Abstract
Treatment with sodium-glucose cotransporter-2 (SGLT2) inhibitors may have pleiotropic and beneficial effects in terms of ameliorating of risk factors for the progression of autosomal dominant polycystic kidney disease (ADPKD). However, there is insufficient evidence regarding the use of these drugs in patients with ADPKD, as they were excluded from several clinical trials conducted to explore kidney protection provided by SGLT2 inhibitors. This retrospective single-arm case series study was performed to investigate the effects of dapagliflozin, a selective SGLT2 inhibitor administered at 10 mg/day, on changes in height-adjusted kidney volume (htTKV) and estimated glomerular filtration rate (eGFR) in ADPKD patients. During a period of 102 ± 20 days (range 70–156 days), eGFR was decreased from 47.9 (39.7–56.9) to 40.8 (33.7–44.5) mL/min/1.73 m2 (p < 0.001), while htTKV was increased from 599 (423–707) to 617 (446–827) mL/m (p = 0.002) (n = 20). The annual increase in htTKV rate was significantly promoted, and urinary phosphate change was found to be correlated with the change in htTKV (rs = 0.575, p = 0.020). In the examined patients, eGFR was decreased and htTKV increased during short-term administration of dapagliflozin. To confirm the possibility of the effects of dapagliflozin on ADPKD, additional interventional studies are required.
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive development and enlargement of kidney cysts, the fourth greatest cause worldwide of kidney failure in patients who have undergone kidney replacement therapy [1,2]. Furthermore, several previous clinical studies have revealed that fasting plasma glucose levels [3], obesity [4], and hypertension [5,6] are risk factors for kidney enlargement and function deterioration in ADPKD patients. In vivo results have also demonstrated that a ketogenic diet and oral administration of the ketone β-hydroxybutyrate inhibit kidney enlargement [7].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors were originally introduced as a novel class of antidiabetic drugs, while several recent clinical trials have shown that they have heart and kidney protective effects in chronic kidney disease (CKD) patients both with and without diabetes [8,9,10]. In the Dapagliflozin in Patients with Chronic Kidney Disease (DAPA-CKD) trial, dapagliflozin was administered to CKD patients with an estimated glomerular filtration rate (eGFR) of 25 to 75 mL/min/1.73 m2 and a urinary albumin-to-creatinine ratio of 200 to 5000 mg/gCr [8]. Additionally, Empagliflozin in the Patients with Chronic Kidney Disease (EMPA-Kidney) trial, empagliflozin was given to those with an eGFR of 20 to 45 mL/min/1.73 m2, or an eGFR of at least 45 to 90 mL mL/min/1.73 m2, and a urinary albumin-to-creatinine ratio of at least 200 mg/gCr [10]. Results of the DAPA-CKD trial showed a greater reduction in eGFR in the dapagliflozin group as compared to the placebo group (−3.97 ± 0.15 vs. −0.82 ± 0.15 mL/min/1.73 m2) during the first two weeks, namely an initial dip [8], while an initial dip was also recognized in the EMPA-Kidney trial [10]. Thus, it is considered that a favorable and reversible hemodynamic change in the glomerulus is possible with the use of an SGLT2 inhibitor. In the DAPA-CKD trial, a smaller annual change in mean eGFR was observed in the dapagliflozin group as compared with the placebo group (−1.67 ± 0.11 and −3.59 ± 0.11 mL/min/1.73 m2, respectively) at two weeks after initiation of treatment. Additionally, the eGFR slope from baseline to 30 months in the dapagliflozin and placebo groups was −2.86 ± 0.11 and −3.79 ± 0.11 mL/min/1.73 m2 per year, respectively [8]. Results indicating a decline in eGFR from two months to the final follow-up examination in the EMPA-Kidney trial showed a between-group difference of 1.37 mL/min/1.73 m2 (95% CI, 1.16 to 1.59) per year [10]. In a pre-specified analysis of DAPA-CKD trial subjects, effects of dapagliflozin on eGFR were observed not only in participants with type 2 diabetes but also in those affected by glomerulonephritis, including IgA nephropathy [11]. Thus, SGLT2 inhibitors are now widely prescribed to CKD patients with various conditions to slow kidney function decline. Additionally, SGLT2 inhibitors have been reported to reduce body weight and blood pressure, risk factors for the progression of ADPKD, in non-diabetic patients with CKD [10] and also in those with heart failure [12], while in vivo results have shown increased endogenous ketone body levels [13]. Thus, it is considered possible that SGLT2 inhibitors have benefits for patients with ADPKD in terms of kidney protection.
Cardiovascular disease (CVD) is the major cause of mortality in ADPKD cases and contributes to a significant disease burden [14,15,16,17]. Patients with ADPKD experience CVD events with greater severity and have an increased risk of CVD-related death as compared to the general population, with 33% of those mortalities mainly due to ischemic heart disease or congestive heart failure [18]. Furthermore, results from the DAPA-CKD and EMPA-Kidney trials demonstrated the clinical benefits of SGLT2 inhibitors for improving CVD outcomes in CKD patients [8,10]. Thus, SGLT2 inhibitors may have benefits for patients with ADPKD in terms of preventing CVD events.
Tolvaptan, a vasopressin V2 receptor (V2R) antagonist, is recognized as a disease-specific treatment option for ADPKD [19]. It is also known that its administration can lead to a rapid decline in eGFR induced by hemodynamic change in the glomerulus [20]. Thus, cotreatment with an SGLT2 inhibitor, and tolvaptan may have increased diuretic effects and cause glomerular hemodynamic changes and subsequent acute kidney injury (AKI). Previously presented metanalysis findings [21] and also those of a pre-specified analysis of DAPA-CKD trial subjects [22] revealed that SGLT 2 inhibitors, including dapagliflozin, reduced the risk of an abrupt decline in kidney function in CKD patients. However, the interactions between SGLT2 inhibitors and tolvaptan have not been well investigated.
Several studies have used murine models of polycystic kidney disease (PKD) to evaluate the effects of SGLT2 inhibition. Five weeks of treatment with phlorizin, which has inhibitory effects on both sodium-glucose cotransporter-1 (SGLT1) and SLGT2, inhibited cyst growth in Han:SPRD rats [23]. Furthermore, dapagliflozin, a selective SGLT2 inhibitor, improved kidney function and albuminuria in Han:SPRD rats, though it failed to slow cyst growth [24]. Surprisingly, another study found that dapagliflozin administration led to increased cyst volume in PCK rats [25]. Thus, results from the studies of SGLT2 inhibitors using animal models of PKD have provided conflicting results.
To date, there is insufficient evidence regarding the use of these drugs in patients with ADPKD, as those were excluded from several clinical trials conducted to explore the kidney protection provided by SGLT2 inhibitors [8,10]. Currently, dapagliflozin is approved for patients with CKD in Japan. However, whether it is beneficial or harmful for ADPKD patients in terms of kidney function preservation is unclear. Hence, the present short-term retrospective observational study of patients with ADPKD was conducted to investigate the effects of dapagliflozin on changes in eGFR and height-adjusted kidney volume (htTKV).
2. Materials and Methods
2.1. Ethics Statement
This study was performed according to ethical guidelines for medical and health research involving human subjects provided by the Japanese Ministry of Health, Labor, and Welfare, as well as the Declaration of Helsinki. The protocol of this investigation was approved by the Ethics Committee of Osaka Metropolitan University (No. 2022-081).
2.2. Patients
A flow chart of the study participants is presented in Figure 1. A total of 102 ADPKD patients who were regularly examined by nephrologists at Osaka Metropolitan University Hospital between October 2021 and August 2022 were initially considered subjects. ADPKD was diagnosed using previously described criteria [26]. Those undergoing hemodialysis (n = 3), with prior kidney transplantation (n = 1), pregnant (n = 1), under 18 years of age (n = 1), or who were participants in other clinical trials (n = 7) were excluded. Furthermore, ADPKD patients with an eGFR <25 or >75 mL/min/1.73 m2 were generally excluded, according to criteria noted in a previous study [10], though one whose eGFR was 24.6 mL/min/1.73 m2 strongly requested dapagliflozin treatment, thus being included. Thirty-three of the remaining 56 patients declined to receive dapagliflozin treatment after an explanation regarding a lack of specific data showing effects for ADPKD and also potential side effects, including ketoacidosis, volume depletion, urosepsis, and pyelonephritis. Twenty-three patients were selected to receive dapagliflozin, administered at 10 mg/day. Of those, 20 who underwent computed tomography (CT) scanning between October 2021 and August 2022, at the start of dapagliflozin administration, were enrolled.
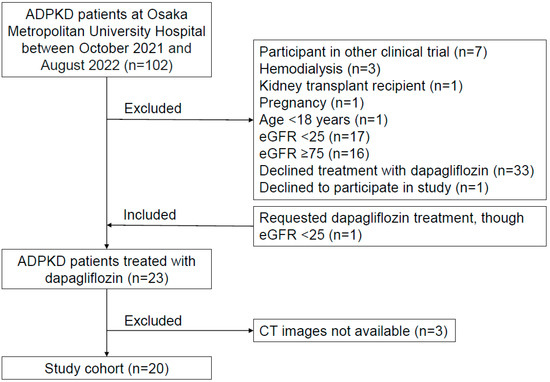
Figure 1.
Flow chart of the study. A total of 20 patients who received dapagliflozin treatment and had CT images available were enrolled.
2.3. Physical and Laboratory Measurements and Other Clinical Information
All blood and urine samples were collected in the morning after overnight fasting on the same day as the CT examinations. Laboratory measurements were performed using routine assays with an automated method [27]. Hemoglobin A1c was assessed using the National Glycohemoglobin Standardization Program (NGSP) equivalent value according to the guidelines of the Japan Diabetes Society [28]. The diagnosis of type 2 diabetes mellitus was based on medical records and criteria for diabetes mellitus defined in the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [29]. Kidney function was assessed by eGFR using a formula for Japanese individuals [30]. When serum albumin was <4.0 g/dL, then the following formula was used: corrected calcium (mg/dL) = measured Ca (mg/dL) + 4 − measured albumin (g/dL) [31]. Urinary protein and phosphate levels were normalized to those of creatinine (Cr), and then expressed as g/gCr, as previously described [32].
Age, gender, height, weight, family history, complications of ADPKD (liver cysts, intracranial aneurysms), and tolvaptan administration details were collected by a review of their medical records.
2.4. Computed Tomography Imaging for Total Kidney Volume (TKV)
CT imaging was used for kidney examinations in the enrolled patients. Total kidney volume (TKV) was estimated based on linear dimensions using an ellipsoid formula, as follows: length × width × thickness × π/6 [33,34,35]. Annual changes were calculated as shown below. At our department, an abdominal CT examination is generally performed for ADPKD patients every two to three years. Furthermore, that is also performed for such patients within two to four months after starting the administration of a new drug to determine its efficacy and safety.
2.4.1. Pre-Dapagliflozin Treatment
Annual change in TKV rate (%/year) = [(TKV at most recent examination before starting dapagliflozin) − (TKV at second most recent examination before starting dapagliflozin)]/[(TKV at second recent before starting dapagliflozin)/(days between CT examinations/365 days)] × 100.
2.4.2. Post-Dapagliflozin Treatment
Annual change in TKV rate (%/year) = [(TKV after dapagliflozin administration) − (TKV at start of administration of dapagliflozin)]/[(TKV at start of dapagliflozin administration)/(days between CT examinations/365 days)] × 100.
2.5. Statistical Analysis
Continuous variables are expressed as medians (interquartile ranges) and categorical variables as numbers (percentages). A paired Student’s t-test or Wilcoxon signed-rank test was used for comparisons of clinical parameters of ADPKD patients who received dapagliflozin between pre-administration and follow-up examinations. A correlation analysis was conducted to examine the relationships between annual changes in htTKV rate and changes in clinical parameters during dapagliflozin treatment. Statistical analyses were performed using the JMP software package, version 10 (SAS Institute, Inc., Cary, NC, USA). p values < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Clinical Characteristics of ADPKD Patients
The baseline clinical characteristics of the present ADPKD patients are shown in Table 1. Nine (45%) were male, and the median age at the initiation of dapagliflozin was 51 (46–57) years. Only two (10%) patients did not have a family history of ADPKD. Body mass index was 23.0 (20.9–24.8) kg/m2, with one (5%) affected by diabetes. eGFR, serum phosphate, and calcium levels were 47.9 (39.7–56.9) mL/min/1.73 m2, 3.5 (3.3–3.7) mg/dL, and 9.4 (9.2–9.6) mg/dL, respectively. The numbers of patients receiving tolvaptan, renin-angiotensin-aldosterone system inhibitor, calcium channel blocker, beta blocker, anti-diabetic agent, or phosphorus binder treatments at the time of the baseline examination were 11 (55%), 11 (55%), 12 (60%), 4 (20%), 1 (5%), and 1 (5%), respectively. During the dapagliflozin administration period, none made a change in regard to those other medications.

Table 1.
Clinical characteristics of study participants.
3.2. Changes in Clinical Parameters after Dapagliflozin Treatment
Changes in clinical parameters after administration of dapagliflozin are presented in Table 2. During a total period of 102 ± 20 days (range 70–156 days), eGFR decreased from 47.9 (39.7–56.9) mL/min/1.73 m2 at the baseline to 40.8 (33.7–44.5) mL/min/1.73 m2 at the end of the observation period (p < 0.001). Changes in eGFR in all patients are shown in Figure 2 and Table 3. Furthermore, hemoglobin and serum creatinine levels were significantly increased [13.1 (12.2–14.4) to 14.0 (13.1–15.1) g/L (p <0.001) and 1.13 (0.93–1.34) to 1.37 (1.05–1.55) mg/dL (p < 0.001)], respectively (Table 2). Blood pressure, serum albumin, calcium, phosphate, urinary protein, and urinary phosphate levels were not significantly different between the baseline and final follow-up examinations.

Table 2.
Changes in characteristics of study participants after treatment with dapagliflozin.
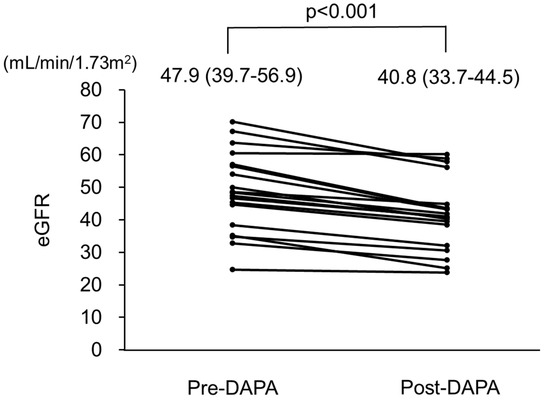
Figure 2.
Changes in estimated glomerular filtration rate (eGFR) in patients with autosomal dominant polycystic kidney disease (ADPKD) treated with dapagliflozin. eGFR was significantly decreased after treatment with dapagliflozin. Pre-DAPA: pre-dapagliflozin treatment; Post-DAPA: post-dapagliflozin treatment.

Table 3.
Changes in height-adjusted total kidney volume (htTKV) and estimated glomerular filtration rate (eGFR) of individual study participants after treatment with dapagliflozin.
3.3. Changes in eGFR before and after Dapagliflozin Treatment
In consideration of the initial dip in eGFR after dapagliflozin administration, values obtained at the most recent evaluation before starting dapagliflozin, on the day of starting dapagliflozin administration, and one month after starting administration were examined (n = 14) (Figure 3). From the first day of dapagliflozin administration until the first visit one month later, eGFR was reduced from 48.5 to 45.2 mL/min/1.73 m2, which can be considered the initial dip. At the second visit after starting dapagliflozin administration, a significant decline was noted (p = 0.002). In contrast, no such significant decline was seen during the pre-dapagliflozin treatment period from the final evaluation before starting dapagliflozin until the first day of administration (p = 0.325).
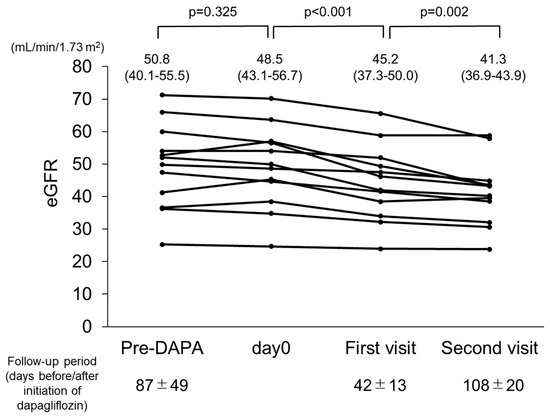
Figure 3.
Changes in estimated glomerular filtration rate (eGFR) in patients with autosomal dominant polycystic kidney disease (ADPKD) treated with dapagliflozin (n = 14). eGFR was significantly decreased during treatment with dapagliflozin.
3.4. Changes in htTKV before and after Dapagliflozin Treatment
Changes in htTKV in all patients are shown in Figure 4 and Table 3. During the observational period, htTKV significantly increased from 599 (423–707) to 617 (446–827) mL/m (p = 0.002), with findings of a representative case presented in Supplemental Figure S1.
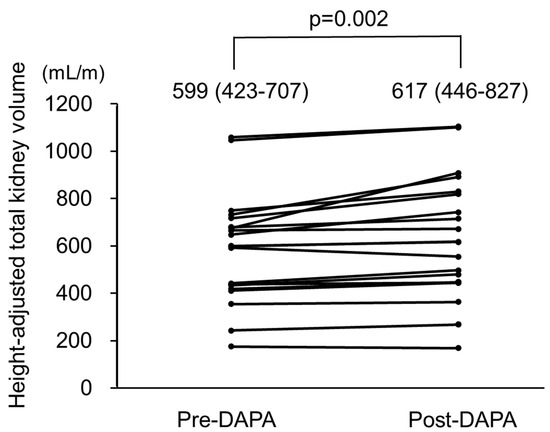
Figure 4.
Height-adjusted total kidney volume (htTKV) in autosomal dominant polycystic kidney disease (ADPKD) patients treated with dapagliflozin. htTKV was significantly increased after treatment with dapagliflozin. pre-DAPA: pre-dapagliflozin treatment; post-DAPA: post-dapagliflozin treatment.
3.5. Changes in eGFR and htTKV in ADPKD Patients with and without Tolvaptan Treatment
In ADPKD patients without tolvaptan treatment (n = 9), eGFR was decreased from 46.7 (44.2–60.6) mL/min/1.73 m2 at the baseline to 40.7 (39.0–49.7) mL/min/1.73 m2 at the end of the observation period (p = 0.004), while htTKV was significantly increased from 598 (387–678) to 619 (404–767) mL/m (p = 0.008). In those patients with tolvaptan treatment (n = 11), eGFR was decreased from 48.6 (34.8–57.0) mL/min/1.73 m2 at the baseline to 41.9 (30.6–44.8) mL/min/1.73 m2 at the end of the observation period (p = 0.001), and htTKV was significantly increased from 600 (434–750) to 616 (479–892) mL/m (p = 0.007).
3.6. Changes in Annual htTKV Rate before and after Dapagliflozin Treatment
The annual change in htTKV rate before dapagliflozin treatment was −1.4% (−11.9–5.0%) during a total period of 725 ± 569 days (range 91–1750 days), while that after the start of dapagliflozin treatment was 23.9% (10.2–44.7%) (Supplemental Figure S2). During the treatment period, the annual change in htTKV rate was significantly increased (p < 0.001).
3.7. Correlations between Changes in htTKV and Changes in Clinical Parameters during Dapagliflozin Treatment
Correlations between changes in htTKV and changes in various clinical parameters are shown in Table 4. While eGFR and serum phosphate levels were not significantly correlated with changes in htTKV, urinary phosphate level changes showed a significant positive correlation (rs = 0.575, p = 0.020) (Table 4, Supplemental Figure S3).

Table 4.
Correlation of change in height-adjusted total kidney volume (htTKV) with clinical factors in ADPKD patients treated with dapagliflozin.
4. Discussion
The present study was conducted to investigate whether dapagliflozin is beneficial or harmful for patients with ADPKD in regard to changes in eGFR and htTKV. In the enrolled participants, short-term administration of dapagliflozin was associated with decreased eGFR and increased htTKV. Considering our findings, prudent informed consent, including written consent, is necessary when administering dapagliflozin to ADPKD patients.
At two to four weeks after SGLT2 inhibitor administration, a modest dip in eGFR was observed in CKD patients with diabetes, and an acute dip was seen in those without diabetes [36]. In the DAPA-CKD trial, CKD patients, whose eGFR was 43.2 mL/min/1.73 m2, showed a dip in that ratio of approximately 10% [8]. In the present study, at one month after administration of dapagliflozin, eGFR was reduced from 48.5 to 45.2 mL/min/1.73 m2 in 14 of the ADPKD patients, similar to the reduction seen in that trial, which can be explained as an initial dip caused by dapagliflozin. In the DAPA-CKD trial, after the initial dip, eGFR decline became mild in those CKD patients, whereas eGFR continued to show a decrease in the present ADPKD patients up to approximately three months after administration of dapagliflozin. This discrepancy in rate of eGFR decline after the initial dip between that trial and the present study may have been due to increased cyst volume after dapagliflozin administration.
Impairment of glucose metabolism has been highlighted as a key feature and important disease modulator in ADPKD cases [37] and reported to be associated with cystogenesis in vitro [38,39] and in vivo [40,41]. Under normoglycemia, up to 97% of filtered glucose is reabsorbed via SGLT2 in early proximal tubules, while the remaining less than 3% is reabsorbed by SGLT1 in late proximal tubules [42]. In instances of SGLT2 knockout or inhibition, approximately 40% of glucose has been reported to be reabsorbed by SGLT1 [43,44]. It has also been reported that dual inhibition of SGLT1 and SGLT2 by phlorizin decreased cyst growth in Han:SPRD rats [23], whereas dapagliflozin was not associated with a decrease in cyst growth in those rats [24], suggesting that increased glucose reabsorption via SGLT1 by treatment with an SGLT2 inhibitor possibly increases cyst growth in late proximal tubules.
It has been speculated that increased intratubular osmotic pressure caused by increased glucose concentration in late proximal tubules was a factor that promoted cyst enlargement in PCK rats treated with dapagliflozin [25]. In addition, a recent study that used a human organoid-on-chip model of PKD demonstrated that fluid flow and solute concentrations can be positive regulators of cyst expansion [38]. In contrast, tolvaptan, a vasopressin V2-receptor antagonist used for disease-specific treatment worldwide, was found to significantly reduce urinary osmolality [20], while another study showed that a greater decrease in urinary osmolality in subjects treated with tolvaptan was associated with slower eGFR decline [45]. These findings indicated that urinary osmotic pressure increased by the use of SGLT2 inhibitors can promote cystogenesis in late proximal tubules.
Recently, findings presented by the Consortium for Radiologic Imaging Studies of PKD (CRISP) regarding fibroblast growth factor 23 (FGF23), a phosphaturic hormone [46], demonstrated its association with the rate of increase in htTKV and that of decline in GFR in APDKD patients [47]. Although the mechanism of FGF23 for acceleration of ADPKD progression was unclear in that study, we speculate that calcium-phosphate crystal depositions mediated by FGF23 lead to acceleration of ADPKD progression. Findings obtained in vivo with PCK rats showed deposits of calcium-phosphate crystals induced by a high-phosphate diet in tubule lumens along the corticomedullary junction, which led to increased cytogenesis and disease progression [48]. The present retrospective analysis of urinary phosphate excretion in ADPKD patients treated with dapagliflozin showed that greater changes in urinary phosphate levels were significantly correlated with disease progression, as reflected by the change in htTKV during dapagliflozin treatment. In addition, the findings suggest that monitoring urinary phosphate levels is important during dapagliflozin treatment for patients with ADPKD.
The present study has some limitations. First, the number of patients examined was relatively few, mainly due to the fact that all of the enrolled subjects were treated at a single institution and also because of the low prevalence of ADPKD patients. Second, this was a retrospective observational study, which possibly limits the ability to draw conclusions from the findings. Due to the limited number of participants and retrospective observational design, our findings only demonstrated an association, not direct causality, between dapagliflozin and the disease progression of ADPKD. To confirm the possibility of the effects of dapagliflozin on that progression, additional interventional studies are required. In addition, the observational period was short. Supplemental Table S1 shows eGFR changes after dapagliflozin initiation for relatively long terms. In patients with long-term administration, the decline in eGFR was mild. However, in 8 (40%), dapagliflozin administration was discontinued within six months of initiation due to a decline in eGFR and an increase in TKV. Moreover, TKV change was not evaluated for the entire cohort. Therefore, it is difficult to draw a definitive conclusion based on the present findings regarding the long-term effects of dapagliflozin on kidney function and TKV in ADPKD patients. A large-scale study with a long-term follow-up period would be needed to determine whether the association of dapagliflozin treatment with the progression of ADPKD continues for a long period of time. Third, the method used for measuring TKV in the present study was simple, though a more precise quantitative assessment technique has recently become available. The present measurements of TKV during dapagliflozin administration showed a close similarity to those obtained using the SYNAPSE VINCENT semi-automatic method, recognized to provide accurate results [49] (r = 0.963, p < 0.001) (Supplemental Figure S4). Unfortunately, CT images obtained during pre-dapagliflozin treatment could not be analyzed with a newer, more advanced system, as that was not available at our institution. Fourth, this was a single-arm case series study. The absence of a control group cannot distinguish between the effect of the treatment and the natural evaluation of the disease. The reason for the single-arm design of the study was that it was not possible to obtain an appropriate control group. Clinically, repeated CT examinations during a short period are conducted exclusively for ADPKD patients administered with novel treatments including SGLT2 inhibitors. Therefore, short-term TKV changes cannot be evaluated in ADPKD patients without novel treatment. Indeed, a single-arm design has been accepted for recent reports in the field of ADPKD [50,51]. However, it is important to compare our findings with the natural course of ADPKD. A study of disease progression of Japanese ADPKD patients using the Japanese Polycystic Kidney Disease registry (J-PKD) indicated an annual TKV increase of 4.78% and eGFR decline of 5.0% [3], with age, eGFR, and htTKV of the cases in the J-PKD 49 years, 56.7 mL/min/1.73 m2, and 825 mL/m, respectively, similar to those for the present patients. In addition, interim results obtained in post-marketing surveillance of tolvaptan in real-world clinical settings, namely SLOW-PKD surveillance, showed an annual TKV increase of 11.7% in rapidly progressive Japanese ADPKD patients whose age, eGFR, and htTKV were 49.7 years, 44.4 mL/min/1.73 m2, and 1301 mL/m, respectively [52]. Differences between the present results and the two prior studies of the natural course of ADPKD patients in Japan may support the notion of an accelerated TKV increase during administration of dapagliflozin over a short-term period. Fifth, the effects of SGLT2 inhibitors other than dapagliflozin were not evaluated. A previous study noted that among SGLT2 inhibitors, including ipragliflozin, dapagliflozin, tofogliflozin, canagliflozin, empagliflozin, and luseogliflozin, patients treated with dapagliflozin and ipragliflozin exhibited increased urinary glucose excretion up to 18 h after administration, while that period in patients treated with the others was approximately 12 h [53]. While dapagliflozin is selective for SGLT2, canagliflozin may also have a capacity for SGLT1 inhibition [54]. In consideration of the various pharmacokinetic effects as well as the effect on glucose metabolism of renal cysts of SGLT2 inhibitors, disease progression in ADPKD patients may be more apparent in those treated with dapagliflozin as compared to the others. Sixth, CKD and mineral and bone disorder (CKD-MBD)-related markers were not evaluated in the present study, though they have been reported to be changed after administration of dapagliflozin in both healthy volunteers [55] and type 2 diabetes patients [56]. Serum phosphate, FGF-23, and parathyroid hormone (PTH) levels have been shown to significantly increase, and 1,25-dihydroxyvitamin D levels have decreased with dapagliflozin treatment in type 2 diabetes patients with CKD stages ranging from 2 to 4 [56]. However, those CKD-MBD-related marker levels could not be measured because serum samples were not stored and thus not available. Seventh, the effects of dapagliflozin on heart involvement, including improvement of diastolic dysfunction, could not be investigated since heart ultrasound examinations were not performed during the observation period. SGLT2 inhibitors have been reported to suppress CVD events in CKD patients and improve diastolic dysfunction in patients with type 2 diabetes and those with heart failure [57]. Clinical studies that investigate the effects of SGLT2 inhibitors on heart involvement in ADPKD patients are needed. Eighth, the side effects of SGLT2 inhibitor administration, including infections, could not be appropriately evaluated during the observation period. Although no infection events were noted, a previously published systematic review found that administration of dapagliflozin at 10 mg/day was associated with a significantly increased risk of urinary tract and genital tract infections [58]. Since an ascending urinary tract infection with effects on kidney cysts may have potentially severe consequences for ADPKD patients [59], the detrimental consequences of possible complications, including infections associated with SGLT2 inhibitors given to ADPKD patients, should be carefully evaluated in future studies. Finally, some data, including urinary osmolality, urinary phosphate, urine output, and body weight, were lacking for some of the participants, while changes in food intake such as phosphate load were not evaluated.
5. Conclusions
In conclusion, short-term dapagliflozin treatment may be associated with a decrease in eGFR and an increase in htTKV in ADPKD patients. SGLT2 inhibitors may have pleiotropic effects and are considered to be beneficial for patients with ADPKD. To confirm the possibility of the effects of dapagliflozin on ADPKD, additional interventional studies are required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12196341/s1, Figure S1. Abdominal computed tomography images of representative case; Figure S2. Changes in annual height-adjusted total kidney volume (htTKV) rate in autosomal dominant polycystic kidney disease (ADPKD) patients treated with dapagliflozin; Figure S3. Correlation between change in height-adjusted total kidney volume (htTKV) during dapagliflozin treatment and urinary phosphate in autosomal dominant polycystic kidney disease (ADPKD) patients during dapagliflozin treatment; Figure S4. Correlations between total kidney volume (TKV) calculated using ellipsoid formula and SYNAPSE VINCENT in autosomal dominant polycystic kidney disease (ADPKD) patients during dapagliflozin treatment; Table S1. Long-term changes in estimated glomerular filtration rate (eGFR) in autosomal dominant polycystic kidney disease (ADPKD) patients from pre- to post-dapagliflozin treatment.
Author Contributions
F.M. contributed to data acquisition, analysis, and writing the manuscript. S.N. contributed to study concept and design, data acquisition, patient recruitment, interpretation of results, and writing the manuscript. H.U. and A.T. contributed to patient recruitment and reviewing the manuscript. K.M. and M.E. contributed to the study concept and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the latest version of the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies by the Ministry of Health, Labor, and Welfare, Japan. The protocol of this study was reviewed and approved by the ethics committee of Osaka Metropolitan University (Approval No. 2022-081).
Informed Consent Statement
Informed consent was obtained by using an opt-out method. Written informed consent was waived due to the observational nature of this study.
Data Availability Statement
Data obtained for this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Eiji Ishimura, Director of Meijibashi Hospital, for assistance with patient recruitment and reviewing the manuscript. We also thank Kozo Nishide of Kaizuka Nishide Clinic for assistance with measuring TKV.
Conflicts of Interest
S.N., K.M., and M.E. have received honorariums for lecturing from Ono Pharma Inc. and Astra Zeneca PLC. F.M., H.U., and A.T. have no conflicts of interest to declare in regard to this case report.
References
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2008, 359, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Mochizuki, T.; Shimada, Y.; Nishio, S.; Kataoka, H.; Mitobe, M.; Tsuchiya, K.; Hanaoka, K.; Ubara, Y.; Suwabe, T.; et al. Factors predicting decline in renal function and kidney volume growth in autosomal dominant polycystic kidney disease: A prospective cohort study (Japanese Polycystic Kidney Disease registry: J-PKD). Clin. Exp. Nephrol. 2021, 25, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; You, Z.; Gitomer, B.; Brosnahan, G.; Torres, V.E.; Chapman, A.B.; Perrone, R.D.; Steinman, T.I.; Abebe, K.Z.; Rahbari-Oskoui, F.F.; et al. Overweight and Obesity Are Predictors of Progression in Early Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Gabow, P.A.; Johnson, A.M.; Kaehny, W.D.; Kimberling, W.J.; Lezotte, D.C.; Duley, I.T.; Jones, R.H. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992, 41, 1311–1319. [Google Scholar] [CrossRef]
- Cornec-Le Gall, E.; Audrezet, M.P.; Rousseau, A.; Hourmant, M.; Renaudineau, E.; Charasse, C.; Morin, M.P.; Moal, M.C.; Dantal, J.; Wehbe, B.; et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.A.; Kruger, S.L.; Broderick, C.; Amarlkhagva, T.; Agrawal, S.; Dodam, J.R.; Mrug, M.; Lyons, L.A.; Weimbs, T. Ketosis Ameliorates Renal Cyst Growth in Polycystic Kidney Disease. Cell Metab. 2019, 30, 1007–1023.e1005. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Kohn, O.F. In CKD, the effect of dapagliflozin on kidney outcomes did not vary by T2DM status or CKD cause. Ann. Intern. Med. 2021, 174, JC53. [Google Scholar] [CrossRef]
- The, E.-K.C.G.; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefansson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlavek, J.; Bohm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients with Heart Failure with and without Diabetes. JAMA 2020, 323, 1353–1368. [Google Scholar] [CrossRef]
- Tomita, I.; Kume, S.; Sugahara, S.; Osawa, N.; Yamahara, K.; Yasuda-Yamahara, M.; Takeda, N.; Chin-Kanasaki, M.; Kaneko, T.; Mayoux, E.; et al. SGLT2 Inhibition Mediates Protection from Diabetic Kidney Disease by Promoting Ketone Body-Induced mTORC1 Inhibition. Cell Metab. 2020, 32, 404–419.e406. [Google Scholar] [CrossRef]
- Gigante, A.; Perrotta, A.M.; Tinti, F.; Assanto, E.; Muscaritoli, M.; Lai, S.; Cianci, R. Assessment of cardiovascular disease in Autosomal dominant polycystic kidney disease. Appl. Sci. 2023, 13, 7175. [Google Scholar] [CrossRef]
- Luciano, R.L.; Dahl, N.K. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): Considerations for routine screening and management. Nephrol. Dial. Transplant. 2013, 29, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gorriz, J.L.; Arroyo, D.; D’Marco, L.; Torra, R.; Tomás, P.; Puchades, M.J.; Panizo, N.; Pantoja, J.; Montomoli, M.; Llisterri, J.L.; et al. Cardiovascular risk factors and the impact on prognosis in patients with chronic kidney disease secondary to autosomal dominant polycystic kidney disease. BMC Nephrol. 2021, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- González Martínez, M.Á.; Hernández García, E.; Morales García, A.I. Autosomal dominant polycystic kidney disease: Cardiovascular risk factor. Med. Clin. 2023, 161, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.D.; Ruthazer, R.; Terrin, N.C. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: Contribution of extrarenal complications to mortality. Am. J. Kidney Dis. 2001, 38, 777–784. [Google Scholar] [CrossRef]
- Muller, R.U.; Messchendorp, A.L.; Birn, H.; Capasso, G.; Cornec-Le Gall, E.; Devuyst, O.; van Eerde, A.; Guirchoun, P.; Harris, T.; Hoorn, E.J.; et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: Consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol. Dial. Transplant. 2022, 37, 825–839. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Menne, J.; Dumann, E.; Haller, H.; Schmidt, B.M.W. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002983. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Cherney, D.; Postmus, D.; Stefansson, B.V.; Chertow, G.M.; Dwyer, J.P.; Greene, T.; Kosiborod, M.; Langkilde, A.M.; McMurray, J.J.V.; et al. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022, 101, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Liu, Y.; Spichtig, D.; Kapoor, S.; Koepsell, H.; Mohebbi, N.; Segerer, S.; Serra, A.L.; Rodriguez, D.; et al. Targeting of sodium-glucose cotransporters with phlorizin inhibits polycystic kidney disease progression in Han:SPRD rats. Kidney Int. 2013, 84, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Kapoor, S.; Edenhofer, I.; Segerer, S.; Riwanto, M.; Kipar, A.; Yang, M.; Mei, C.; Wuthrich, R.P. Inhibition of Sodium-Glucose Cotransporter 2 with Dapagliflozin in Han: SPRD Rats with Polycystic Kidney Disease. Kidney Blood Press. Res. 2015, 40, 638–647. [Google Scholar] [CrossRef]
- Kapoor, S.; Rodriguez, D.; Riwanto, M.; Edenhofer, I.; Segerer, S.; Mitchell, K.; Wuthrich, R.P. Effect of Sodium-Glucose Cotransport Inhibition on Polycystic Kidney Disease Progression in PCK Rats. PLoS ONE 2015, 10, e0125603. [Google Scholar] [CrossRef]
- Nishio, S.; Tsuchiya, K.; Nakatani, S.; Muto, S.; Mochizuki, T.; Kawano, H.; Hanaoka, K.; Hidaka, S.; Ichikawa, D.; Ishikawa, E.; et al. A digest from evidence-based Clinical Practice Guideline for Polycystic Kidney Disease 2020. Clin. Exp. Nephrol. 2021, 25, 1292–1302. [Google Scholar] [CrossRef]
- Nakatani, S.; Nakatani, A.; Tsugawa, N.; Yamada, S.; Mori, K.; Imanishi, Y.; Ishimura, E.; Okano, T.; Inaba, M. Fibroblast Growth Factor-23 and Vitamin D Metabolism in Subjects with eGFR >/= 60 mL/min/1.73 m(2). Nephron 2015, 130, 119–126. [Google Scholar] [CrossRef]
- Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus; Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef]
- Expert Committee on the, D.; Classification of Diabetes, M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5–S20. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Guideline Working Group, J.S.f.D.T. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther. Apher. Dial. 2008, 12, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Al Ghali, R.; El-Mallah, C.; Obeid, O.; El-Saleh, O.; Smail, L.; Haroun, D. Urinary minerals excretion among primary schoolchildren in Dubai-United Arab Emirates. PLoS ONE 2021, 16, e0255195. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Britz-Cunningham, S.; Kumamaru, K.K.; Malek, S.K.; Tullius, S.G.; Rybicki, F.J. Comprehensive comparative study of computed tomography-based estimates of split renal function for potential renal donors: Modified ellipsoid method and other CT-based methods. J. Comput. Assist. Tomogr. 2012, 36, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.B.; Wei, W. Imaging approaches to patients with polycystic kidney disease. Semin. Nephrol. 2011, 31, 237–244. [Google Scholar] [CrossRef]
- Geraghty, E.M.; Boone, J.M.; McGahan, J.P.; Jain, K. Normal organ volume assessment from abdominal CT. Abdom. Imaging 2004, 29, 482–490. [Google Scholar] [CrossRef]
- Shibata, R.; Taguchi, K.; Kaida, Y.; Fukami, K. Effect of dapagliflozin on the initial estimated glomerular filtration rate dip in chronic kidney disease patients without diabetes mellitus. Clin. Exp. Nephrol. 2023, 27, 44–53. [Google Scholar] [CrossRef]
- Dachy, A.; Decuypere, J.P.; Vennekens, R.; Jouret, F.; Mekahli, D. Is autosomal dominant polycystic kidney disease an early sweet disease? Pediatr. Nephrol. 2022, 37, 1945–1955. [Google Scholar] [CrossRef]
- Li, S.R.; Gulieva, R.E.; Helms, L.; Cruz, N.M.; Vincent, T.; Fu, H.; Himmelfarb, J.; Freedman, B.S. Glucose absorption drives cystogenesis in a human organoid-on-chip model of polycystic kidney disease. Nat. Commun. 2022, 13, 7918. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Riwanto, M.; Kapoor, S.; Rodriguez, D.; Edenhofer, I.; Segerer, S.; Wuthrich, R.P. Inhibition of Aerobic Glycolysis Attenuates Disease Progression in Polycystic Kidney Disease. PLoS ONE 2016, 11, e0146654. [Google Scholar] [CrossRef]
- Chiaravalli, M.; Rowe, I.; Mannella, V.; Quilici, G.; Canu, T.; Bianchi, V.; Gurgone, A.; Antunes, S.; D’Adamo, P.; Esposito, A.; et al. 2-Deoxy-d-Glucose Ameliorates PKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. 2020, 472, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Renal Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J. Am. Soc. Nephrol. 2011, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Chapman, A.B.; Gansevoort, R.T.; Higashihara, E.; Perrone, R.D.; Torres, V.E.; Blais, J.D.; Zhou, W.; Ouyang, J.; Czerwiec, F.S. Urine Osmolality, Response to Tolvaptan, and Outcome in Autosomal Dominant Polycystic Kidney Disease: Results from the TEMPO 3:4 Trial. J. Am. Soc. Nephrol. 2017, 28, 1592–1602. [Google Scholar] [CrossRef]
- Yamashita, T.; Konishi, M.; Miyake, A.; Inui, K.; Itoh, N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 2002, 277, 28265–28270. [Google Scholar] [CrossRef]
- El Ters, M.; Lu, P.; Mahnken, J.D.; Stubbs, J.R.; Zhang, S.; Wallace, D.P.; Grantham, J.J.; Chapman, A.B.; Torres, V.E.; Harris, P.C.; et al. Prognostic Value of Fibroblast Growth Factor 23 in Autosomal Dominant Polycystic Kidney Disease. Kidney Int. Rep. 2021, 6, 953–961. [Google Scholar] [CrossRef]
- Torres, J.A.; Rezaei, M.; Broderick, C.; Lin, L.; Wang, X.; Hoppe, B.; Cowley, B.D., Jr.; Savica, V.; Torres, V.E.; Khan, S.; et al. Crystal deposition triggers tubule dilation that accelerates cystogenesis in polycystic kidney disease. J. Clin. Investig. 2019, 129, 4506–4522. [Google Scholar] [CrossRef]
- Muto, S.; Kawano, H.; Isotani, S.; Ide, H.; Horie, S. Novel semi-automated kidney volume measurements in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 2018, 22, 583–590. [Google Scholar] [CrossRef]
- Sorohan, B.M.; Ismail, G.; Andronesi, A.; Micu, G.; Obrișcă, B.; Jurubiță, R.; Sinescu, L.; Baston, C. A single-arm study of metformin in patients with autosomal dominant polycystic kidney disease. BMC Nephrol. 2019, 20, 276. [Google Scholar] [CrossRef]
- Strubl, S.; Oehm, S.; Torres, J.A.; Grundmann, F.; Haratani, J.; Decker, M.; Vuong, S.; Bhandal, A.K.; Methot, N.; Haynie-Cion, R.; et al. Ketogenic dietary interventions in autosomal dominant polycystic kidney disease-a retrospective case series study: First insights into feasibility, safety and effects. Clin. Kidney J. 2021, 15, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Muto, S.; Miyake, M.; Tanaka, T.; Wang, W. Safety and efficacy of Tolvaptan in real-world patients with autosomal dominant polycystic kidney disease- interim results of SLOW-PKD surveillance. Clin. Exp. Nephrol. 2021, 25, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Takasu, T.; Yokono, M.; Imamura, M.; Kurosaki, E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors: Part 2. Antidiabetic effects in type 2 diabetic mice. J. Pharmacol. Sci. 2016, 131, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.; Yakovleva, T.; Chu, L.; Tang, W.; Greasley, P.J.; Johansson, S.; Peskov, K.; Helmlinger, G.; Boulton, D.W.; Penland, R.C. Differentiating the Sodium-Glucose Cotransporter 1 Inhibition Capacity of Canagliflozin vs. Dapagliflozin and Empagliflozin Using Quantitative Systems Pharmacology Modeling. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 222–229. [Google Scholar] [CrossRef]
- Blau, J.E.; Bauman, V.; Conway, E.M.; Piaggi, P.; Walter, M.F.; Wright, E.C.; Bernstein, S.; Courville, A.B.; Collins, M.T.; Rother, K.I.; et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 2018, 3, e99123. [Google Scholar] [CrossRef]
- de Jong, M.A.; Petrykiv, S.I.; Laverman, G.D.; van Herwaarden, A.E.; de Zeeuw, D.; Bakker, S.J.L.; Heerspink, H.J.L.; de Borst, M.H. Effects of Dapagliflozin on Circulating Markers of Phosphate Homeostasis. Clin. J. Am. Soc. Nephrol. 2019, 14, 66–73. [Google Scholar] [CrossRef]
- Sakai, T.; Miura, S. Effects of Sodium-Glucose Cotransporter 2 Inhibitor on Vascular Endothelial and Diastolic Function in Heart Failure with Preserved Ejection Fraction―Novel Prospective Cohort Study. Circ. Rep. 2019, 1, 286–295. [Google Scholar] [CrossRef]
- Puckrin, R.; Saltiel, M.P.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Filion, K.B. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 503–514. [Google Scholar] [CrossRef]
- Idrizi, A.; Barbullushi, M.; Koroshi, A.; Dibra, M.; Bolleku, E.; Bajrami, V.; Xhaferri, X.; Thereska, N. Urinary tract infections in polycystic kidney disease. Med. Arh. 2011, 65, 213–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).