Redo Surgical Aortic Valve Replacement versus Valve-In-Valve Transcatheter Aortic Valve Implantation: A Systematic Review and Reconstructed Time-To-Event Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
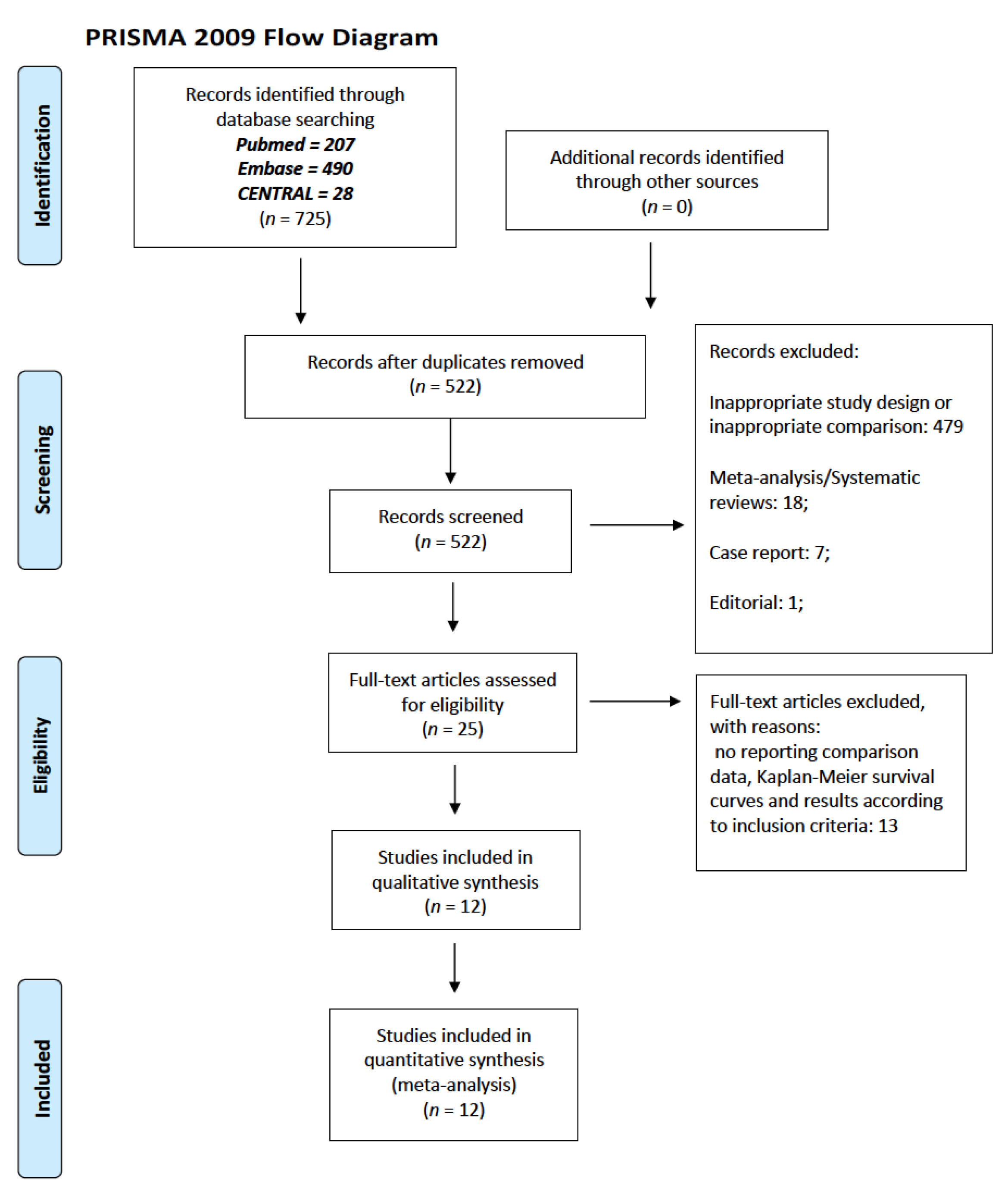
2.1. Eligibility Criteria, Search Strategy and Selection Process
2.2. Data Extraction and Collection
2.3. Risk of Bias Assessment
2.4. Primary and Secondary Endpoints
2.5. Statistical Analysis
3. Results
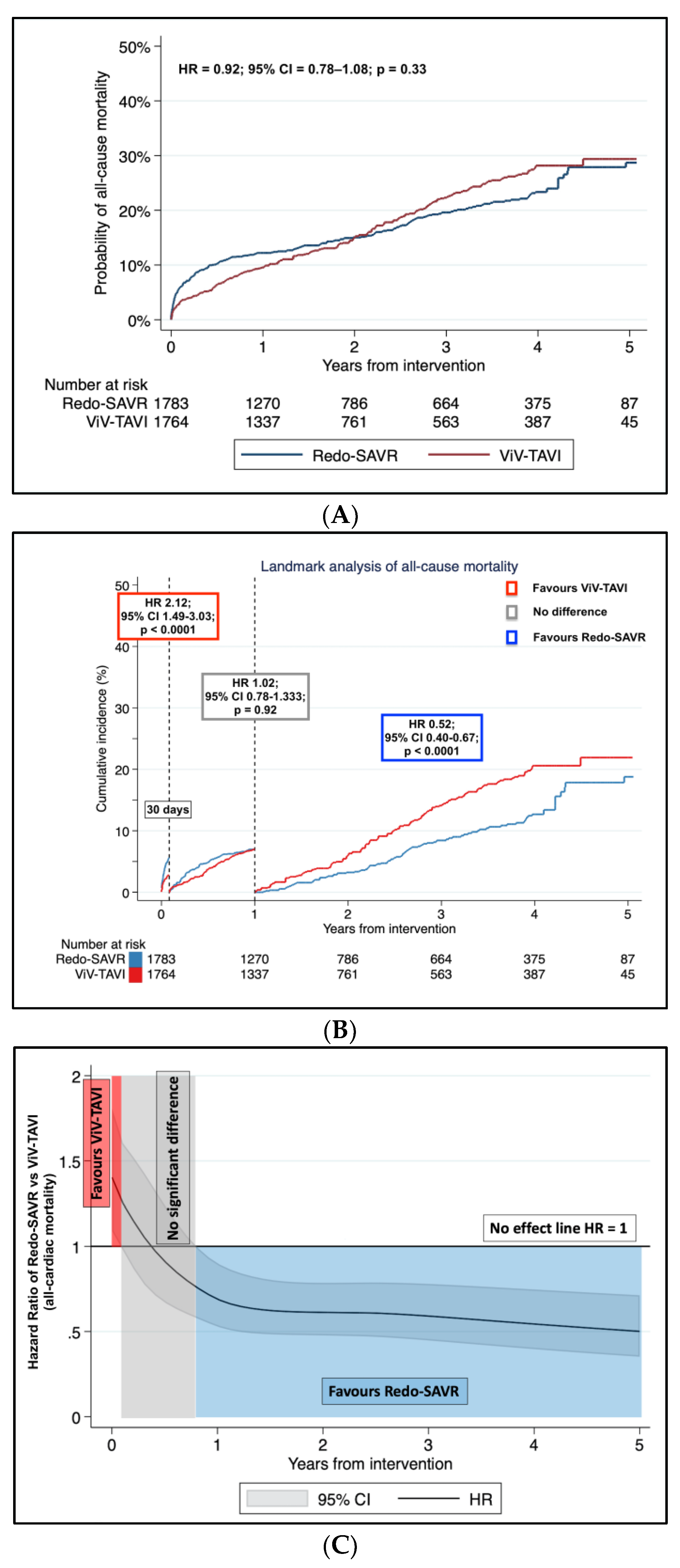
3.1. Primary Endpoint: Long-Term Mortality
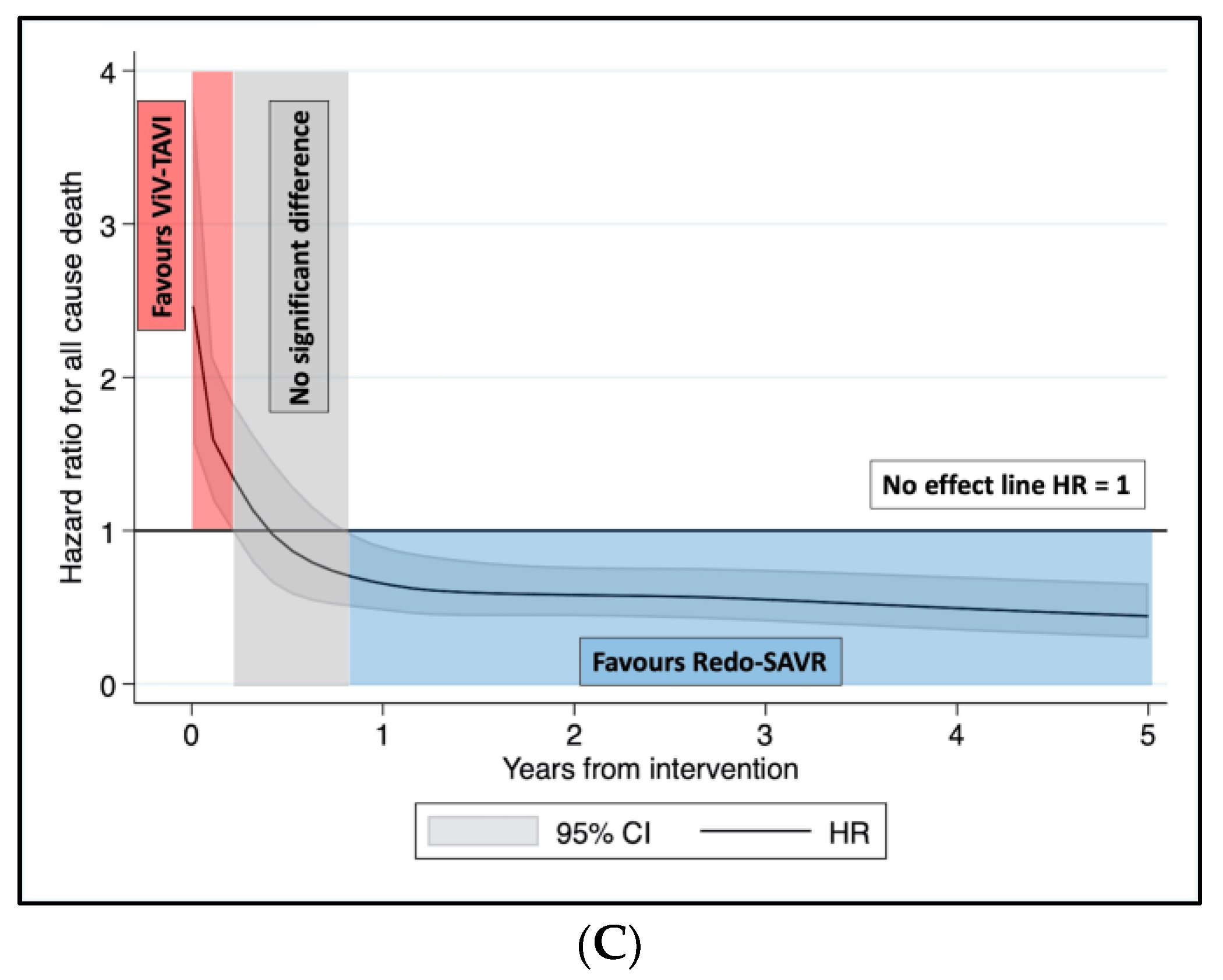
3.2. Sensitivity Analysis
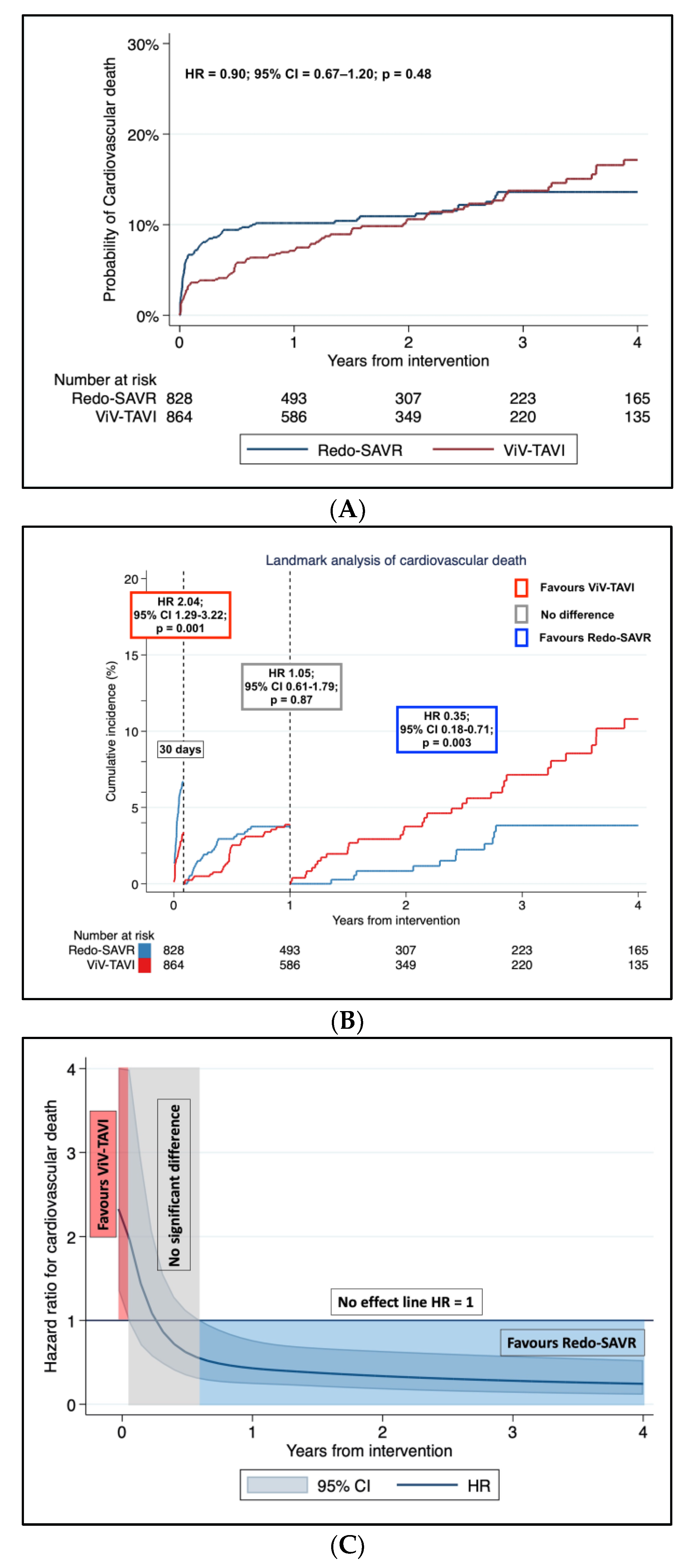
3.3. Secondary Endpoint: Incidence of Cardiovascular Death
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyersdorf, F.; Vahanian, A.; Milojevic, M.; Praz, F.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Joseph, W.Y. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.J.; Shuhaiber, J.; Salemi, A.; Isom, O.W.; Sedrakyan, A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J. Thorac. Cardiovasc Surg. 2015, 149, 1262–1269.e3. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F., 3rd; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gabella, T.; Voisine, P.; Dagenais, F.; Mohammadi, S.; Perron, J.; Dumont, E.; Puri, R.; Asmarats, L.; Côté, M.; Bergeron, S.; et al. Long-Term Outcomes Following Surgical Aortic Bioprosthesis Implantation. J. Am. Coll. Cardiol. 2018, 71, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. PARTNER 2 Investigators. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Landes, U.; Sathananthan, J.; Witberg, G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Holzhey, D.; Kim, W.-K.; Hamm, C.; Buzzatti, N.; et al. Transcatheter Replacement of Transcatheter Versus Surgically Implanted Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2021, 77, 1–14. [Google Scholar] [CrossRef]
- van Nieuwkerk, A.C.; Santos, R.B.; Fernandez-Nofrerias, E.; Tchétché, D.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Outcomes in Valve-in-Valve Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 172, 81–89. [Google Scholar] [CrossRef]
- Majmundar, M.; Doshi, R.; Kumar, A.; Johnston, D.; Brockett, J.; Kanaa’N, A.; Lahorra, J.A.; Svensson, L.G.; Krishnaswamy, A.; Reed, G.W.; et al. Valve-in-valve transcatheter aortic valve implantation versus repeat surgical aortic valve replacement in patients with a failed aortic bioprosthesis. EuroIntervention 2022, 17, 1227–1237. [Google Scholar] [CrossRef]
- Saleem, S.; Ullah, W.; Syed, M.A.; Megaly, M.; Thalambedu, N.; Younas, S.; Zahid, S.; Alam, M.; Virani, S.S.; Verma, D.R.; et al. Meta-analysis comparing valve-in-valve TAVR and redo-SAVR in patients with degenerated bioprosthetic aortic valve. Catheter. Cardiovasc. Interv. 2021, 98, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Al-Abcha, A.; Saleh, Y.; Boumegouas, M.; Prasad, R.; Herzallah, K.; Baloch, Z.Q.; Abdelkarim, O.; Rayamajhi, S.; Abela, G.S. Meta-Analysis of Valve-in-Valve Transcatheter Aortic Valve Implantation Versus Redo-surgical Aortic Valve Replacement in Failed Bioprosthetic Aortic Valve. Am. J. Cardiol. 2021, 146, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.B.O.; Van den Eynde, J.; Simonato, M.; Cavalcanti, L.R.P.; Doulamis, I.P.; Weixler, V.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; Zhigalov, K.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement: An Updated Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Elia, E.; D’Ascenzo, F.; Marengo, G.; Deharo, P.; Kaneko, T.; Cuisset, T.; Fauchier, L.; De Filippo, O.; Gallone, G.; et al. Valve-in-valve transcatheter aortic valve replacement or re-surgical aortic valve replacement in degenerated bioprostheses: A systematic review and meta-analysis of short and midterm results. Catheter. Cardiovasc. Interv. 2022, 100, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wei, Y.; Royston, P.; Tierney, J.F.; Parmar, M.K. Meta-analysis of time-to-event outcomes from randomized trials using restricted mean survival time: Application to individual participant data. Stat. Med. 2015, 34, 2881–2898. [Google Scholar] [CrossRef]
- Wei, Y.; Royston, P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata. J. 2017, 17, 786–802. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Ejiofor, J.I.; Yammine, M.; Harloff, M.T.; McGurk, S.; Muehlschlegel, J.D.; Shekar, P.S.; Cohn, L.H.; Shah, P.; Kaneko, T. Reoperative Surgical Aortic Valve Replacement Versus Transcatheter Valve-in-Valve Replacement for Degenerated Bioprosthetic Aortic Valves. Ann. Thorac. Surg. 2016, 102, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Silaschi, M.; Wendler, O.; Seiffert, M.; Castro, L.; Lubos, E.; Schirmer, J.; Blankenberg, S.; Reichenspurner, H.; Schäfer, U.; Treede, H.; et al. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Spaziano, M.; Mylotte, D.; Thériault-Lauzier, P.; De Backer, O.; Søndergaard, L.; Bosmans, J.; Debry, N.; Modine, T.; Barbanti, M.; Tamburino, C.; et al. Transcatheter aortic valve implantation versus redo surgery for failing surgical aortic bioprostheses: A multicentre propensity score analysis. EuroIntervention 2017, 13, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vaquero, D.; Díaz, R.; Pascual, I.; Avanzas, P.; Silva, J.; Moris, C. Long-term Survival After Surgery Versus Transcatheter Technique to Treat Degenerated Aortic Bioprostheses. Rev. Esp. Cardiol. 2019, 72, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, A.F.; Greason, K.L.; Sandhu, G.S.; Dearani, J.A.; Holmes, D.R., Jr.; Schaff, H.V. Transcatheter Valve-in-Valve Vs Surgical Replacement of Failing Stented Aortic Biological Valves. Ann. Thorac. Surg. 2019, 108, 424–430. [Google Scholar] [CrossRef]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Etienne, C.S.; Porto, A.; Theron, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Transcatheter Valve-in-Valve Aortic Valve Replacement as an Alternative to Surgical Re-Replacement. J. Am. Coll. Cardiol. 2020, 76, 489–499. [Google Scholar] [CrossRef]
- Stankowski, T.; Aboul-Hassan, S.S.; Zinab, F.S.; Herwig, V.; Stępiński, P.; Grimmig, O.; Just, S.; Harnath, A.; Muehle, A.; Fritzsche, D.; et al. Femoral transcatheter valve-in-valve implantation as alternative strategy for failed aortic bioprostheses: A single-centre experience with long-term follow-up. Int. J. Cardiol. 2020, 306, 25–34. [Google Scholar] [CrossRef]
- Tam, D.Y.; Dharma, C.; Rocha, R.V.; Ouzounian, M.; Wijeysundera, H.C.; Austin, P.C.; Chikwe, J.; Gaudino, M.; Fremes, S.E. Transcatheter ViV Versus Redo Surgical AVR for the Management of Failed Biological Prosthesis: Early and Late Outcomes in a Propensity-Matched Cohort. JACC Cardiovasc. Interv. 2020, 13, 765–774. [Google Scholar] [CrossRef]
- Woitek, F.J.; Stachel, G.; Kiefer, P.; Haussig, S.; Leontyev, S.; Schlotter, F.; Mende, M.; Hommel, J.; Crusius, L.; Spindler, A.; et al. Treatment of failed aortic bioprostheses: An evaluation of conventional redo surgery and transfemoral transcatheter aortic valve-in-valve implantation. Int. J. Cardiol. 2020, 300, 80–86. [Google Scholar] [CrossRef]
- Dokollari, A.; Cameli, M.; Mandoli, G.E.; Kalra, D.-K.S.; Poston, R.; Coku, L.; Pernoci, M.; Miri, M.; Bonacchi, M.; Gelsomino, S. Early and Midterm Clinical Outcomes of Transcatheter Valve-in-Valve Implantation Versus Redo Surgical Aortic Valve Replacement for Aortic Bioprosthetic Valve Degeneration: Two Faces of the Same Medal. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3223–3231. [Google Scholar] [CrossRef]
- van Steenbergen, G.J.; van Straten, B.; Lam, K.Y.; van Veghel, D.; Dekker, L.; Tonino, P.A. Report on outcomes of valve-in-valve transcatheter aortic valve implantation and redo surgical aortic valve replacement in the Netherlands. Neth. Heart J. 2022, 30, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Chiou, E.; Cao, Y.; Binongo, J.; Guyton, R.A.; Leshnower, B.; Grubb, K.J.; Chen, E.P. Isolated Redo Aortic Valve Replacement Versus Valve-in-Valve Transcatheter Valve Replacement. Ann. Thorac. Surg. 2021, 112, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- UK TAVI Trial Investigators; Toff, W.D.; Hildick-Smith, D.; Kovac, J.; Mullen, M.J.; Wendler, O.; Mansouri, A.; Rombach, I.; Abrams, K.R.; Conroy, S.P.; et al. Effect of Transcatheter Aortic Valve Implantation vs Surgical Aortic Valve Replacement on All-Cause Mortality in Patients With Aortic Stenosis: A Randomized Clinical Trial. JAMA 2022, 327, 1875–1887. [Google Scholar]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, F.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. SURTAVI Trial Investigators. Self-expanding Transcatheter vs. Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar]
- Barili, F.; Freemantle, N.; Musumeci, F.; Martin, B.; Anselmi, A.; Rinaldi, M.; Kaul, S.; Rodriguez-Roda, J.; Di Mauro, M.; Folliguet, T.; et al. Five-year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: A pooled meta-analysis of reconstructed time-to-event data. Eur. J. Cardiothorac. Surg. 2022, 61, 977–987. [Google Scholar] [CrossRef]
- Beyersdorf, F.; Bauer, T.; Freemantle, N.; Walther, T.; Frerker, C.; Herrmann, E.; Bleiziffer, S.; Möllmann, H.; Landwehr, S.; Ensminger, S.; et al. Five-year outcome in 18 010 patients from the German Aortic Valve Registry. Eur. J. Cardiothorac. Surg. 2021, 60, 1139–1146. [Google Scholar] [CrossRef]
- Sá, M.P.; Ramlawi, B.; Sicouri, S.; Torregrossa, G.; Al Abri, Q.; Kempfert, J.; Kofler, M.; Falk, V.; Unbehaun, A.; Van Praet, K.M.; et al. Lifetime management of aortic valve disease: Aligning surgical and transcatheter armamentarium to set the tone for the present and the future. J. Card. Surg. 2022, 37, 205–213. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Daneshvar, S.A.; Fonarow, G.C.; Stebbins, A.; Velumapalli, S.; Desai, N.D.; Malenka, D.J.; Thourani, V.H.; Rymer, J.; Kosinski, A.S. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2018, 72, 2701–2711. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Erlebach, M.; Simonato, M.; Pibarot, P.; Webb, J.; Capek, L.; Windecker, S.; George, I.; Sinning, J.M.; Horlick, E.; et al. Incidence, predictors and clinical outcomes of residual stenosis after aortic valve-in-valve. Heart 2018, 104, 828–834. [Google Scholar] [CrossRef]
- Fallon, J.M.; DeSimone, J.P.; Brennan, J.M.; O’Brien, S.; Thibault, D.P.; DiScipio, A.W.; Pibarot, P.; Jacobs, J.P.; Malenka, D.J. The Incidence and Consequence of Prosthesis-Patient Mismatch After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 14–22. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Simonato, M.; Webb, J.G.; Rodés-Cabau, J.; Pibarot, P.; Kornowski, R.; Windecker, S.; Erlebach, M.; Duncan, A.; Seiffert, M.; et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur. Heart J. 2020, 41, 2731–2742. [Google Scholar] [CrossRef]
- Pibarot, P.; Simonato, M.; Barbanti, M.; Linke, A.; Kornowski, R.; Rudolph, T.; Spence, M.; Moat, N.; Aldea, G.; Mennuni, M.; et al. Impact of Pre-Existing Prosthesis-Patient Mismatch on Survival Following Aortic Valve-in-Valve Procedures. JACC Cardiovasc. Interv. 2018, 11, 133–141. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, F.; Gallingani, A.; Tuttolomondo, D.; Hernandez-Vaquero, D.; D’Alessandro, S.; Pattuzzi, C.; Çelik, M.; Singh, G.; Ceccato, E.; Niccoli, G.; et al. Redo Surgical Aortic Valve Replacement versus Valve-In-Valve Transcatheter Aortic Valve Implantation: A Systematic Review and Reconstructed Time-To-Event Meta-Analysis. J. Clin. Med. 2023, 12, 541. https://doi.org/10.3390/jcm12020541
Formica F, Gallingani A, Tuttolomondo D, Hernandez-Vaquero D, D’Alessandro S, Pattuzzi C, Çelik M, Singh G, Ceccato E, Niccoli G, et al. Redo Surgical Aortic Valve Replacement versus Valve-In-Valve Transcatheter Aortic Valve Implantation: A Systematic Review and Reconstructed Time-To-Event Meta-Analysis. Journal of Clinical Medicine. 2023; 12(2):541. https://doi.org/10.3390/jcm12020541
Chicago/Turabian StyleFormica, Francesco, Alan Gallingani, Domenico Tuttolomondo, Daniel Hernandez-Vaquero, Stefano D’Alessandro, Claudia Pattuzzi, Mevlüt Çelik, Gurmeet Singh, Evelina Ceccato, Giampaolo Niccoli, and et al. 2023. "Redo Surgical Aortic Valve Replacement versus Valve-In-Valve Transcatheter Aortic Valve Implantation: A Systematic Review and Reconstructed Time-To-Event Meta-Analysis" Journal of Clinical Medicine 12, no. 2: 541. https://doi.org/10.3390/jcm12020541
APA StyleFormica, F., Gallingani, A., Tuttolomondo, D., Hernandez-Vaquero, D., D’Alessandro, S., Pattuzzi, C., Çelik, M., Singh, G., Ceccato, E., Niccoli, G., Lorusso, R., & Nicolini, F. (2023). Redo Surgical Aortic Valve Replacement versus Valve-In-Valve Transcatheter Aortic Valve Implantation: A Systematic Review and Reconstructed Time-To-Event Meta-Analysis. Journal of Clinical Medicine, 12(2), 541. https://doi.org/10.3390/jcm12020541









