Predictive Factors Associated with Successful Response to Percutaneous Adhesiolysis in Chronic Lumbar Radicular Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Interventions
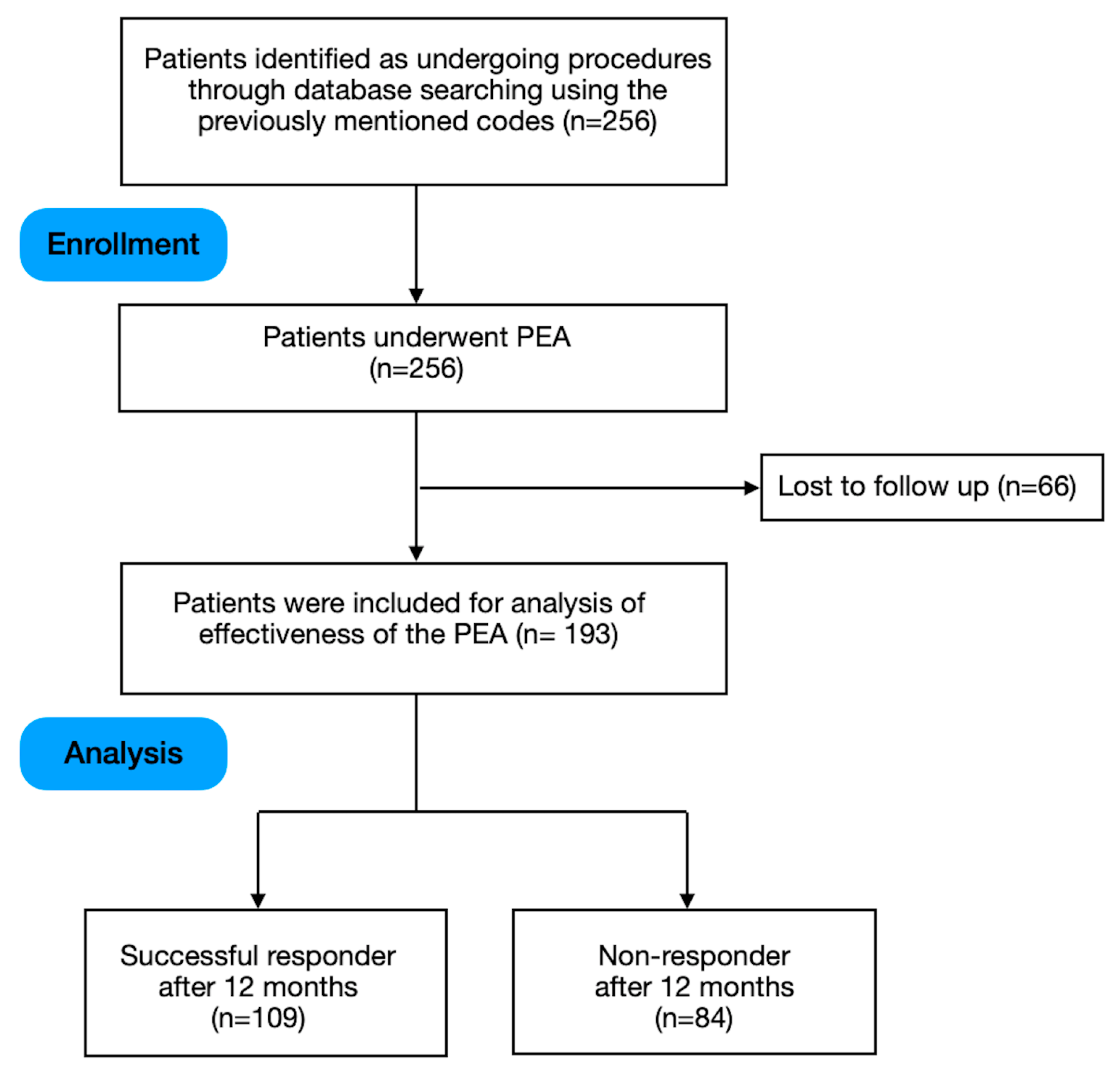
2.3. Outcome Data and Follow-Up Period
2.4. Statistical Analysis
3. Results
3.1. Univariate Logistic Regression Analysis
3.2. Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Traeger, A.; Buchbinder, R.; Harris, I.; Maher, C. Diagnosis and management of low-back pain in primary care. Can. Med Assoc. J. 2017, 189, E1386–E1395. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.S.; Hu, H.T.; Lin, R.M.; Huang, K.Y.; Lin, P.C.; Zhong, Z.C.; Hseih, M.L. Biomechanical analysis of the lumbar spine on facet joint force and intradiscal pressure—A finite element study. BMC Musculoskelet. Disord. 2010, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.; Candido, K.; Vlaeyen, J.; Van Zundert, J.; Cohen, S. Low Back Pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.A.; Elia, C.; Saini, H.S.; Miulli, D.E. A Review of Lumbar Radiculopathy, Diagnosis, and Treatment. Cureus 2019, 11, e5934. [Google Scholar] [CrossRef]
- North, R.B.; Levy, R.M. Chronic Low Back Pain and Failed Back Surgery Syndrome. In Neurosurgical Management of Pain; North, R.B., Levy, R.M., Eds.; Springer: New York, NY, USA, 1997. [Google Scholar]
- Lall, M.; Restrepo, E. The Biopsychosocial Model of Low Back Pain and Patient-Centered Outcomes Following Lumbar Fusion. Orthop. Nurs. 2017, 36, 213–221. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.; McLean, R.; Forciea, M. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Hussain, A.; Erdek, M. Interventional Pain Management for Failed Back Surgery Syndrome. Pain Pr. 2014, 14, 64–78. [Google Scholar] [CrossRef]
- Inoue, S.; Kamiya, M.; Nishihara, M.; Arai, Y.; Ikemoto, T.; Ushida, T. Prevalence, characteristics, and burden of failed back surgery syndrome: The influence of various residual symptoms on patient satisfaction and quality of life as assessed by a nationwide Internet survey in Japan. J. Pain Res. 2017, 10, 811–823. [Google Scholar] [CrossRef]
- Kobayashi, S.; Baba, H.; Uchida, K.; Kokubo, Y.; Kubota, C.; Yamada, S.; Suzuki, Y.; Yoshizawa, H. Effect of Mechanical Compression on the Lumbar Nerve Root: Localization and Changes of Intraradicular Inflammatory Cytokines, Nitric Oxide, and Cyclooxygenase. Spine 2005, 30, 1699–1705. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Kloth, D.; Slipman, C.W.; Jasper, J.F.; Trescot, A.M.; Varley, K.G.; Atluri, S.L.; Giron, C.; Curran, M.J.; et al. Interventional techniques in the management of chronic pain: Part 2.0. Pain Physician 2001, 4, 24–96. [Google Scholar] [CrossRef]
- Park, Y.; Lee, W.; Ahn, J.; Nam, H.; Lee, K. Percutaneous Adhesiolysis Versus Transforaminal Epidural Steroid Injection for the Treatment of Chronic Radicular Pain Caused by Lumbar Foraminal Spinal Stenosis: A Retrospective Comparative Study. Ann. Rehabil. Med. 2015, 39, 941–949. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Lee, S. Clinical Effectiveness of Percutaneous Adhesiolysis and Predictive Factors of Treatment Efficacy in Patients with Lumbosacral Spinal Stenosis. Pain Med. 2013, 14, 1497–1504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manchikanti, L.; Singh, V.; Bakhit, C.; Fellows, B. Interventional techniques in the management of chronic pain: Part 1.0. Pain Physician 2000, 3, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.; Jamison, D.; Hurley, R.; Cohen, S. Epidural Lysis of Adhesions. Korean J. Pain 2014, 27, 3–15. [Google Scholar] [CrossRef]
- Jamison, D.; Hsu, E.; Cohen, S. Epidural adhesiolysis: An evidence-based review. J. Neurosurg. Sci. 2014, 58, 65–76. [Google Scholar]
- Brito-García, N.; García-Pérez, L.; Kovacs, F.M.; del Pino-Sedeño, T.; Pérez-Ramos, J.; Imaz-Iglesia, I.; Serrano-Aguilar, P. Efficacy, Effectiveness, Safety, and Cost-effectiveness of Epidural Adhesiolysis for Treating Failed Back Surgery Syndrome. A Systematic Review. Pain Med. 2019, 20, 692–706. [Google Scholar] [CrossRef]
- Helm, S.; Racz, G.; Gerdesmeyer, L.; Justiz, R.; Hayek, S.M.; Kaplan, E.D.; El Terany, M.A.; Knezevic, N.N. Percutaneous and Endoscopic Adhesiolysis in Managing Low Back and Lower Extremity Pain: A Systematic Review and Meta-analysis. Pain Physician 2016, 19, E245–E281. [Google Scholar] [CrossRef]
- Devulder, J.; Bogaert, L.; Castille, F.; Moerman, A.; Rolly, G. Relevance of Epidurography and Epidural Adhesiolysis in Chronic Failed Back Surgery Patients. Clin. J. Pain 1995, 11, 147–150. [Google Scholar] [CrossRef]
- Racz, G.B.; Day, M.R.; Heavner, J.E.; Smith, J.P. The Racz Procedure: Lysis of Epidural Adhesions (Percutaneous Neuroplasty). In Treatment of Chronic Pain by Interventional Approaches; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Manchikanti, L.; Rivera, J.J.; Pampati, V.; Damron, K.S.; McManus, C.D.; Brandon, D.E.; Wilson, S.R. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: A randomized, double-blind trial. Pain Physician 2004, 7, 177–186. [Google Scholar] [CrossRef]
- Manchikanti, L.; Cash, K.A.; McManus, C.D.; Pampati, V.; Singh, V.; Benyamin, R. The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: A randomized, equivalence controlled trial. Pain Physician 2009, 12, E341–E354. [Google Scholar] [CrossRef]
- Lee, G.Y.; Lee, J.W.; Choi, H.S.; Oh, K.-J.; Kang, H.S. A new grading system of lumbar central canal stenosis on MRI: An easy and reliable method. Skelet. Radiol. 2011, 40, 1033–1039. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Yeom, J.S.; Kim, K.-J.; Kim, H.-J.; Chung, S.K.; Kang, H.S. A Practical MRI Grading System for Lumbar Foraminal Stenosis. Am. J. Roentgenol. 2010, 194, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Lee, K.W.; Lee, J.H.; Kim, M.A.; An, B.W. The Effect of Hyaluronidase in Interlaminar Lumbar Epidural Injection for Failed Back Surgery Syndrome. Ann. Rehabil. Med. 2012, 36, 466–473. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.; Lee, J.; Kim, M.; Kim, B. The Additional Effect of Hyaluronidase in Lumbar Interlaminar Epidural Injection. Ann. Rehabil. Med. 2011, 35, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.H.; Yoo, S.; Kim, J.Y.; Joo, M.; Park, H.J. Factors Predicting the Success of Adhesiolysis Using a Steerable Catheter in Lumbar Failed Back Surgery Syndrome: A Retrospective Study. J. Clin. Med. 2021, 10, 913. [Google Scholar] [CrossRef]
- Oh, Y.; Kim, D.; Park, J.Y.; Ji, G.Y.; Shin, D.A.; Lee, S.W.; Park, J.K.; Shin, J.W.; Choi, S.S. Factors Associated with Successful Response to Balloon Decompressive Adhesiolysis Neuroplasty in Patients with Chronic Lumbar Foraminal Stenosis. J. Clin. Med. 2019, 8, 1766. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, S.H. Effectiveness of percutaneous transforaminal adhesiolysis in patients with lumbar neuroforaminal spinal stenosis. Pain Physician 2013, 16, E37. [Google Scholar] [CrossRef]
- Chen, Y.; Vu, T.-N.H.; Chinchilli, V.M.; Farrag, M.; Roybal, A.R.; Huh, A.; Cohen, Z.O.; Becker, A.B.; Arvanaghi, B.; Agrawal, M.; et al. Clinical and technical factors associated with knee radiofrequency ablation outcomes: A multicenter analysis. Reg. Anesth. Pain Med. 2021, 46, 298–304. [Google Scholar] [CrossRef]
- House, L.M.; Korn, M.A.; Garg, A.; Jung, M.J.; Kendall, M.C.; Walega, D.R.; McCormick, Z.L. Severity of Knee Osteoarthritis and Pain Relief After Cooled Radiofrequency Ablation of the Genicular Nerves. Pain Med. 2019, 20, 2601–2603. [Google Scholar] [CrossRef]
- Philip, A.; Williams, M.; Davis, J.; Beeram, A.; Feng, C.; Poli, J.; Vangellow, A.; Gewandter, J. Evaluating predictors of pain reduction after genicular nerve radiofrequency ablation for chronic knee pain. Pain Manag. 2021, 11, 669–677. [Google Scholar] [CrossRef]
- Kose, S.; Kose, H.; Celikel, F.; Akkaya, O. Predictive factors associated with successful response to utrasound guided genicular radiofrequency ablation. Korean J. Pain 2022, 35, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S. Clinical effectiveness of percutaneous adhesiolysis using Navicath for the management of chronic pain due to lumbosacral disc herniation. Pain Physician 2012, 15, 213–221. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Cash, K.; Pampati, V.; Datta, S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: A randomized, equivalence controlled trial. Pain Physician 2009, 12, E355–E368. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Singh, V.; Cash, K.; Pampati, V. Assessment of effectiveness of percutaneous adhesiolysis and caudal epidural injections in managing post lumbar surgery syndrome: 2-year follow-up of a randomized, controlled trial. J. Pain Res. 2012, 5, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Hunter, D.J. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur. Spine J. 2008, 17, 327–335. [Google Scholar] [CrossRef]
- Roughan, W.; Campos, A.; García-Marín, L.; Cuéllar-Partida, G.; Lupton, M.; Hickie, I.; Medland, S.; Wray, N.; Byrne, E.; Ngo, T.; et al. Comorbid Chronic Pain and Depression: Shared Risk Factors and Differential Antidepressant Effectiveness. Front. Psychiatry 2021, 12, 643609. [Google Scholar] [CrossRef]
- Lumley, M.; Cohen, J.; Borszcz, G.; Cano, A.; Radcliffe, A.; Porter, L.; Schubiner, H.; Keefe, F. Pain and emotion: A biopsychosocial review of recent research. J. Clin. Psychol. 2011, 67, 942–968. [Google Scholar] [CrossRef]
- Manchikanti, L.; Pampati, V.; Damron, K. The role of placebo and nocebo effects of perioperative administration of sedatives and opioids in interventional pain management. Pain Physician 2005, 8, 349–355. [Google Scholar] [CrossRef]
| Variable | n = 193 |
|---|---|
| Age (yr) | 61.9 ± 10.4 |
| Sex Female Male | - 97 (50.2%) 96 (49.8%) |
| History of smoking Current Former Never | - 28 (14.5%) 76 (39.3%) 89 (46.1%) |
| Working status Employed Unemployed (retired, housewives, etc) | - 74 (38.3%) 119 (61.6%) |
| Obesity Present Absent | - 65 (33.7%) 128 (66.3%) |
| History of depression Yes No | - 39 (20.2%) 154 (79.8%) |
| History of spondylolisthesis Yes No | - 45 (23.3%) 148 (76.7%) |
| History of lumbar surgery Yes No | - 58 (30%) 135 (70%) |
| Use of opioid Yes No | - 72 (37.3%) 121 (62.7%) |
| Duration of pain (months) | 10.1 ± 3.72 |
| Baseline VAS score | 7.06 ± 1.30 |
| Positive Outcome (n = 109) | Negative Outcome (n = 84) | Odds Ratio (95% CI) | p | |
|---|---|---|---|---|
| Age | 62.3 ± 9.2 | 61.3 ± 10.2 | 1.013 (0.97, 1.05) | 0.561 |
| Sex Female Male | - 57 (52.3%) 52 (47.7%) | - 40 (52.3%) 44 (52.4%) | - 1.026 (0.47, 2.2) 1 (Ref) | 0.947 |
| Smoking status Current Former Never | - 12 (11%) 46 (42.2%) 51 (46.8%) | - 16 (19%) 30 (35.7%) 38 (45.3%) | - 1 (Ref) 1.548 (0.51, 4.64) 1.844 (0.61, 5.51) | - - 0.436 0.273 |
| Working status Employed Unemployed (re-tired, housewives, etc.) | - 41 (37.6%) 68 (62.4%) | - 33 (39.3%) 51 (60.7%) | - 1 (Ref) 1.116 (0.44, 2.80) | - - 0.815 |
| Obesity Present Absent | - 36 (33%) 73 (67%) | - 29 (34.5%) 55 (65.5%) | - 1.165 (0.53, 2.56) 1 (Ref) | 0.702 |
| Depression Yes No | - 8 (7.3%) 101 (92.7%) | - 31 (36.9%) 53 (63.1%) | - 1 (Ref) 2.750 (0.95, 8.33) | - - 0.062 |
| Spondylolisthesis Yes No | - 13 (11.9%) 96 (88.1%) | - 32 (38.1%) 52 (61.9%) | - 1 (Ref) 3.831 (1.40, 11.11) | - - 0.009 |
| History of lumbar surgery Yes No | - - 23 (21.1%) 86 (78.9%) | - - 35(41.7%) 49 (58.3%) | - - 1 (Ref) 2.257 (1.03, 5) | - - - 0.043 |
| Use of opioid Yes No | - 35 (32.1%) 74 (67.9%) | - 37 (44%) 47 (56%) | 1 (Ref) 2.090 (0.961, 4.76) | - - 0.064 |
| Duration of pain (months) | 9.87 ± 3.16 | 10.3 ± 4.34 | 0.985 (0.88, 1.09) | 0.787 |
| Grade of central stenosis Mild Moderate Severe | - 50 (46.7%) 43 (40.2%) 14(13.1%) | - 35 (44.3%) 31 (39.2%) 13 (16.5%) | - 1.209 (0.40, 3.57) 1.077 (0.47, 2.5) 1 (Ref) | - 0.759 0.859 |
| Grade of foraminal stenosis Mild Moderate Severe | - 55 (51.4%) 32 (29.9%) 20 (18.7%) | - 18 (22.5%) 26 (32.5%) 36 (45%) | - 4.670 (1.81, 12.50) 2.182 (0.90, 5.55) 1 (Ref) | - 0.002 0.085 |
| Baseline VAS score | 6.75 ± 1.17 | 7.50 ± 1.28 | 0.626 (0.59, 4.14) | 0.127 |
| Positive Outcome (n = 109) | Negative Outcome (n = 84) | Odds Ratio (95% CI) | p | |
|---|---|---|---|---|
| Target level 1 level 2 levels 3 levels | - 35 (32.1%) 62 (56.8%) 12 (11.0%) | - 25 (29.7%) 48 (57.1%) 11 (13.0%) | - 1.398 (0.520–3.759) 1.082 (0.538–2.164) Ref | - 0.506 0.824 |
| Target side Left Right Both Central Left, central Right, central Both, central | - 12 (11.0%) 15 (13.7%) 30 (27.5%) 4 (3.6%) 10 (9.1%) 14 (12.8%) 24 (22.0%) | - 10 (11.5%) 7 (8.3%) 23 (27.3%) 4 (4.7%) 11 (13.0%) 11 (13.0%) 18 (21.4%) | - Ref 1.832 (0.525–6.402) 1.111 (0.405–3.053) 0.840 (0.163–4.328) 0.765 (0.228–2.575) 1.065 (0.329–3.454) 1.160 (0.401–3.359) | - - 0.343 0.838 0.834 0.666 0.916 0.784 |
| Number of target side 1–2 3–4 >4 | - 30 (27.5%) 56 (51.3%) 23 (21.1%) | - 25(29.7%) 44 (52.2%) 15 (17.8%) | - Ref 1.020 (0.519–2.003) 1.274 (0.527–3.080 | - - 0.954 0.591 |
| Predictor | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Grade of foraminal stenosis Mild Moderate Severe | - 3.460 1.890 1 (Ref) | 1.436, 8.333 0.843, 4.329 | - 0.006 0.121 |
| Use of opioid Yes No | - 1 (Ref) 1.782 | - - 0.854, 3.717 | - - 0.123 |
| Baseline VAS score | 0.787 | 0.583, 1.064 | 0.120 |
| History of spondylolisthesis Yes No | - 1 (Ref) 2.976 | - - 1.246, 7.092 | - - 0.014 |
| History of lumbar surgery Yes No | - 1 (Ref) 2.242 | - - 1.067, 4.716 | - - 0.033 |
| History of depression Yes No | - 1 (Ref) 3.105 | - - 1.127, 8.547 | - - 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, H.C.; Akkaya, O.T. Predictive Factors Associated with Successful Response to Percutaneous Adhesiolysis in Chronic Lumbar Radicular Pain. J. Clin. Med. 2023, 12, 6337. https://doi.org/10.3390/jcm12196337
Kose HC, Akkaya OT. Predictive Factors Associated with Successful Response to Percutaneous Adhesiolysis in Chronic Lumbar Radicular Pain. Journal of Clinical Medicine. 2023; 12(19):6337. https://doi.org/10.3390/jcm12196337
Chicago/Turabian StyleKose, Halil Cihan, and Omer Taylan Akkaya. 2023. "Predictive Factors Associated with Successful Response to Percutaneous Adhesiolysis in Chronic Lumbar Radicular Pain" Journal of Clinical Medicine 12, no. 19: 6337. https://doi.org/10.3390/jcm12196337
APA StyleKose, H. C., & Akkaya, O. T. (2023). Predictive Factors Associated with Successful Response to Percutaneous Adhesiolysis in Chronic Lumbar Radicular Pain. Journal of Clinical Medicine, 12(19), 6337. https://doi.org/10.3390/jcm12196337





