The Influence of Methods for Cardiac Output Determination on the Diagnosis of Precapillary Pulmonary Hypertension: A Mathematical Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Mathematical Model
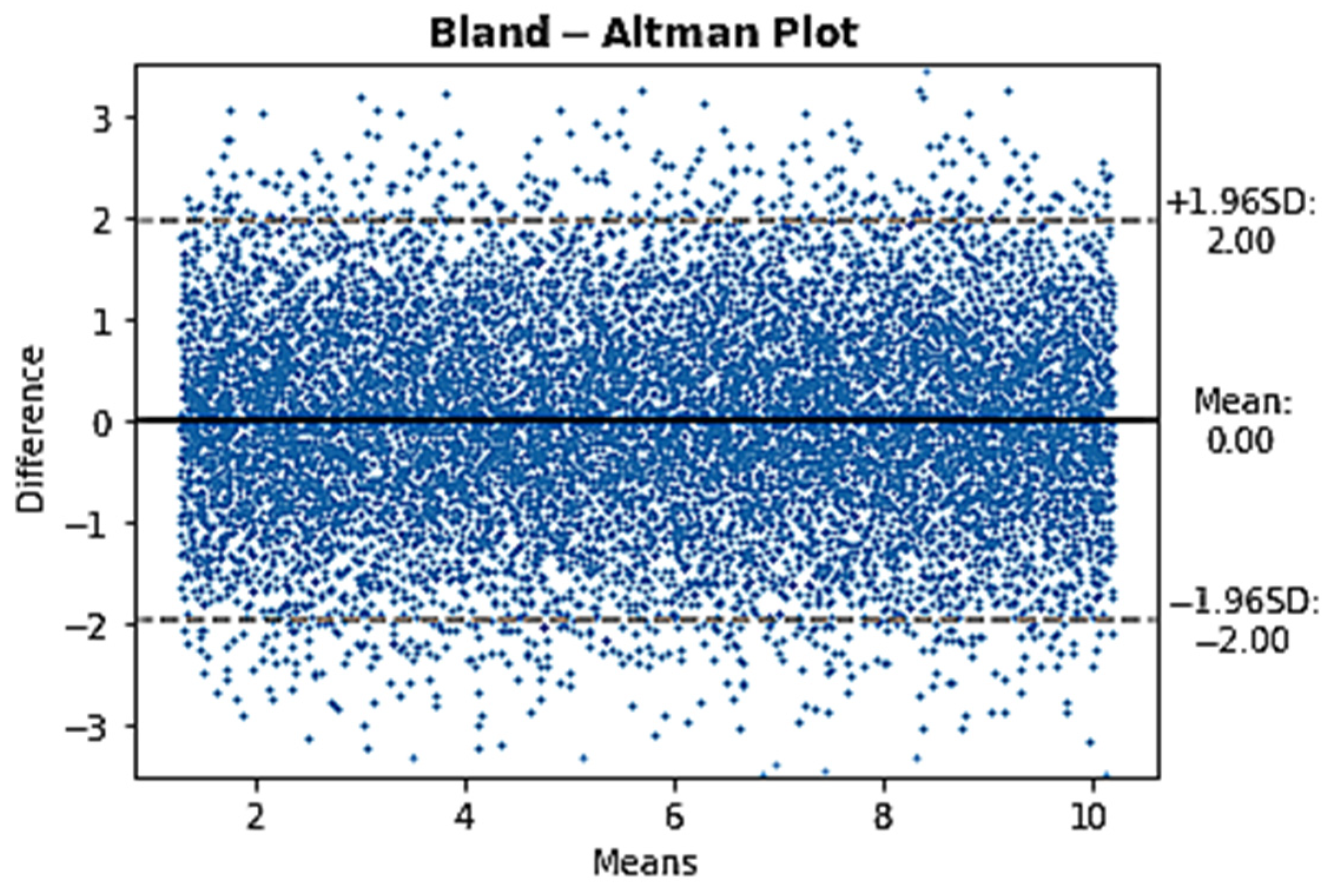
2.2.1. Comparison between COTD and CODF at Rest in PcPH Patients
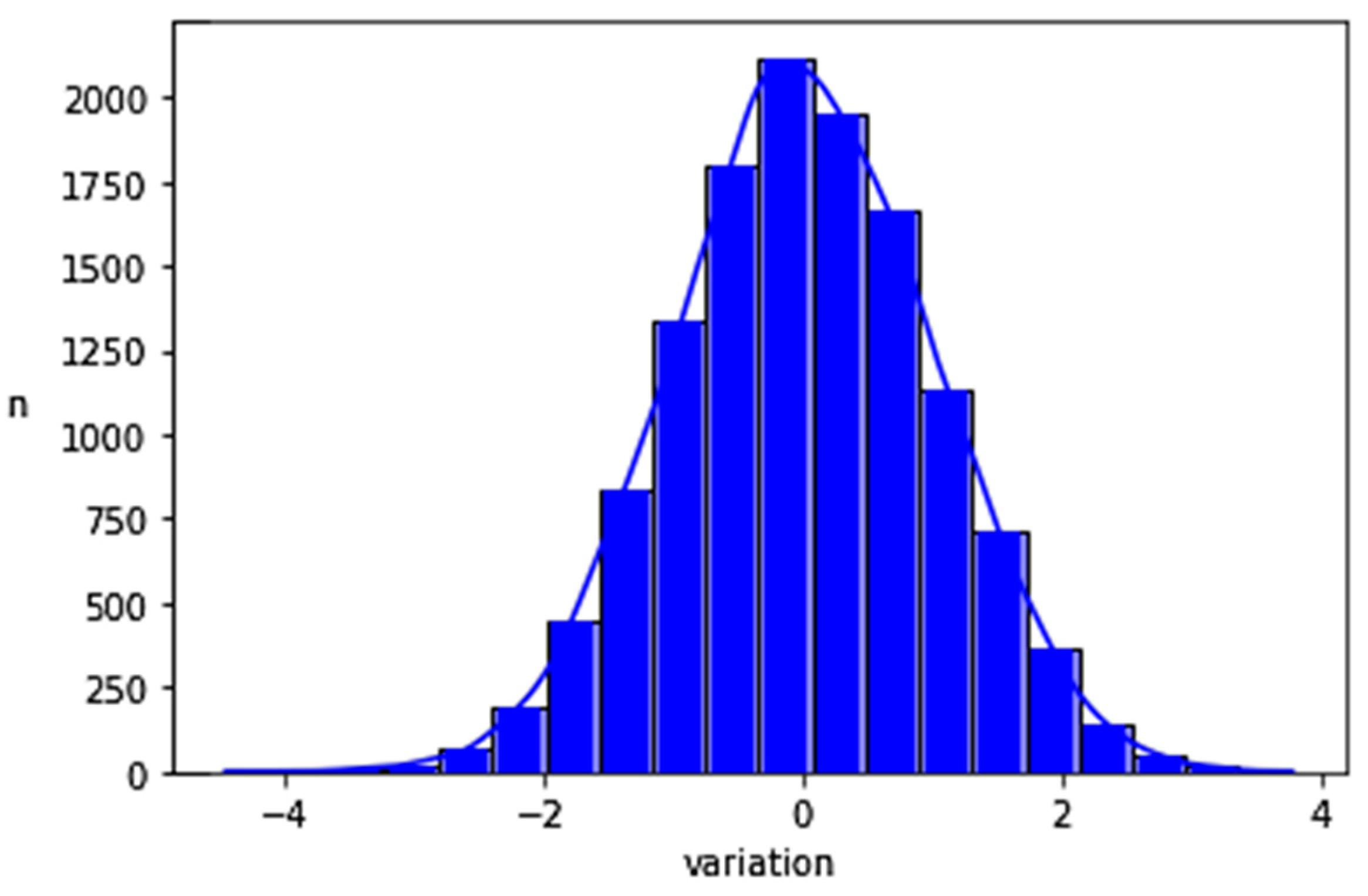
2.2.2. Creation of a Set of Data with a Normal Distribution of CO Differences within a Plausible CO Range
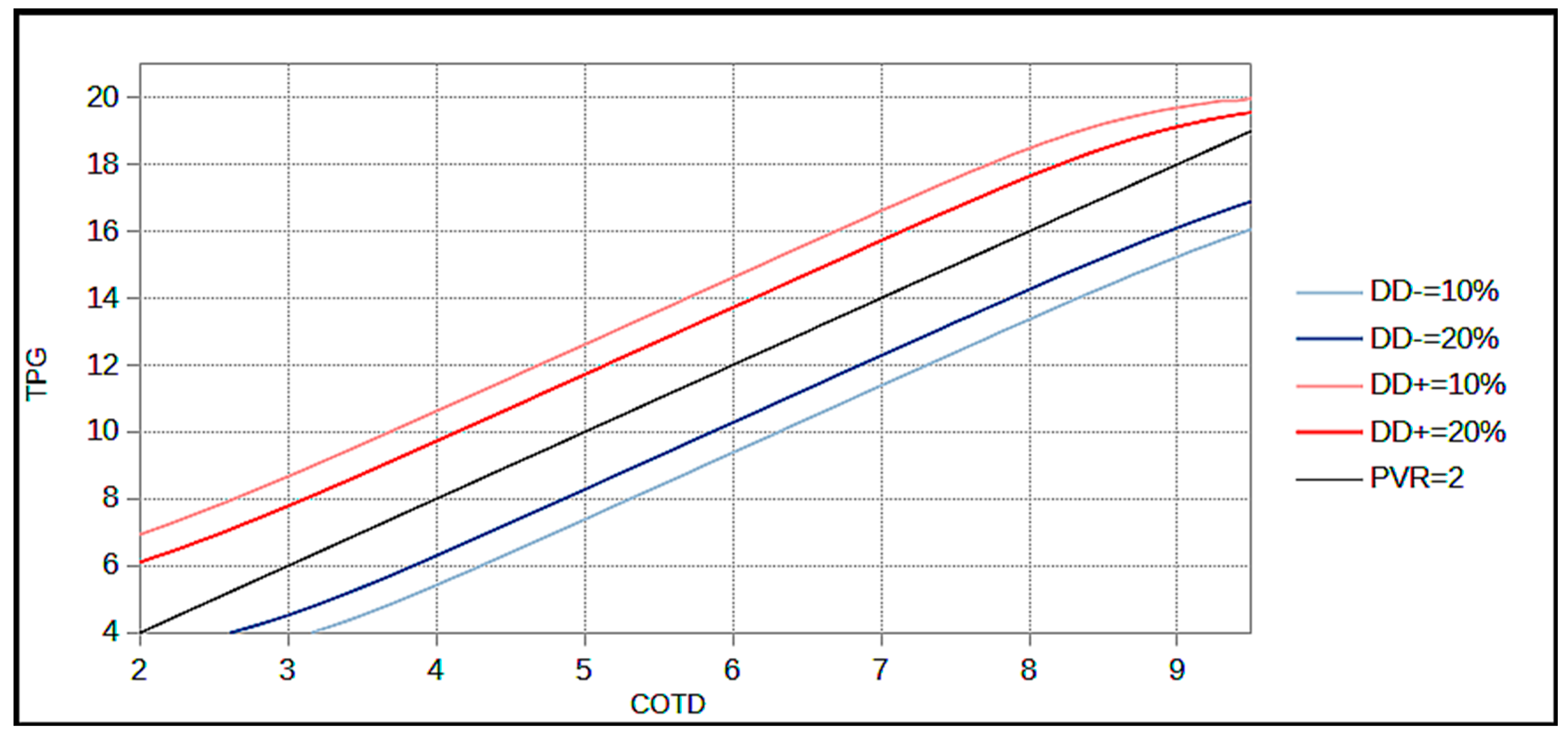
2.2.3. Diagnostic Disagreement Calculation with Limits of Plausible CO Values
3. Results
| Patients | mPAP | Mean CODF | Mean COTD | Bias | 1.96 SD of Bias | |
|---|---|---|---|---|---|---|
| Hoeper et al. [2] | 35 (31 PAH, 4 CTEPH) | 56 ± 12 | 3.7 ± 1.2 | 3.7 ± 1.3 | 0.01 | 1.10 |
| Khirfan et al. * [4] | 75 PAH patients | 48 ± 14 | 4.6 ± 1.5 | 4.6 ± 1.6 | 0.02 | 2.02 |
| Duknic et al. [3] | 24 (7 PAH 17 CTEPH) | 37 ± 11 | 5.9 ± 1.5 | 5.5 ± 1.2 | 0.45 | 2.48 |
4. Discussion
- For a DgDis < 10%, the clinician can be quite confident of a diagnosis using COTD and measurement of CODF is not systematically required.
- For a DgDis between 10 and 20% the clinician should consider the use of CODF.
- For a DgDis > 20% the clinician should use CODF because the probability that the patient would be classified differently using CODF is high.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Maier, R.; Tongers, J.; Niedermeyer, J.; Hohlfeld, J.M.; Hamm, M.; Fabel, H. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 160, 535–541. [Google Scholar] [CrossRef]
- Duknic, M.; Lichtblau, M.; Saxer, S.; Berlier, C.; Schneider, S.R.; Schwarz, E.I.; Carta, A.F.; Furian, M.; Bloch, K.E.; Ulrich, S. Comparison of Repetitive Cardiac Output Measurements at Rest and End-Exercise by Direct Fick Using Pulse Oximetry vs. Blood Gases in Patients with Pulmonary Hypertension. Front. Med. 2021, 8, 776956. [Google Scholar] [CrossRef] [PubMed]
- Khirfan, G.; Ahmed, M.K.; Almaaitah, S.; Almoushref, A.; Agmy, G.M.; Dweik, R.A.; Tonelli, A.R. Comparison of Different Methods to Estimate Cardiac Index in Pulmonary Arterial Hypertension. Circulation 2019, 140, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Desole, S.; Obst, A.; Habedank, D.; Opitz, C.F.; Knaack, C.; Hortien, F.; Heine, A.; Stubbe, B.; Ewert, R. Comparison between thermodilution and Fick methods for resting and exercise-induced cardiac output measurement in patients with chronic dyspnea. Pulm. Circ. 2022, 12, e12128. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Genecand, L.; Adler, D.; Beghetti, M.; Lador, F. Cardiac Output Determination in Precapillary Pulmonary Hypertension: A Systematic Review. Respiration 2021, 100, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Noel-Savina, E.; Prevot, G.; Tetu, L.; Pillard, F.; Riviere, D.; Didier, A. Determination of Cardiac Output in Pulmonary Hypertension Using Impedance Cardiography. Respiration 2018, 96, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Farina, S.; Teruzzi, G.; Cattadori, G.; Ferrari, C.; De Martini, S.; Bussotti, M.; Calligaris, G.; Bartorelli, A.; Agostoni, P. Noninvasive cardiac output measurement by inert gas rebreathing in suspected pulmonary hypertension. Am. J. Cardiol. 2014, 113, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Yung, G.L.; Fedullo, P.F.; Kinninger, K.; Johnson, W.; Channick, R.N. Comparison of impedance cardiography to direct Fick and thermodilution cardiac output determination in pulmonary arterial hypertension. Congest. Heart Fail. 2004, 10, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Brusca, S.B.; Rhodes, P.S.; Kolb, T.M.; Mathai, S.C.; Tedford, R.J. Use of thermodilution cardiac output overestimates diagnoses of exercise-induced pulmonary hypertension. Pulm. Circ. 2017, 7, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Fares, W.H.; Blanchard, S.K.; Stouffer, G.A.; Chang, P.P.; Rosamond, W.D.; Ford, H.J.; Aris, R.M. Thermodilution and Fick cardiac outputs differ: Impact on pulmonary hypertension evaluation. Can. Respir. J. 2012, 19, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Alkhodair, A.; Tsang, M.Y.C.; Cairns, J.A.; Swiston, J.R.; Levy, R.D.; Lee, L.; Huckell, V.F.; Brunner, N.W. Comparison of thermodilution and indirect Fick cardiac outputs in pulmonary hypertension. Int. J. Cardiol. 2018, 258, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.D.; Archer, S.L.; Rich, S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur. Respir. J. 2013, 42, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.A.; Genecand, L.; Hachulla, A.L.; Noble, S.; Beghetti, M.; Vallee, J.P.; Lador, F. Non-Invasive Cardiac Output Determination Using Magnetic Resonance Imaging and Thermodilution in Pulmonary Hypertension. J. Clin. Med. 2022, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, G.J.; Marcus, J.T.; Boonstra, A.; Postmus, P.E.; Westerhof, N.; Vonk-Noordegraaf, A. Non-invasive stroke volume assessment in patients with pulmonary arterial hypertension: Left-sided data mandatory. J. Cardiovasc. Magn. Reson. 2008, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Deux, J.F.; Crowe, L.A.; Genecand, L.; Hachulla, A.L.; Glessgen, C.G.; Noble, S.; Beghetti, M.; Ning, J.; Giese, D.; Lador, F.; et al. Correlation between Pulmonary Artery Pressure and Vortex Duration Determined by 4D Flow MRI in Main Pulmonary Artery in Patients with Suspicion of Chronic Thromboembolic Pulmonary Hypertension (CTEPH). J. Clin. Med. 2022, 11, 5237. [Google Scholar] [CrossRef] [PubMed]

| TPG | COTD | PVRTD | DgDis− |
|---|---|---|---|
| 6 | 3.5 | 1.7 | 30.0% |
| 7 | 4 | 1.75 | 29.4% |
| 7 | 5 | 1.4 | 6.9% |
| 8 | 5 | 1.6 | 16.2% |
| 8 | 6 | 1.3 | 2.5% |
| 9 | 5 | 1.8 | 31.1% |
| 9 | 6 | 1.5 | 7.1% |
| 10 | 6 | 1.7 | 15.4% |
| 10 | 7 | 1.4 | 2.5% |
| 11 | 6 | 1.8 | 31.2% |
| 11 | 7 | 1.6 | 7.07% |
| TPG | COTD | PVRTD | DgDis+ |
|---|---|---|---|
| 6 | 2.5 | 2.4 | 45.4% |
| 7 | 2.5 | 2.8 | 23.8% |
| 7 | 3 | 2.3 | 37.3% |
| 8 | 3 | 2.7 | 19.6% |
| 8 | 3.5 | 2.3 | 33.6% |
| 9 | 3 | 3 | 8.5% |
| 9 | 4 | 2.3 | 32.0% |
| 10 | 3 | 3.3 | 3.0% |
| 10 | 4 | 2.5 | 16.8% |
| 11 | 4 | 2.75 | 7.3% |
| 11 | 5 | 2.2 | 31.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genecand, L.; Simian, G.; Desponds, R.; Wacker, J.; Ulrich, S.; Lechartier, B.; Fellrath, J.-M.; Sitbon, O.; Beghetti, M.; Lador, F. The Influence of Methods for Cardiac Output Determination on the Diagnosis of Precapillary Pulmonary Hypertension: A Mathematical Model. J. Clin. Med. 2023, 12, 410. https://doi.org/10.3390/jcm12020410
Genecand L, Simian G, Desponds R, Wacker J, Ulrich S, Lechartier B, Fellrath J-M, Sitbon O, Beghetti M, Lador F. The Influence of Methods for Cardiac Output Determination on the Diagnosis of Precapillary Pulmonary Hypertension: A Mathematical Model. Journal of Clinical Medicine. 2023; 12(2):410. https://doi.org/10.3390/jcm12020410
Chicago/Turabian StyleGenecand, Léon, Gaëtan Simian, Roberto Desponds, Julie Wacker, Silvia Ulrich, Benoit Lechartier, Jean-Marc Fellrath, Olivier Sitbon, Maurice Beghetti, and Frédéric Lador. 2023. "The Influence of Methods for Cardiac Output Determination on the Diagnosis of Precapillary Pulmonary Hypertension: A Mathematical Model" Journal of Clinical Medicine 12, no. 2: 410. https://doi.org/10.3390/jcm12020410
APA StyleGenecand, L., Simian, G., Desponds, R., Wacker, J., Ulrich, S., Lechartier, B., Fellrath, J.-M., Sitbon, O., Beghetti, M., & Lador, F. (2023). The Influence of Methods for Cardiac Output Determination on the Diagnosis of Precapillary Pulmonary Hypertension: A Mathematical Model. Journal of Clinical Medicine, 12(2), 410. https://doi.org/10.3390/jcm12020410






