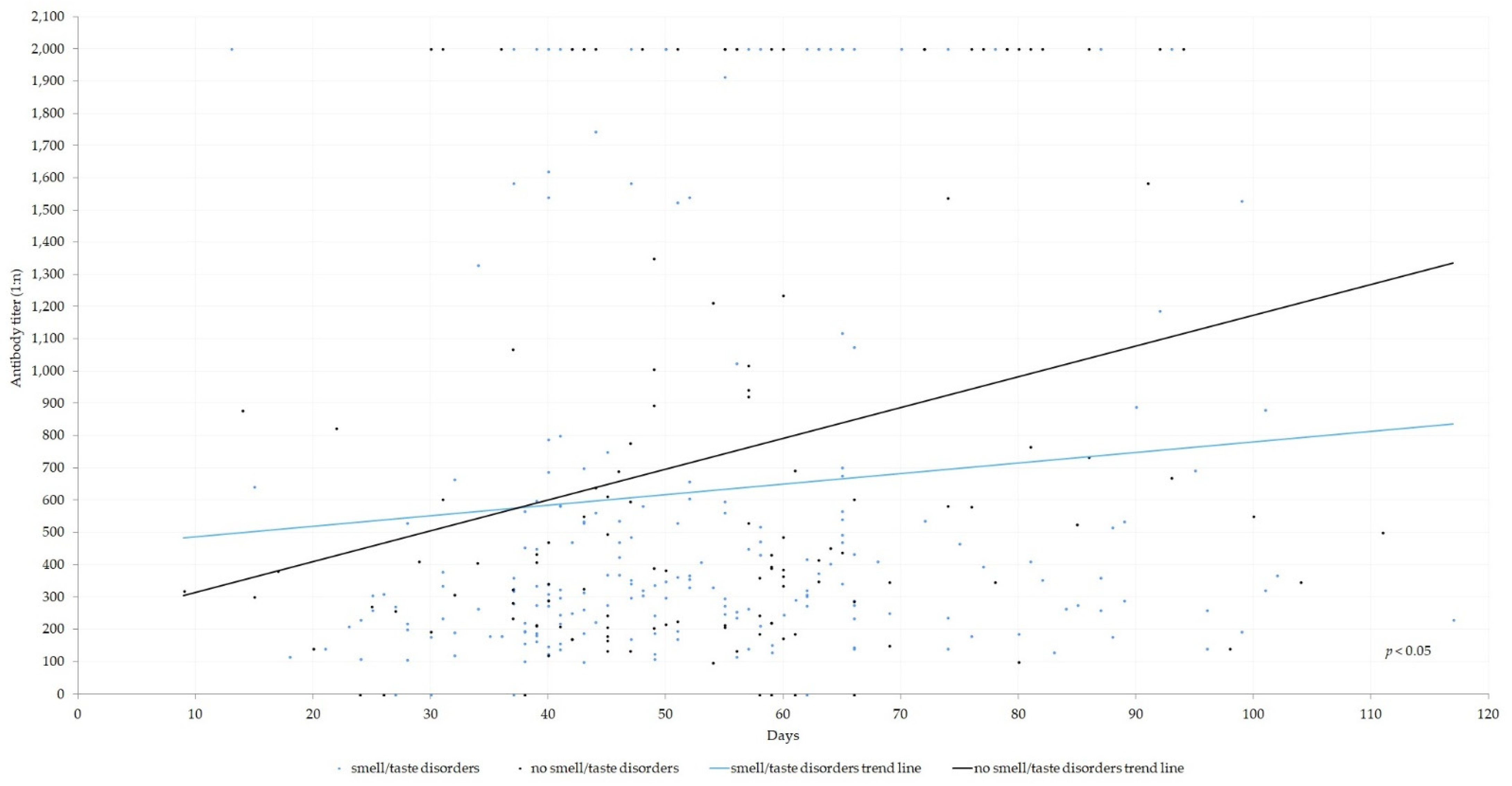
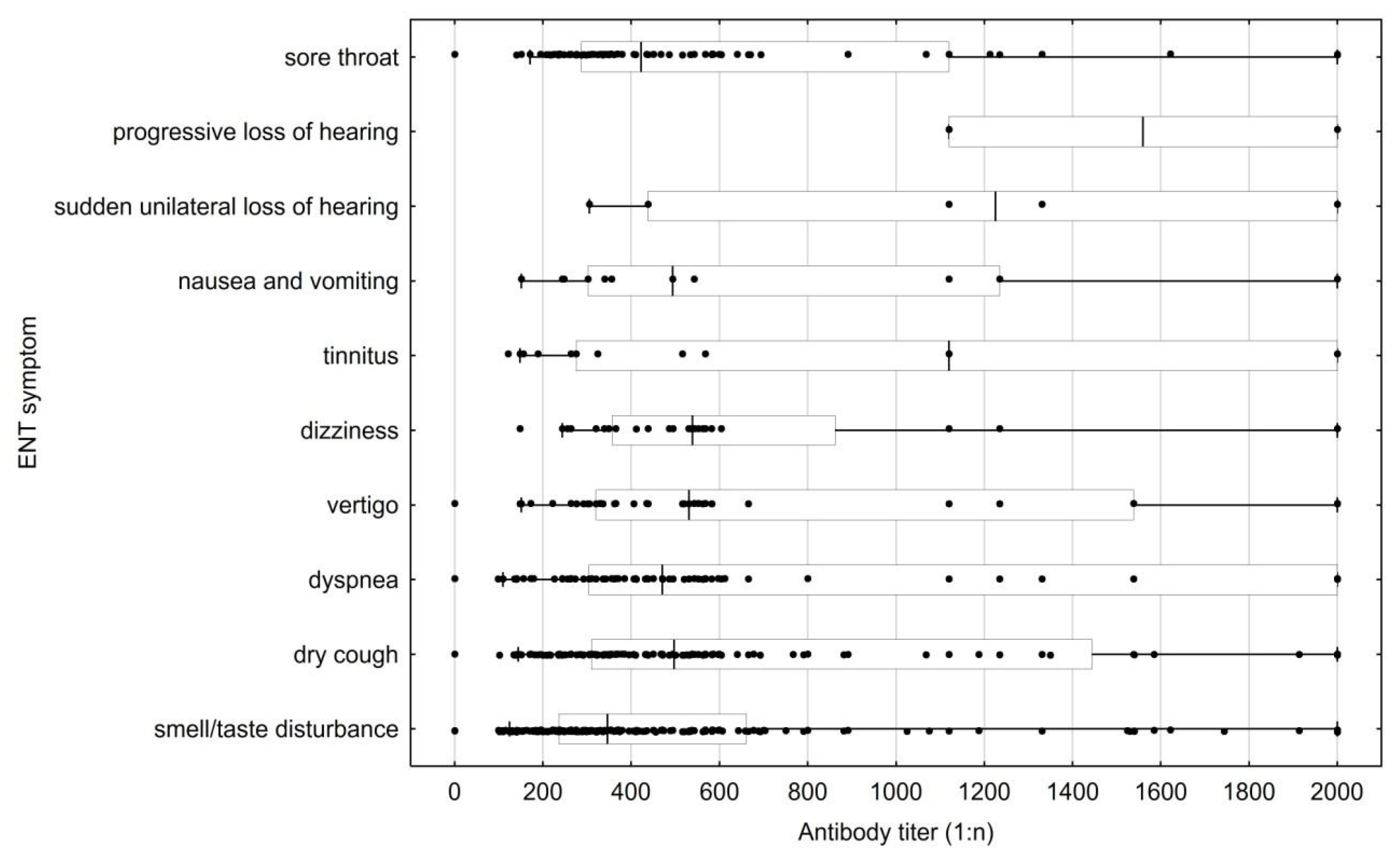
| Index | N |
|---|---|
| Mean age (95% CI) | 39.80 (38.79–40.81) |
| Gender | |
| Male | 302 |
| Female | 44 |
| Antibody titer | |
| <1:368.5 (male:female ratio) | 6:1 |
| >1:368.5 (male:female ratio) | 9:1 |
| Clinical information | |
| Hypertension | 9 (only males) |
| Familial hypercholesterolemia | 1 (only females) |
| No comorbidities (male:female ratio) | 7:1 |
| Ethnicity | |
| Polish (%) | 346 (100%) |
| ENT Symptom | <1:368.5 (N = 173) | >1:368.5 (N = 173) | p Value | Cohen’s h | Males (N = 302) | Females (N = 44) | p Value | Cohen’s h | Age < 39.8 (N = 167) | Age > 39.8 (N = 179) | p Value | Cohen’s h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| smell/taste disturbance | 117 | 99 | 0.046 a | 0.215 | 184 | 32 | 0.131 a | 0.252 | 119 | 97 | 0.001 a | 0.355 |
| dry cough | 51 | 89 | <0.0001 a | 0.452 | 125 | 15 | 0.357 a | 0.151 | 65 | 75 | 0.573 a | 0.061 |
| sore throat | 39 | 47 | 0.32 a | 0.107 | 72 | 14 | 0.253 a | 0.178 | 38 | 48 | 0.382 a | 0.094 |
| dyspnea | 30 | 52 | 0.005 a | 0.302 | 72 | 10 | 0.871 a | 0.026 | 45 | 37 | 0.17 a | 0.148 |
| vertigo | 15 | 26 | 0.067 a | 0.198 | 37 | 4 | 0.544 a | 0.103 | 19 | 22 | 0.793 a | 0.028 |
| dizziness | 8 | 20 | 0.018 a | 0.26 | 26 | 2 | 0.554 b | 0.166 | 15 | 13 | 0.558 a | 0.063 |
| tinnitus | 8 | 13 | 0.26 a | 0.122 | 19 | 2 | 1 b | 0.077 | 8 | 13 | 0.336 a | 0.104 |
| nausea and vomiting | 6 | 7 | 0.786 a | 0.03 | 10 | 3 | 0.221 b | 0.162 | 6 | 7 | 0.885 a | 0.017 |
| sudden unilateral loss of hearing | 1 | 5 | 0.215 b | 0.189 | 6 | 0 | 1 b | 0.283 | 2 | 4 | 0.686 b | 0.081 |
| progressive loss of hearing | 0 | 2 | 0.499 b | 0.215 | 2 | 0 | 1 b | 0.163 | 1 | 1 | 1 b | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśniewska, A.; Kwaśniewski, K.; Skorek, A.; Tretiakow, D.; Jaźwińska-Curyłło, A.; Burduk, P. Correlation of ENT Symptoms with Age, Sex, and Anti-SARS-CoV-2 Antibody Titer in Plasma. J. Clin. Med. 2023, 12, 610. https://doi.org/10.3390/jcm12020610
Kwaśniewska A, Kwaśniewski K, Skorek A, Tretiakow D, Jaźwińska-Curyłło A, Burduk P. Correlation of ENT Symptoms with Age, Sex, and Anti-SARS-CoV-2 Antibody Titer in Plasma. Journal of Clinical Medicine. 2023; 12(2):610. https://doi.org/10.3390/jcm12020610
Chicago/Turabian StyleKwaśniewska, Aleksandra, Krzysztof Kwaśniewski, Andrzej Skorek, Dmitry Tretiakow, Anna Jaźwińska-Curyłło, and Paweł Burduk. 2023. "Correlation of ENT Symptoms with Age, Sex, and Anti-SARS-CoV-2 Antibody Titer in Plasma" Journal of Clinical Medicine 12, no. 2: 610. https://doi.org/10.3390/jcm12020610
APA StyleKwaśniewska, A., Kwaśniewski, K., Skorek, A., Tretiakow, D., Jaźwińska-Curyłło, A., & Burduk, P. (2023). Correlation of ENT Symptoms with Age, Sex, and Anti-SARS-CoV-2 Antibody Titer in Plasma. Journal of Clinical Medicine, 12(2), 610. https://doi.org/10.3390/jcm12020610







