A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.4. Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Outcomes
3.3. Adverse Effects
4. Discussion
5. Implications for Clinical Practice
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aes | adverse events |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| BC | breast cancer |
| CIPN | chemotherapy-induced peripheral neuropathy |
| ECOG | Eastern Cooperative Oncology Group |
| EORTC QLQ-CIPN20 | the European Organization for the Research and Treatment of Cancer-Chemotherapy-induced peripheral neuropathy 20 |
| HGWD | Huangqi Guizhi Wuwu decoction |
| IL-4 | interleukin-4 |
| iNOS | inducible nitric oxide synthase |
| ITT | intention-to-treat |
| nab-PTX | albumin-bound paclitaxel |
| NCI-CTCAE | Common terminology criteria for adverse events |
| PN | peripheral neuropathy; |
| PS | performance status; |
| PTX | paclitaxel |
| TNBC | triple negative breast cancer |
| TRAEs | treatment-related adverse events |
| ULN | upper limit of normal |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Mi, W.L.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Prevention and treatment for chemotherapy-induced peripheral neuropathy: Therapies based on cipn mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, H.; Dong, J.; Feng, Y.; Li, H.; Zhuang, R.; Wang, P.; Cai, W.; Zhou, Y. Does nab-paclitaxel have a higher incidence of peripheral neuropathy than solvent-based paclitaxel? Evidence from a systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2019, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase iii trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Bu, Z.; Ye, X.; Zhou, Y.; Zhao, Q. Incidence and risk of peripheral neuropathy with nab-paclitaxel in patients with cancer: A meta-analysis. Eur. J. Cancer Care 2017, 26, e12407. [Google Scholar] [CrossRef]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (geparsepto-gbg 69): A randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [CrossRef]
- Yamashita, Y.; Egashira, N.; Masuguchi, K.; Ushio, S.; Kawashiri, T.; Oishi, R. Comparison of peripheral neuropathy induced by standard and nanoparticle albumin-bound paclitaxel in rats. J. Pharmacol. Sci. 2011, 117, 116–120. [Google Scholar] [CrossRef]
- Klein, I.; Lehmann, H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Sakaguchi, K.; Ishikawa, T.; Ouchi, Y.; Nakatsukasa, K.; Tabuchi, Y.; Kanehisa, F.; Hiramatsu, M.; Takagi, R.; Yokota, I.; et al. Comparison of the efficacy of cryotherapy and compression therapy for preventing nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: A prospective self-controlled trial. Breast 2020, 49, 219–224. [Google Scholar] [CrossRef]
- Schloss, J.M.; Colosimo, M.; Airey, C.; Masci, P.; Linnane, A.W.; Vitetta, L. A randomised, placebo-controlled trial assessing the efficacy of an oral b group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (cipn). Support. Care Cancer 2017, 25, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Shen, J.; Gao, X.; Ruan, Y.; Ling, J.; Sun, R.; Dai, J.; Fan, H.; Cheng, X.; Cao, P. Herbal formula huangqi guizhi wuwu decoction attenuates paclitaxel-related neurotoxicity via inhibition of inflammation and oxidative stress. Chin. Med. 2021, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Chen, X.F.; Shu, P.; Jiang, Z.W.; Wu, X.Y.; Zou, X.; Chen, K.; Shen, B.; Hu, W.W.; Lu, W.; et al. Study on efficacy and safety of huangqi guizhi wuwu decoction treatment for oxaliplatin induced peripheral neurotoxicity: A protocol for a randomized, controlled, double-blind, multicenter trial. Medicine 2020, 99, e19923. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, F.; Han, L.; Gou, X.; Lian, F.; Liu, W.; Zhao, L.; Pang, B.; Zhao, X.; Tong, X. Efficacy of chinese herbal medicine in the treatment of moderate-severe painful diabetic peripheral neuropathy: A retrospective study. J. Diabetes Res. 2019, 2019, 4035861. [Google Scholar] [CrossRef]
- Lavoie, S.E.; Barton, D.L.; Qin, R.; Steen, P.D.; Aaronson, N.K.; Loprinzi, C.L. Assessing patient-reported peripheral neuropathy: The reliability and validity of the european organization for research and treatment of cancer qlq-cipn20 questionnaire. Qual. Life Res. 2013, 22, 2787–2799. [Google Scholar] [CrossRef]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lantéri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an eortc quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The qlq-cipn20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Kerckhove, N.; Busserolles, J.; Stanbury, T.; Pereira, B.; Plence, V.; Bonnetain, F.; Krakowski, I.; Eschalier, A.; Pezet, D.; Balayssac, D. Effectiveness assessment of riluzole in the prevention of oxaliplatin-induced peripheral neuropathy: Riluzox-01: Protocol of a randomised, parallel, controlled, double-blind and multicentre study by the unicancer-afsos supportive care intergroup. Bmj Open 2019, 9, e27770. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; Mckenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef]
- Lees, J.G.; Makker, P.G.; Tonkin, R.S.; Abdulla, M.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur. J. Cancer 2017, 73, 22–29. [Google Scholar] [CrossRef]
- Mallet, M.L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The role of oxidative stress in peripheral neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W. Alphalipoic acid prevents oxidative stress and peripheral neuropathy in nab-paclitaxel-treated rats through the nrf2 signalling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 3142732. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: Asco guideline update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Beijers, A.; Bonhof, C.S.; Mols, F.; Ophorst, J.; de Vos-Geelen, J.; Jacobs, E.; van de Poll-Franse, L.V.; Vreugdenhil, G. Multicenter randomized controlled trial to evaluate the efficacy and tolerability of frozen gloves for the prevention of chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2020, 31, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.; Guo, L.; Hamre, J.R. A comparative review of chemotherapy-induced peripheral neuropathy in in vivo and in vitro models. Toxicol. Pathol. 2020, 48, 190–201. [Google Scholar] [CrossRef]
- Poupon, L.; Kerckhove, N.; Vein, J.; Lamoine, S.; Authier, N.; Busserolles, J.; Balayssac, D. Minimizing chemotherapy-induced peripheral neuropathy: Preclinical and clinical development of new perspectives. Expert Opin. Drug Saf. 2015, 14, 1269–1282. [Google Scholar] [CrossRef]
- Cheng, X.; Huo, J.; Wang, D.; Cai, X.; Sun, X.; Lu, W.; Yang, Y.; Hu, C.; Wang, X.; Cao, P. Herbal medicine ac591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front. Pharmacol. 2017, 8, 344. [Google Scholar] [CrossRef]
- Liang, L.; Wei, X.; Feng, M.; Zhu, L.; Yu, J.; Yang, G.; Yin, X.; Zhou, S.; Li, K.; Yang, M.; et al. Huangqi guizhi wuwu decoction for treating cervical radiculopathy: A systematic review and meta-analysis of randomized controlled trials. Medicine 2020, 99, e19137. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, T.Y.; Zhao, L.H.; Wan, F.; Ye, R.; Zhou, Q.; Tian, F.; Tong, X.L. Huangqi guizhi wuwu decoction for treating diabetic peripheral neuropathy: A meta-analysis of 16 randomized controlled trials. Neural Regen. Res. 2016, 11, 1347–1358. [Google Scholar]
- Lee, S.; Wong, A.R.; Yang, A.; Hung, A. Interaction of compounds derived from the chinese medicinal formula huangqi guizhi wuwu tang with stroke-related numbness and weakness targets: An in-silico docking and molecular dynamics study. Comput. Biol. Med. 2022, 146, 105568. [Google Scholar] [CrossRef]
- Lee, S.; Hung, A.; Li, H.; Yang, A. Mechanisms of action of a herbal formula huangqi guizhi wuwu tang for the management of post-stroke related numbness and weakness: A computational molecular docking study. J. Evid.-Based Integr. Med. 2022, 27, 2515690X–221082989X. [Google Scholar] [CrossRef]
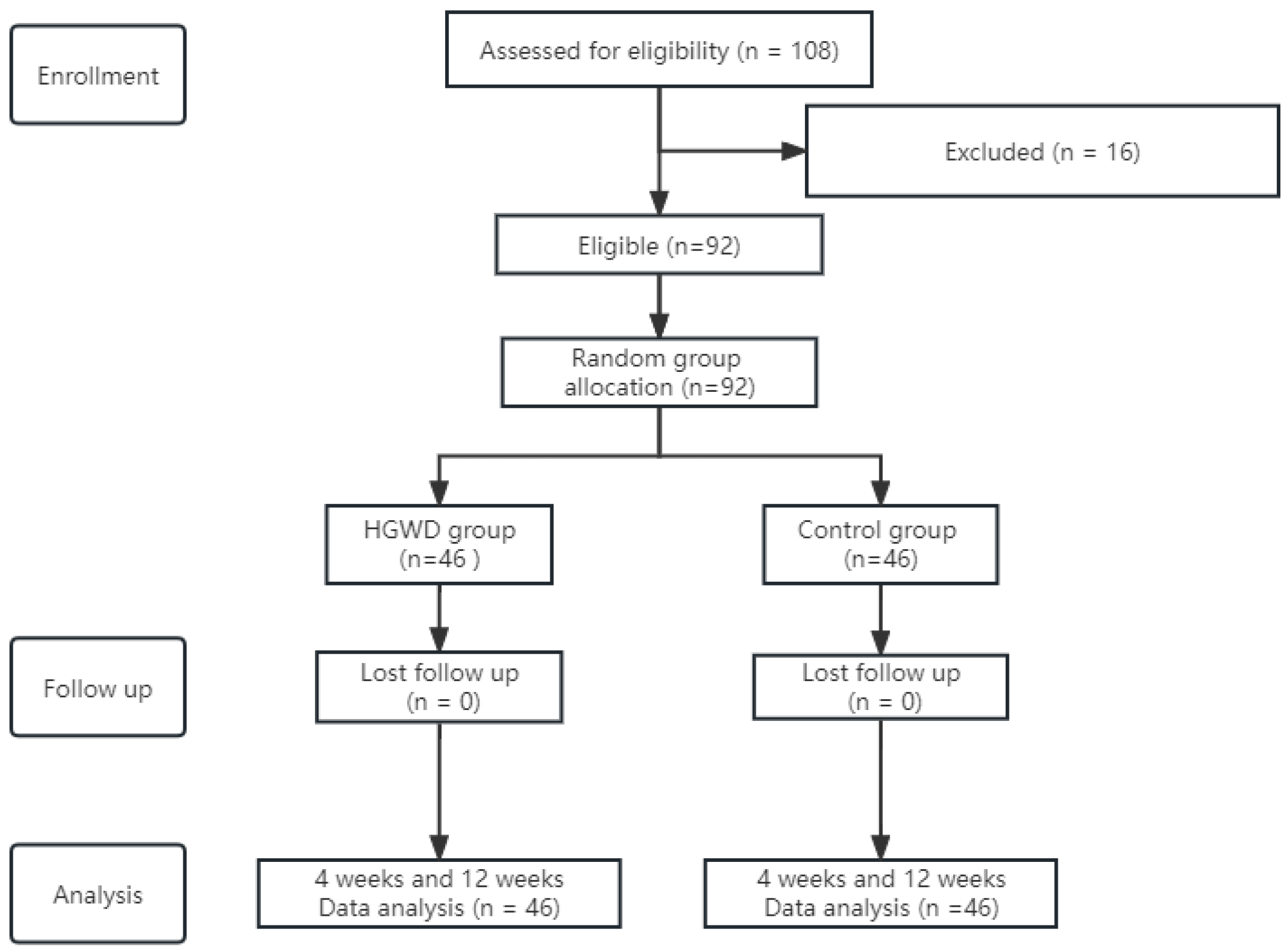
| Variables | HGWD Group (n = 46) | Control Group (n = 46) | p * |
|---|---|---|---|
| Age (range) | 50 (23–65) | 52 (29–65) | |
| ECOG score | 0.669 | ||
| 0 | 29 (63.0) | 27 (58.7) | |
| 1 | 17 (37.0) | 19 (41.3) | |
| Menstruation status | 0.381 | ||
| Pre | 27 (58.7) | 28 (60.9) | |
| Post | 19 (41.3) | 18 (39.1) | |
| Lymph node metastasis | 0.804 | ||
| Yes | 36 (78.3) | 35 (76.1) | |
| No | 10 (21.7) | 11 (30.4) | |
| Pathologic tumor stage a | 0.620 | ||
| I | 8 (17.4) | 6 (13.0) | |
| II | 8 (17.4) | 10 (21.7) | |
| III | 23 (50.0) | 19 (41.3) | |
| IV | 7 (15.2) | 11 (23.9) | |
| Molecular classification | 0.513 | ||
| Luminal A | 3 (6.5) | 5 (10.9) | |
| Luminal B | 25 (54.3) | 18 (39.1) | |
| HER-2 | 2 (4.3) | 3 (6.5) | |
| TNBC | 16 (34.8) | 20 (43.5) | |
| Surgery | 0.559 | ||
| MRM | 29 (63.0) | 24 (52.2) | |
| BCS | 11 (23.9) | 15 (32.6) | |
| SLN | 6 (13.0) | 7 (15.2) | |
| Radiotherapy | 0.369 | ||
| Yes | 38 (82.6) | 41 (89.1) | |
| No | 8 (17.4) | 5 (10.9) | |
| Endocrine therapy | 0.294 | ||
| Yes | 28 (60.9) | 23 (50.0) | |
| No | 18 (39.1) | 23 (50.0) | |
| Anti-Her2 therapy | 0.748 | ||
| Yes | 5 (10.9) | 6 (13.0) | |
| No | 41 (89.1) | 40 (87.0) |
| Outcomes | Median (Range) a | Estimate of Median Change (Range, p Value) b | Comparison of Change between Groups (p Value) c | ||
|---|---|---|---|---|---|
| HGWD Group | Control Group | HGWD Group | Control Group | ||
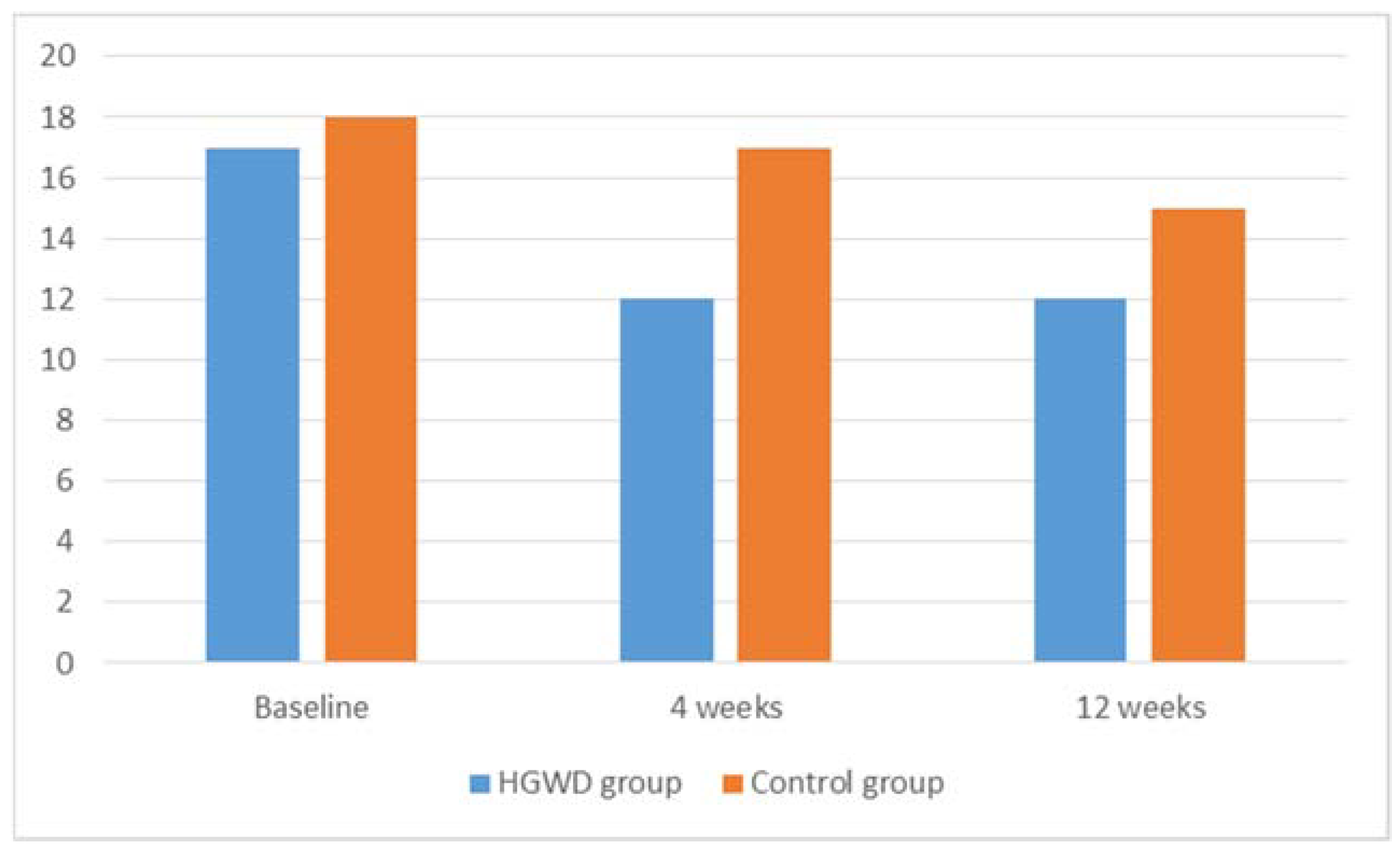
| CIPN sensory | |||||
| Baseline | 17 (12–24) | 18 (12–24) | - | ||
| 4 weeks | 12 (9–15) | 17 (12–22) | −4 (−10 to 0, p < 0.001) * | −1 (−4 to 0, p = 0.006) * | p < 0.001 * |
| 12 weeks | 12 (9–15) | 15 (11–19) | −6 (−10 to 0, p < 0.001) * | −3 (−9 to 0, p < 0.001) * | p < 0.001 * |
| CIPN motor | |||||
| Baseline | 12 (10–15) | 12 (10–14) | - | ||
| 4 weeks | 10 (8–12) | 12 (10–13) | −2 (−5 to 0, p < 0.001) * | 0 (−2 to 0, p = 0.003) * | p < 0.001 * |
| 12 weeks | 10 (8–11) | 12 (10–13) | −2 (−5 to 0, p < 0.001) * | 0 (−3 to 0, p = 0.004) * | p < 0.001 * |
| Autonomic CIPN | |||||
| Baseline | 2 (1–4) | 2 (2–4) | - | ||
| 4 weeks | 2 (1–3) | 2 (1–3) | 0 (−2 to 0, p = 0.157) | 0 (−2 to 0, p = 0.102) | p = 0.633 |
| 12 weeks | 2 (1–3) | 2 (1–3) | 0 (−2 to 0, p = 0.102) | 0 (−2 to 0, p = 0.059) | p = 0.702 |
| Adverse Events | Grade 1/2 | Grade 3/4 | p-Value | |||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | |||||
| HGWD Group (N = 46) | Control Group (N = 46) | p-Value | HGWD Group (N = 46) | Control Group (N = 46) | ||
| Fatigue | 20 (43.5) | 23 (50.0) | 0.531 | 0 | 0 | - |
| Reduced appetite | 14 (30.4) | 11 (23.9) | 0.482 | 0 | 0 | - |
| Nausea/Vomiting | 5 (10.9) | 3 (6.5) | 0.714 | 0 | 0 | - |
| Constipation | 2 (4.3) | 2 (4.3) | 1.000 | 0 | 0 | - |
| Diarrhea | 4 (8.7) | 1 (2.2) | 0.361 | 0 | 0 | - |
| Skin rash | 1 (2.2) | 1 (2.2) | 1.000 | 0 | 0 | - |
| Oral ulcer | 4 (8.7) | 1 (2.2) | 0.361 | 0 | 0 | - |
| Alopecia | 46 (100.0) | 46 (100.0) | 1.000 | 0 | 0 | - |
| leucopenia | 3 (6.5) | 11 (23.9) | 0.020 * | 1 (2.2) | 0 | - |
| Neutropenia | 7 (15.2) | 8 (17.4) | 0.778 | 3 (6.5) | 3 (6.5) | 1.000 |
| Anemia | 2 (4.3) | 3 (6.5) | 1.000 | 0 | 0 | - |
| Thrombocytopenia | 3 (6.5) | 0 | - | 0 | 0 | - |
| Creatinine elevation | 1 (2.2) | 4 (8.7) | 0.361 | 0 | 0 | - |
| ALT/AST elevation | 9 (19.6) | 11 (23.9) | 0.613 | 1 (2.2) | 1 (2.2) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.; Zhao, F.; Ye, P.; Ma, F.; Wang, J.; Zhang, P.; Li, Q.; Wang, J.; Wang, W.; Li, Q.; et al. A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy. J. Clin. Med. 2023, 12, 505. https://doi.org/10.3390/jcm12020505
Chai Y, Zhao F, Ye P, Ma F, Wang J, Zhang P, Li Q, Wang J, Wang W, Li Q, et al. A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy. Journal of Clinical Medicine. 2023; 12(2):505. https://doi.org/10.3390/jcm12020505
Chicago/Turabian StyleChai, Yue, Fang Zhao, Peizhi Ye, Fei Ma, Jiayu Wang, Pin Zhang, Qing Li, Jiani Wang, Wenna Wang, Qiao Li, and et al. 2023. "A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy" Journal of Clinical Medicine 12, no. 2: 505. https://doi.org/10.3390/jcm12020505
APA StyleChai, Y., Zhao, F., Ye, P., Ma, F., Wang, J., Zhang, P., Li, Q., Wang, J., Wang, W., Li, Q., & Xu, B. (2023). A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy. Journal of Clinical Medicine, 12(2), 505. https://doi.org/10.3390/jcm12020505








