Abstract
Background: Recirculation during veno-venous extracorporeal membrane oxygenation reduces extracorporeal oxygen exchange and patient oxygenation. To minimize recirculation and maximize oxygen delivery (DO2) the interaction of cannulation, ECMO flow and cardiac output requires careful consideration. We investigated this interaction in an observational trial. Methods: In 19 patients with acute respiratory distress syndrome and ECMO, we measured recirculation with the ultrasound dilution technique and calculated extracorporeal oxygen transfer (VO2), extracorporeal oxygen delivery (DO2) and patient oxygenation. To assess the impact of cardiac output (CO), we included CO measurement through pulse contour analysis. Results: In all patients, there was a median recirculation rate of approximately 14–16%, with a maximum rate of 58%. Recirculation rates >35% occurred in 13–14% of all cases. In contrast to decreasing extracorporeal gas exchange with increasing ECMO flow and recirculation, patient oxygenation increased with greater ECMO flows. High CO diminished recirculation by between 5–20%. Conclusions: Extracorporeal gas exchange masks the importance of DO2 and its effects on patients. We assume that increasing DO2 is more important than reduced VO2. A negative correlation of recirculation to CO adds to the complexity of this phenomenon. Patient oxygenation may be optimized with the direct measurement of recirculation.
1. Introduction
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is an important option in the treatment of patients suffering from severe forms of acute respiratory distress syndrome (ARDS). It is known to allow pulmonary recovery based on extracorporeal gas exchange [1]. Although thought to completely replace the native pulmonary gas exchange, the interaction between cannulation, ECMO blood flow (QECMO) and cardiac output (CO) affects the efficiency of extracorporeal gas exchange, as well as limits delivered oxygen (DO2) and patient arterial oxygenation [2,3,4].
Reduced extracorporeal gas exchange efficiency is initiated by the phenomenon of recirculation, which causes a hardly predictable return of extracorporeal oxygenated blood into the ECMO system because of the position of both ECMO cannulas [5]. The return of oxygenated blood thus decreases effective extracorporeal blood flow. Several determinants known to influence both recirculation and extracorporeal gas exchange have been described [6,7]. The position of the cannulas is one major determinant of the recirculation fraction (REC): a closer cannula position would increase recirculation, while a broader position would decrease the recirculation fraction. Despite the fixed cannula position, recirculation may be further affected by the native cardiac output of the patients and show two different results. A cardiac output close to the extracorporeal blood flow with a low CO/QECMO ratio will increase recirculation, and a cardiac output higher than extracorporeal blood flow with a higher CO/QECMO ratio will decrease recirculation. Both conditions reduce the fraction of venous blood oxygenated by the extracorporeal system and can cause systemic hypoxia.
As recirculation rises with increasing QECMO, it is thought that a corresponding higher recirculation reduces the oxygen delivery and leads to a further decrease of arterial patient oxygenation. To avoid this inefficient oxygen delivery with decreased patient oxygenation it was recommended to use a low QECMO regime in clinical practice [6,8,9,10].
Several authors have attempted to estimate the aforementioned determinants to minimize recirculation. It may be required to either exclude high recirculation with blood gas analysis, to indirectly recalculate native venous saturation, or to directly measure recirculation with an appropriate device such as the thermodilution technique [9,10,11,12,13,14,15]. At least one of these determinants must be known to resolve the recirculation and its impact on patient oxygenation, otherwise only an approximation is possible during clinical routine. An estimation may not allow the discrimination of whether low patient arterial oxygenation may be caused by a close cannula position, an assumed inefficient oxygen delivery with high QECMO or a patient-related high CO [16]. Systematic, but retrospective data, was provided by Zanella: he reported an increase in patient arterial oxygenation with increasing QECMO and increasing REC with a mathematical model fitted to retrospective patient data [17]. Prospective clinical and systematic directly measured data beyond the mathematical approximations, and fitted data was so far not carried out. In our prospective clinical trial, we sought to address the questions of whether (1) higher blood flows and increased recirculation are automatically associated with an inefficient oxygen delivery and reduced arterial patient oxygenation and (2) if patient cardiac output affects this dynamic condition. To systematically investigate the determinants of recirculation, we conducted an observational study with patients suffering from ARDS and requiring VV-ECMO.
2. Materials and Methods
2.1. Study Design and Participants
The study was an observational and prospective clinical study that consecutively enrolled adult patients. They were eligible if, according to the Berlin definition of ARDS, they failed to reach acceptable blood oxygenation and decarboxylation levels with optimized ventilator settings, inspired O2 fraction ≥ 90%, peak airway pressure [30 cm H2O, inverse-ratio ventilation] and supportive treatment such as inhaled nitric oxide (NO), and required extracorporeal veno-venous ECMO support [18]. The study was approved by the local medical ethics committee (Justus Liebig University Giessen, 22/2012) and registered with the German Clinical Trials Register (DRKS, DRKS00005106). Due to its observational design, a control group was not required. Written informed consent was obtained from all patients or substitute decision makers for participation in the trial.
2.2. ECMO Management and Patient Interventions
We either set up a standard two-cannula femoro-jugular or double lumen cannulation approach for the patients with a standardized ECMO circuit. In all cases, the cannulation was guided by transoesophageal echocardiography (TEE). The sizes of the cannulas were in the range 19–31 F depending on the patients’ anatomical features. The ECMO equipment employed was a standard centrifugal pump-based system (PLS-Rotaflow; Maquet Cardiopulmonary AG, Rastatt, Germany) with a hollow-fiber oxygenator (Quadrox-D; Maquet Cardiopulmonary AG, Rastatt, Germany). Once optimal and stable, QECMO and patient oxygenation and decarboxylation levels were discerned, and ventilation was gradually reduced for protective ventilation [19,20].
2.3. Patient Monitoring and Gas-Transfer Calculation
Besides standard ICU treatment, the patients received a CO-monitoring system based on arterial pulse contour analysis (Vigileo FloTrac™, Edwards, München, Germany) to be independent from right ventricle-based thermodilution principles [21]. Arterial and venous blood samples were drawn from the oxygenator during recirculation measurement to calculate ECMO oxygen transfer (VO2 ECMO). DO2 ECMO was calculated from the blood gas results. To avoid extensive blood sampling, we utilized transcutaneous pulsoxymetry (SpO2) as a surrogate parameter for patient arterial saturation.
VO2 ECMO was calculated according to the following equation:
where cxO2—content of oxygen in arterial (a) and venous (v) blood samples (mL O2/100 mL blood); QECMO—ECMO flow (L × min−1).
VO2 ECMO = (mL O2 × min−1) = caO2 − cvO2 × QECMO
DO2 ECMO was calculated according to the following equation:
where caO2—content of oxygen in arterial blood samples (mL O2/100 mL blood); QECMO – ECMO flow (L × min−1).
DO2 ECMO = (mL O2 × min−1) = caO2 × QECMO
2.4. Recirculation Measurement and Protocol
Recirculation was measured with an ultrasound-based flow dilution principle that makes use of two probes for ECMO draining and returning tubing (ELSA, Transonic Systems Inc., Ithaca, NY, USA) [11,12]. With an injection of a 20 mL NaCl 0.9% bolus, the device computes the REC from upstream- and downstream-derived flow dilution signals. Recirculation, gas exchange and SpO2 were measured daily during ECMO. To determine the impact of recirculation, a series of measurements was carried out for each patient. First, the ECMO flow was decreased before the measurement until SpO2 reached a clinically accepted and safe minimal value of approximately 85% [22]. Then, the ECMO flow was gradually increased with 300–500 mL steps until a maximum stable ECMO flow without any fluctuation was accomplished. During each step, recirculation was assessed with corresponding VO2 ECMO, SpO2 and CO.
2.5. Outcome Measures
The primary outcome was to explore the impact of REC on patient oxygenation and VO2 ECMO at increasing QECMO. We evaluated the clinical relevance of recirculation through investigating whether increasing ECMO flow causes higher recirculation and if it is associated with lower VO2 ECMO and SpO2. Secondary endpoints were the oxygenator gas exchange (DO2, and VO2 ECMO), REC, ECMO flow (QECMO) and patient CO.
2.6. Data Collection and Statistical Analysis
As we did not expect a particular probability distribution of the individual data series, we utilized the known impact of ECMO flow on patient oxygenation for an explorative analysis. The first day of support (Day 1) and 2–3 days before weaning (Day x) were chosen as the representative values for a non-parametrical Spearman rank correlation test to reveal an association between QECMO and SpO2 (first analysis day 1, second analysis day x). We postulated SpO2 to be independent of ECMO flow. Based on the different interindividual flow ranges, selecting five evenly distributed ECMO flow values was required from each patient data set for the correlation test. A repeated analysis at two different days should have shown whether the correlation could be confirmed during the entire ECMO period. A p-value < 5% was defined as significant. All data were collected as worksheets and analysed with Stata/IC 12.1 (Stata Corp., College Station, TX, USA) and Statistical Package for the Social Sciences (SPSS) 22.0 (IBM Corp., Armonk, NY, USA). Data graphing was carried out with OriginPro 2023 (OriginLab, Additive GmbH, Friedrichsdorf, Germany). To model a correlation between recirculation, gas exchange and patient oxygenation, we applied a curve fitting to the data and included the fitting line as well as its 95% CI in the graphs as appropriate.
3. Results
Nineteen patients could be included for assessment of recirculation, VO2 ECMO and SpO2. The median age of the patients was 48 years and the median duration of the ECMO support was 12 days. In total, 52% of the patients could be weaned from ECMO and 42% could be discharged. In 70% of cases, patients received a double lumen cannula (Avalon Elite®, Maquet KG, Rastatt, Germany), whereas the remaining patients were cannulated through a femoro-jugular access with two separate cannulas.
3.1. ECMO Flow and Recirculation
The frequency distribution of all recirculation data showed a median recirculation rate between 14–16% at day 1 and 2–3 days (Day x) before weaning with a maximum rate of approximately 58%. High recirculation rates above 35% were noted in only 13–14% of all measurements (Figure 1). To exemplify the distribution of REC and show the median value we added additional distribution curves (red and grey, day 1 and day x, respectively) to the frequency distribution of REC.
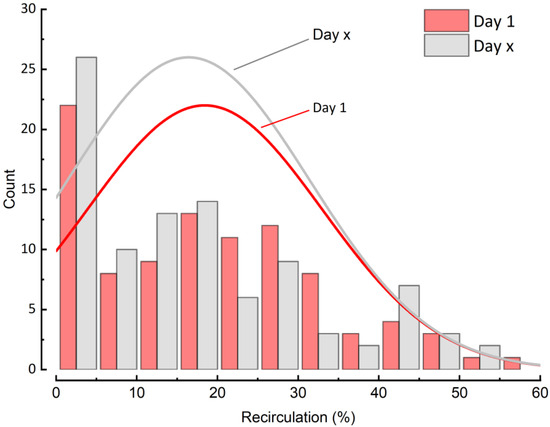
Figure 1.
Distribution of recirculation at Day 1 and 2–3 days before weaning.
3.2. Recirculation and ECMO Oxygenator Transfer
Figure 2 depicts the effect of increasing blood flow on recirculation and ECMO oxygen transfer. With increasing ECMO blood flow and the corresponding increasing recirculation (Figure 2A), VO2 ECMO rose from 50 mL O2 × min−1 to a maximum value of approximately 220 mL O2 × min−1 (Figure 2B). Above a plateau at 4 L × min−1 ECMO blood flow, VO2 ECMO r reached either a plateau (10 out 19 patients) or showed reduced oxygen transfer (9 out of 19 patients) simultaneous to a further rising recirculation rate.
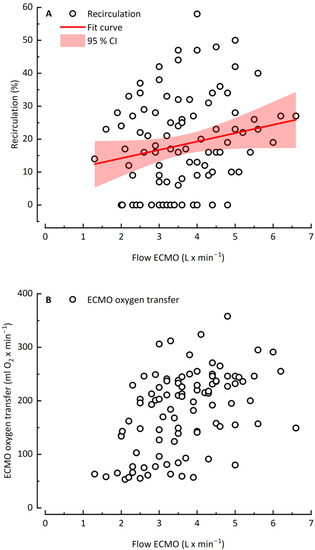
Figure 2.
Recirculation rate (A) and ECMO oxygen transfer (B) as a function of ECMO flow rate.
3.3. ECMO Blood Flow, Recirculation, ECMO Oxygen Delivery and Arterial Patient Saturation
In contrast to VO2 ECMO with its mostly plateau reaching values, DO2 ECMO rose linearly as recirculation increased with higher QECMO. Even with high QECMO, no decrease was observed (Figure 3B). Analogous to the linear trend of DO2, SpO2 increased linearly with increasing QECMO and recirculation without any relevant decrease at high QECMO (Figure 3C). The binomial test results showed a strong positive relationship between QECMO and SpO2 on Day 1 and Day x (p = 1.51 × 10 −6 and 7.19 × 10 −7, respectively).
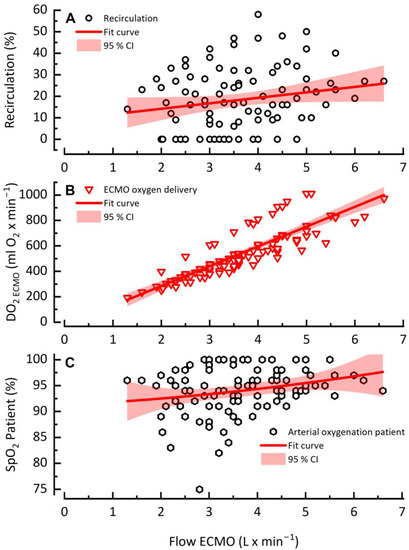
Figure 3.
Recirculation rate (A), DO2 ECMO (B) and patient arterial saturation (C) as a function of ECMO flow rate.
3.4. Cardiac Output and Its Impact on Recirculation
To unravel the impact of CO on recirculation, we investigated patients with high CO variation with a difference >4 L × min−1 between the lowest and highest CO during ECMO. The data was separated into two groups with low and high CO limited by the 0.15 and 0.85 quintiles. A clinically common QECMO of 4 L × min−1 was chosen as a point of reference for comparison of both groups. Exemplified by one data set (patient #10), higher CO decreased recirculation by between 13% (Figure 4).
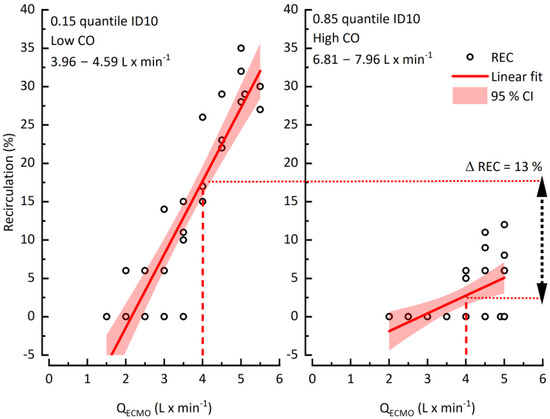
Figure 4.
The impact of cardiac output on recirculation.
4. Discussion
Our findings suggest that recirculation is a clinically variable phenomenon that reduces extracorporeal oxygenation depending on ECMO blood flow. Second, higher blood flow seems to not be negatively associated with patient arterial oxygenation. Third, recirculation appears to be considerably influenced by patient CO.
How can these findings be reconciled with the evidence from numerous studies indicating that recirculation causes decreased efficiency with a negative impact on patients?
4.1. Recirculation, Gas Transfer and Patient Oxygenation
Recirculation is thought to be associated with a decreasing effective pump flow beyond an optimal flow—Fortenberry reported that effective ECMO flow is delivered to patients’ plateaus after an initial increase and is then reduced with the rising pump flow [6,8]. Additionally, several investigators have assumed that high recirculation may markedly diminish extracorporeal gas exchange and cause patient hypoxemia [5,6,9]. We indeed could confirm with our findings that VO2 ECMO in 9 out of 10 patients decreased beyond a variable optimum flow. Nevertheless, high values of QECMO were thought not only to reduce extracorporeal oxygen transfer, but to also cause patient hypoxemia. Consequently, high ECMO flows of approximately 5–6 L × min−1, with obviously reduced extracorporeal DO2, were prevented in the past [8,10,13]. We adopted this assumption previously, calculated an individual optimum flow and recommended this as a reasonable maximum flow before the study period. Despite this calculated optimum flow, our patients clinically often demonstrated an increased arterial saturation with pump flows higher than the computed optimal flow. Togo reported a similar effect with adult goats—higher flow and recirculation resulted in increasing arterial oxygenation [23]. Nunes further described a positive association between arterial blood oxygen content and increasing ECMO blood flow [16].
Because of the lack of systematic data, it remained open whether increasing ECMO blood flow or recirculation was accompanied with changed or reduced patient oxygenation. To disentangle the clinical paradox of increasing patient oxygenation despite reduced oxygenator function, we then focused on patient parameters. Akin to the pre-trial period, SpO2 increased linearly with increasing ECMO blood flow and recirculation in our study. This was reproducible at the beginning, as well as before the weaning of ECMO. Although the patient oxygenation may reach a plateau or even slightly decrease at flows >5–6 L × min−1 beyond an “optimum” flow, the ECMO flow dependent extracorporeal DO2 seemed clinically to be a determining factor of patient oxygenation. ECMO blood flow did not limit but, rather, ensured and increased patient arterial oxygenation. It could cautiously be assumed that increasing ECMO blood flow is much more important in clinical practice than a reduced oxygenator oxygen transfer which strictly affects oxygenator performance. Our prospective and systematic clinical data confirmed the findings of Zanella, who used retrospective patient data to compute a predictive model: with a high accuracy he could show that higher ECMO blood flows are associated with higher patient arterial oxygenation [17]. Although available as a teaching tool a validation of this predictive model with larger and especially prospective clinical data has not been carried out.
4.2. Recirculation and Patient CO
Besides gas exchange, recirculation as a function of CO featured a certain pattern. In patients with a high variation of CO > 4 L × min−1 during support recirculation, it was negatively correlated with CO. Higher CO markedly reduced recirculation. This highlights how recirculation is not a fixed but dynamic process that could be misleading if the affecting CO is not considered. Although low recirculation because of high CO may increase the extracorporeal DO2, the mismatch between higher CO and lower pump flow still leads to prepulmonary shunting and refractory patient hypoxemia as described by Levy [3]. Therefore, it is important to reassess recirculation during daily practice as it may exhibit major changes.
4.3. Clinical Relevance
The estimation of recirculation based on theoretical calculations of the native venous saturation were instruments that allowed for initial insights into the complex interaction between native venous circulation and a technical ECMO system [9,15,24,25]. They permitted a mathematical approximation and resulted in estimated recirculation fractions between 20–30%, which seemed to be clinically prevalent [9]. Several algorithms have been developed for the clinical management and estimation of recirculation phenomena and patient oxygenation [4,26,27]. The existence of several mathematical unknowns in the calculations and a lack of comprehensive clinical validation limited the usability of these methods. The overestimation of ECMO-related technical efficiency further masked the importance of ECMO flow-dependent extracorporeal DO2 and its positive impact on patient oxygenation. Quite recently, the focus has shifted from ECMO efficiency towards an optimized extracorporeal DO2 with higher oxygenation in animals [23]. A more careful delineation of our data, beyond extracorporeal oxygen transfer, revealed extracorporeal DO2 to be independent from extracorporeal gas transfer. The combined use of blood gas analysis and the ultrasound dilution technique fostered our confirmation of recirculation as a clinically prevalent phenomenon with reduced oxygenator efficiency that does not automatically reduce patient arterial oxygenation with higher ECMO blood flows. The positive impact of higher ECMO flow on patient arterial oxygenation is a major contribution to the clinical context, enabling optimization of patient oxygenation.
4.4. Limitations
Based on the observational study character, we were not able to vary cannulation and cannula positions, and thus could not draw any conclusions on the impact of cannulation. Additionally, 68% of the patients received a double-lumen cannulation, which further impedes any assessment. Additionally, a predominant double-lumen cannulation further impedes any assessment. Systematic data on varying cannulation, venous volume and altering CO may only be derived with an in vitro model.
Although the validity of pulse contour analysis applied to CO measurement may be questioned, we could exclude any possible right ventricular mixing phenomena with this method and estimate CO without additional invasive methods. More recent results indeed confirmed that mixing effects during ECMO may impact the accuracy of traditional thermodilution based cardiac output measurement [28]. Russ reported in an animal experiment that during ECMO measurement of cardiac output through thermodilution resulted in an overestimation of measured CO compared to aortic blood flow measured directly with an ultrasound flow probe. Even with low blood flows a mean difference approximately 1.38 L × min−1 could be observed which increased with higher blood flows and increasing recirculation measured with ultrasound dilution. Flow differences up to 2.7 L × min−1 may be assumed to be a high proportion of the cardiac output. The authors concluded that the additional recirculation within the ECMO system caused an additional recirculation signal within the algorithm of CO-calculation and affected the temperature changes which influenced the computed CO values. A higher accuracy during ECMO would require a modification of the current algorithms for the CO measurement. Haller observed an overestimated cardiac output up to a maximum of 300% with conventional thermodilution during ECMO and recommended using dye dilution techniques instead, which were obviously not influenced by recirculation kinetics [21]. The used pulse contour analysis used in our study is classified as an internally calibrated system based on biometric data and pulse wave characteristics depending on vascular resistance and compliance [29]. Depending on vascular tone changes, Slagt reported error rates below 30% with the internally calibrated pulse contour analysis in normo- and hypodynamic conditions and classified this system to be sufficiently accurate [30]. Bond reported a more recent pulse contour analysis during ECMO to be in good agreement to echocardiographic cardiac output measurement with a mean bias of −0.2 L × min−1 and a percentage error of 24% [31]. We, therefore, assume the used pulse contour analysis to be a compromise in estimating cardiac output. Regarding patient oxygenation, traditional blood gas analysis would have resulted in more precise data of the patient gas exchange but would have also caused extra blood loss. Therefore, pulsoxymetry as a surrogate parameter was a justifiable compromise with this trial.
5. Conclusions
The measurement of recirculation as a prevalent clinical phenomenon through the ultrasound dilution technique is a real-time method with higher precision than mathematical approximations. The systematic and direct clinical measurement confirmed empirical ranges of recirculation approximately 10–30%, which reduced extracorporeal oxygen transfer. However, higher ECMO flows allowed increased patient arterial oxygenation, and thus, may prevent refractory hypoxemia. To be of true diagnostic value, recirculation should always be considered multifactorial. The ECMO blood flow itself, its ratio to cardiac output and the cannula position may intricately affect patient oxygenation and should be carefully considered.
Author Contributions
J.G.: trial design, principal investigator, data acquisition, data analysis and interpretation, and drafting the manuscript. D.B.: trial design, principal investigator, data analysis and interpretation, drafting the manuscript, and critical review. K.M. and A.B.: trial design, drafting the manuscript, and critical review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the medical Ethics Committee (Justus Liebig University Giessen, 22/2012; 10 April 2012).
Informed Consent Statement
Written informed consent was obtained from all patients or substitute decision makers for participation in the trial.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge Nikolai Krivitski (Transonic Systems Inc., Ithaca, NY, USA) for technical assistance and stimulating and valuable discussion.
Conflicts of Interest
Johannes Gehron received presentation honoraria from Getinge Deutschland GmbH, Keller Medical GmbH, LivaNova Group Deutschland and Terumo Deutschland GmbH. Dirk Bandorski declared no conflicts of interest. Konstantin Mayer received presentation honoraria from Abbot, Astellas, AstraZeneca, Baxter, BBraun, BerlinChemie, Boehringer, FreseniusKabi, GSK, MST, Novartis, Nestle, Pfizer. Andreas Böning received presentation honoraria from Abbott Zoll, Bayer, B. Braun Maquet, Smith & Nephew, Somahlution, and Spectranetics.
References
- Frenckner, B.; Palmer, P.; Linden, V. Extracorporeal respiratory support and minimally invasive ventilation in severe ARDS. Minerva Anestesiol. 2002, 68, 381–386. [Google Scholar] [PubMed]
- Charbit, J.; Deras, P.; Courvalin, E.; Laumon, T.; Dagod, G.; Martinez, O.; Capdevila, X. Structural recirculation and refractory hypoxemia under femoro-jugular veno-venous extracorporeal membrane oxygenation. Artif. Organs 2021, 45, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Taccone, F.S.; Guarracino, F. Recent developments in the management of persistent hypoxemia under veno-venous ECMO. Intensive Care Med. 2015, 41, 508–510. [Google Scholar] [CrossRef]
- Messai, E.; Bouguerra, A.; Guarracino, F.; Bonacchi, M. Low Blood Arterial Oxygenation During Venovenous Extracorporeal Membrane Oxygenation: Proposal for a Rational Algorithm-Based Management. J. Intensive Care Med. 2016, 31, 553–560. [Google Scholar] [CrossRef]
- Xie, A.; Yan, T.D.; Forrest, P. Recirculation in venovenous extracorporeal membrane oxygenation. J. Crit. Care 2016, 36, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Fortenberry, J.D.; Pettignano, R.; Dykes, F. Principles and Practice of venovenous ECMO. In ECMO Extracorporeal Cardiopulmonary Support in Critical Care, 3rd ed.; Van Meurs, K., Lally, K.P., Peek, G., Zwischenberger, J.B., Eds.; ELSO Extracorporeal Life Support Organisation: Ann Arbor, MI, USA, 2005. [Google Scholar]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Brechot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013, 39, 838–846. [Google Scholar] [CrossRef]
- Broman, M.; Frenckner, B.; Bjallmark, A.; Broome, M. Recirculation during veno-venous extra-corporeal membrane oxygenation—A simulation study. Int. J. Artif. Organs 2015, 38, 23–30. [Google Scholar] [CrossRef]
- Locker, G.J.; Losert, H.; Schellongowski, P.; Thalhammer, F.; Knapp, S.; Laczika, K.F.; Burgmann, H.; Staudinger, T.; Frass, M.; Muhm, M. Bedside exclusion of clinically significant recirculation volume during venovenous ECMO using conventional blood gas analyses. J. Clin. Anesth. 2003, 15, 441–445. [Google Scholar] [CrossRef]
- Sreenan, C.; Osiovich, H.; Cheung, P.Y.; Lemke, R.P. Quantification of recirculation by thermodilution during venovenous extracorporeal membrane oxygenation. J. Pediatr. Surg. 2000, 35, 1411–1414. [Google Scholar] [CrossRef]
- Clements, D.; Primmer, J.; Ryman, P.; Marr, B.; Searles, B.; Darling, E. Measurements of recirculation during neonatal veno-venous extracorporeal membrane oxygenation: Clinical application of the ultrasound dilution technique. J. Extra-Corpor. Technol. 2008, 40, 184–187. [Google Scholar]
- Darling, E.M.; Crowell, T.; Searles, B.E. Use of dilutional ultrasound monitoring to detect changes in recirculation during venovenous extracorporeal membrane oxygenation in swine. ASAIO J. 2006, 52, 522–524. [Google Scholar] [CrossRef]
- van Heijst, A.F.; van der Staak, F.H.; de Haan, A.F.; Liem, K.D.; Festen, C.; Geven, W.B.; van de Bor, M. Recirculation in double lumen catheter veno-venous extracorporeal membrane oxygenation measured by an ultrasound dilution technique. ASAIO J. 2001, 47, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Primmer, J.; Searles, B.E.; Darling, E.M. The potential of accurate SvO2 monitoring during venovenous extracorporeal membrane oxygenation: An in vitro model using ultrasound dilution. Perfusion 2007, 22, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Gelfond, J.; Zarzabal, L.A.; Darling, E. Calculating mixed venous saturation during veno-venous extracorporeal membrane oxygenation. Perfusion 2009, 24, 333–339. [Google Scholar] [CrossRef]
- Nunes, L.B.; Mendes, P.V.; Hirota, A.S.; Barbosa, E.V.; Maciel, A.T.; Schettino, G.P.P.; Costa, E.L.V.; Azevedo, L.C.P.; Park, M.; Group, E. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: Exploring the limits of extracorporeal respiratory support. Clinics 2014, 69, 173–178. [Google Scholar] [CrossRef]
- Zanella, A.; Salerno, D.; Scaravilli, V.; Giani, M.; Castagna, L.; Magni, F.; Carlesso, E.; Cadringher, P.; Bombino, M.; Grasselli, G.; et al. A mathematical model of oxygenation during venovenous extracorporeal membrane oxygenation support. J. Crit. Care 2016, 36, 178–186. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Tonetti, T.; Quintel, M. How best to set the ventilator on extracorporeal membrane lung oxygenation. Curr. Opin. Crit. Care 2017, 23, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Terragni, P.P.; Del Sorbo, L.; Mascia, L.; Urbino, R.; Martin, E.L.; Birocco, A.; Faggiano, C.; Quintel, M.; Gattinoni, L.; Ranieri, V.M. Tidal Volume Lower than 6 ml/kg Enhances Lung Protection Role of Extracorporeal Carbon Dioxide Removal. Anesthesiology 2009, 111, 826–835. [Google Scholar] [CrossRef]
- Haller, M.; Zollner, C.; Manert, W.; Briegel, J.; Kilger, E.; Polasek, J.; Hummel, T.; Forst, H.; Peter, K. Thermodilution cardiac output may be incorrect in patients on venovenous extracorporeal lung assist. Am. J. Respir. Crit. Care Med. 1995, 152, 1812–1817. [Google Scholar] [CrossRef]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; RUBIO Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef]
- Togo, K.; Takewa, Y.; Katagiri, N.; Fujii, Y.; Kishimoto, S.; Date, K.; Miyamoto, Y.; Tatsumi, E. Impact of bypass flow rate and catheter position in veno-venous extracorporeal membrane oxygenation on gas exchange in vivo. J. Artif. Organs 2015, 18, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D. Refinement of a novel empirical formula to determine the “true” mixed venous saturation in patients undergoing veno-venous extracorporeal life support. ASAIO J. 2010, 56, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D.W.; Chambers, S.; Fazzalari, F.; Hirschl, R.; Bartlett, R.H. Determination of native mixed venous saturation during venovenous extracorporeal circulation. ASAIO J. 1995, 41, 838–841. [Google Scholar] [CrossRef]
- Messai, E.; Bouguerra, A.; Harmelin, G.; Di Lascio, G.; Cianchi, G.; Bonacchi, M. A new formula for determining arterial oxygen saturation during venovenous extracorporeal oxygenation. Intensive Care Med. 2013, 39, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Costa, E.L.; Maciel, A.T.; Silva, D.P.; Friedrich, N.; Barbosa, E.V.; Hirota, A.S.; Schettino, G.; Azevedo, L.C. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS ONE 2013, 8, e54954. [Google Scholar] [CrossRef]
- Russ, M.; Steiner, E.; Boemke, W.; Busch, T.; Melzer-Gartzke, C.; Taher, M.; Badulak, J.; Weber-Carstens, S.; Swenson, E.R.; Francis, R.C.E.; et al. Extracorporeal Membrane Oxygenation Blood Flow and Blood Recirculation Compromise Thermodilution-Based Measurements of Cardiac Output. ASAIO J. 2022, 68, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Kouz, K.; Scheeren, T.W.L.; Greiwe, G.; Hoppe, P.; Romagnoli, S.; de Backer, D. Cardiac output estimation using pulse wave analysis—Physiology, algorithms, and technologies: A narrative review. Br. J. Anaesth. 2021, 126, 67–76. [Google Scholar] [CrossRef]
- Slagt, C.; Malagon, I.; Groeneveld, A.B. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br. J. Anaesth. 2014, 112, 626–637. [Google Scholar] [CrossRef]
- Bond, O.; Pozzebon, S.; Franchi, F.; Zama Cavicchi, F.; Creteur, J.; Vincent, J.L.; Taccone, F.S.; Scolletta, S. Comparison of estimation of cardiac output using an uncalibrated pulse contour method and echocardiography during veno-venous extracorporeal membrane oxygenation. Perfusion 2020, 35, 397–401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).