Usefulness of AI-Equipped Endoscopy for Detecting Colorectal Adenoma during Colonoscopy Screening: Confirm That Colon Neoplasm Finely Can Be Identified by AI without Overlooking Study (Confidential Study)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participating Institutes and Patients
2.2. Endoscopic Equipment and AI System
2.3. Study design
2.4. Endoscopists
2.5. Evaluation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Colorectal Observation Time
3.3. Characteristics of Identified Lesions
3.4. Primary Endpoint
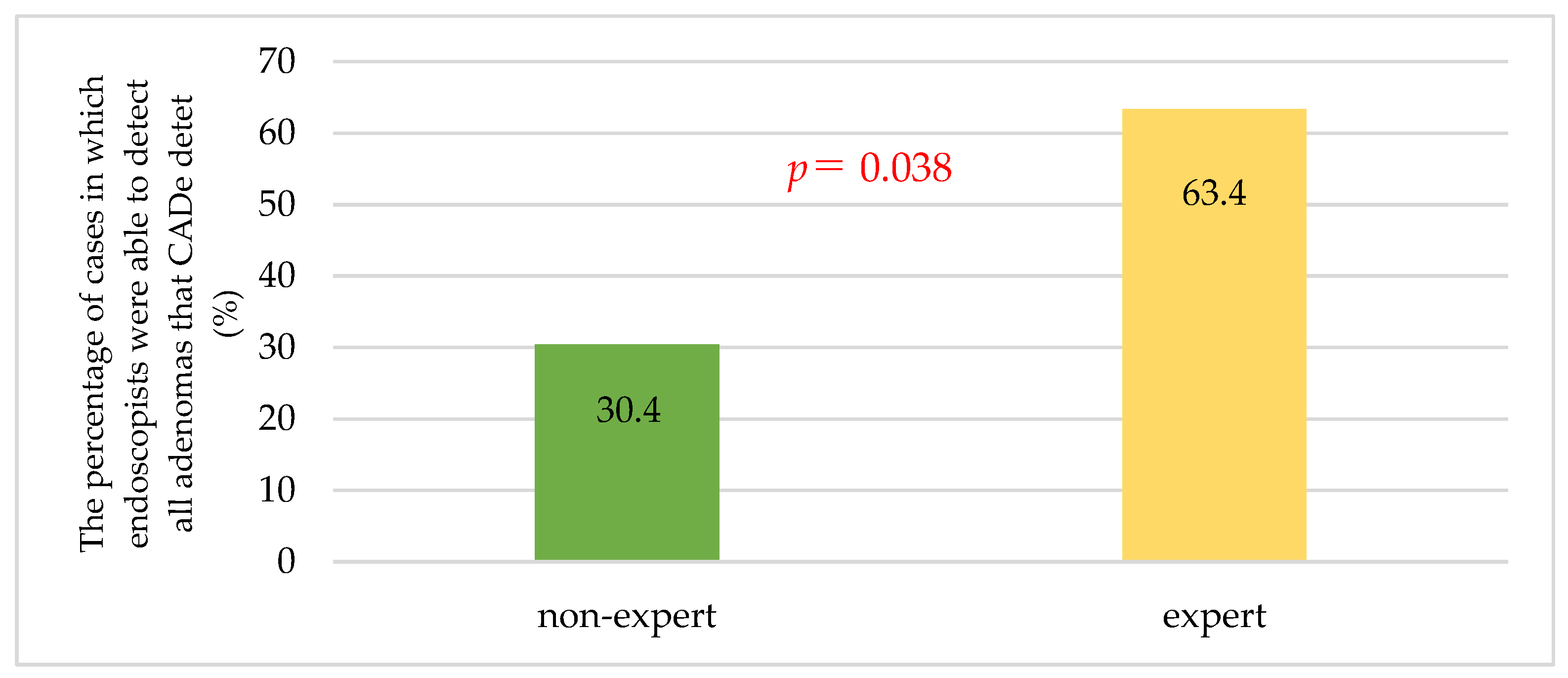
3.5. Secondary Endpoint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Pilonis, N.D.; Bugajski, M.; Wieszczy, P.; Franczyk, R.; Didkowska, J.; Wojciechowska, U.; Pisera, M.; Rupinski, M.; Regula, J.; Kaminski, M.F. Long-term colorectal cancer incidence and mortality after a single negative screening colonoscopy. Ann. Intern. Med. 2020, 173, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Wieszczy, P.; Kaminski, M.F.; Franczyk, R.; Loberg, M.; Kobiela, J.; Rupinska, M.; Kocot, B.; Rupinski, M.; Holme, O.; Wojciechowska, U.; et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology 2020, 158, 875–883.e5. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Provenzale, D.; Ness, R.M.; Llor, X.; Weiss, J.M.; Abbadessa, B.; Cooper, G.; Early, D.S.; Friedman, M.; Giardiello, F.M.; Glaser, K.; et al. NCCN Guidelines insights: Colorectal cancer screening, version 2.2020. J. Natl. Compr. Canc. Netw. 2020, 18, 1312–1320. [Google Scholar] [CrossRef]
- Saito, Y.; Oka, S.; Kawamura, T.; Shimoda, R.; Sekiguchi, M.; Tamai, N.; Hotta, K.; Matsuda, T.; Misawa, M.; Tanaka, S.; et al. Colonoscopy screening and surveillance guidelines. Dig. Endosc. 2021, 33, 486–519. [Google Scholar] [CrossRef]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef]
- Anderson, R.; Burr, N.E.; Valori, R. Causes of post-colonoscopy colorectal cancers based on World Endoscopy Organization System of Analysis. Gastroenterology 2020, 158, 1287–1299.e2. [Google Scholar] [CrossRef]
- Fuccio, L.; Rex, D.; Ponchon, T.; Frazzoni, L.; Dinis-Ribeiro, M.; Bhandari, P.; Dekker, E.; Pellisè, M.; Correale, L.; van Hooft, J.; et al. New and recurrent colorectal cancers after resection: A systematic review and meta-analysis of endoscopic surveillance studies. Gastroenterology 2019, 156, 1309–1323.e3. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W.; et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019, 156, 1661–1674.e11. [Google Scholar] [CrossRef]
- Baxter, N.N.; Sutradhar, R.; Forbes, S.S.; Paszat, L.F.; Saskin, R.; Rabeneck, L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011, 140, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Goldwasser, M.A.; Paszat, L.F.; Saskin, R.; Urbach, D.R.; Rabeneck, L. Association of colonoscopy and death from colorectal cancer. Ann. Intern. Med. 2009, 150, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.T.; Protiva, P.; Nagar, A.; Imaeda, A.; Ciarleglio, M.M.; Deng, Y.; Laine, L. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology 2016, 150, 396–405, quiz e14-5. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Matsuda, T.; Ono, A.; Sekiguchi, M.; Fujii, T.; Saito, Y. Advances in image enhancement in colonoscopy for detection of adenomas. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.S.S.; Ket, S.; Bassett, P.; Aponte, D.; De Aguiar, S.; Gupta, N.; Horimatsu, T.; Ikematsu, H.; Inoue, T.; Kaltenbach, T.; et al. Narrow-Band Imaging for detection of neoplasia at colonoscopy: A meta-analysis of data from individual patients in randomized controlled trials. Gastroenterology 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Yoshida, N.; Hisabe, T.; Ikematsu, H.; Ishihara, H.; Terasawa, M.; Inaba, A.; Sato, D.; Cho, H.; Ego, M.; Tanaka, Y.; et al. Comparison between Linked Color Imaging and Blue Laser Imaging for improving the visibility of flat colorectal polyps: A multicenter pilot study. Dig. Dis. Sci. 2020, 65, 2054–2062. [Google Scholar] [CrossRef]
- Shinozaki, S.; Kobayashi, Y.; Hayashi, Y.; Sakamoto, H.; Sunada, K.; Lefor, A.K.; Yamamoto, H. Colon polyp detection using Linked Color Imaging compared to white light imaging: Systematic review and meta-analysis. Dig. Endosc. 2020, 32, 874–881. [Google Scholar] [CrossRef]
- Weigt, J.; Repici, A.; Antonelli, G.; Afifi, A.; Kliegis, L.; Correale, L.; Hassan, C.; Neumann, H. Performance of a new integrated computer-assisted system (CADe/CADx) for detection and characterization of colorectal neoplasia. Endoscopy 2022, 54, 180–184. [Google Scholar] [CrossRef]
- Aronchick, C.A.; Lipshutz, W.H.; Wright, S.H.; Dufrayne, F.; Bergman, G. A novel tableted purgative for colonoscopic preparation: Efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest. Endosc. 2000, 52, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.J.; Calderwood, A.H.; Doros, G.; Fix, O.K.; Jacobson, B.C. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest. Endosc. 2009, 69, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Fernández-Esparrach, G.; Bernal, J.; López-Cerón, M.; Córdova, H.; Sánchez-Montes, C.; Rodríguez de Miguel, C.; Sánchez, F.J. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy 2016, 48, 837–842. [Google Scholar] [CrossRef]
- Misawa, M.; Kudo, S.E.; Mori, Y.; Cho, T.; Kataoka, S.; Yamauchi, A.; Ogawa, Y.; Maeda, Y.; Takeda, K.; Ichimasa, K.; et al. Artificial intelligence-assisted polyp detection for colonoscopy: Initial experience. Gastroenterology 2018, 154, 2027–2029.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Lei, S.; Liu, X.; Li, L.; Xiao, X. Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Gastroenterology 2020, 159, 1252–1261.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berzin, T.M.; Glissen Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Berzin, T.M.; Glissen Brown, J.R.; Liu, P.; Zhou, C.; Lei, L.; Li, L.; Guo, Z.; Lei, S.; et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): A double-blind randomised study. Lancet Gastroenterol. Hepatol. 2020, 5, 343–351. [Google Scholar] [CrossRef]
- Liu, P.; Wang, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Liu, W.; Xiao, X.; Chen, Z.; Zhang, Z.; Zhou, C.; et al. The single-monitor trial: An embedded CADe system increased adenoma detection during colonoscopy: A prospective randomized study. Therap. Adv. Gastroenterol. 2020, 13, 1756284820979165. [Google Scholar] [CrossRef]
- Gong, D.; Wu, L.; Zhang, J.; Mu, G.; Shen, L.; Liu, J.; Wang, Z.; Zhou, W.; An, P.; Huang, X.; et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): A randomised controlled study. Lancet Gastroenterol. Hepatol. 2020, 5, 352–361. [Google Scholar] [CrossRef]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef]
- Barua, I.; Vinsard, D.G.; Jodal, H.C.; Løberg, M.; Kalager, M.; Holme, Ø.; Misawa, M.; Bretthauer, M.; Mori, Y. Artificial intelligence for polyp detection during colonoscopy: A systematic review and meta-analysis. Endoscopy 2021, 53, 277–284. [Google Scholar] [CrossRef]
- Mehrotra, A.; Morris, M.; Gourevitch, R.A.; Carrell, D.S.; Leffler, D.A.; Rose, S.; Greer, J.B.; Crockett, S.D.; Baer, A.; Schoen, R.E. Physician characteristics associated with higher adenoma detection rate. Gastrointest Endosc. 2018, 87, 778–786.e5. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Hasan, A.G.; Jacobson, N.B.; Austin, G.L. Level of fellowship training increases adenoma detection rates. Clin. Gastroenterol. Hepatol. 2010, 8, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Qayed, E.; Shea, L.; Goebel, S.; Bostick, R.M. Association of trainee participation with adenoma and polyp detection rates. World J. Gastrointest Endosc. 2017, 9, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.; Shimoda, R.; Miyahara, K.; Yukimoto, T.; Sakata, Y.; Takamori, A.; Mizuta, Y.; Fujimura, Y.; Inoue, S.; Tomonaga, M.; et al. Impact of an artificial intelligence-aided endoscopic diagnosis system on improving endoscopy quality for trainees in colonoscopy: Prospective, randomized, multicenter study. Dig. Endosc. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Tonti, P.; Licinio, R.; Del Prete, V.; Neve, V.; Di Maso, M.; Muscatiello, N. Impact of fellow participation on colon adenoma detection rates: A multicenter randomized trial. Gastrointest. Endosc. 2020, 92, 1228–1235. [Google Scholar] [CrossRef]
- Barclay, R.L.; Vicari, J.J.; Doughty, A.S.; Johanson, J.F.; Greenlaw, R.L. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N. Engl. J. Med. 2006, 355, 2533–2541. [Google Scholar] [CrossRef]
| Total | Experts | Non-Experts | p-Value | |
|---|---|---|---|---|
| Number | 100 | 47 | 53 | - |
| Age (median (IQR, range)) | 66.5 (59.75, 37–89) | 68 (62.5, 37–89) | 65 (59, 41–85) | 0.578 |
| Male | 51 | 27 | 24 | 0.32 |
| CS for the first time | 53 | 24 | 29 | 0.843 |
| Obesity (BMI > 25) | 30 | 12 | 18 | 0.388 |
| Insertion time (min) (median (IQR, range)) | 343.5 (224.75, 89–2348) | 265 (212.5, 89–1042) | 399 (278, 150–2348) | 0.002 |
| Total | Experts | Non-Experts | p-Value | ||
|---|---|---|---|---|---|
| Lesion area | 170 | 82 | 88 | ||
| Ascending colon | 46 | 28 | 18 | 0.003 | |
| Transverse colon | 36 | 17 | 19 | ||
| Descending colon | 26 | 4 | 22 | ||
| Sigmoid colon | 53 | 28 | 25 | ||
| Rectum | 9 | 5 | 4 | ||
| Size | |||||
| <5 mm | 77 | 35 | 42 | 0.037 | |
| 5–10 mm | 62 | 37 | 25 | ||
| >10 mm | 31 | 10 | 21 | ||
| Form | |||||
| Ⅰs | 97 | 50 | 47 | 0.783 | |
| Ⅰsp | 54 | 24 | 30 | ||
| Ⅰp | 8 | 3 | 5 | ||
| Others | 11 | 5 | 6 | ||
| Procedure (histology) | |||||
| Biopsy | 2 | 0 | 2 | <0.001 | |
| CSP | 111 | 43 | 68 | ||
| EMR | 56 | 38 | 18 | ||
| Polypectomy | 1 | 1 | 0 | ||
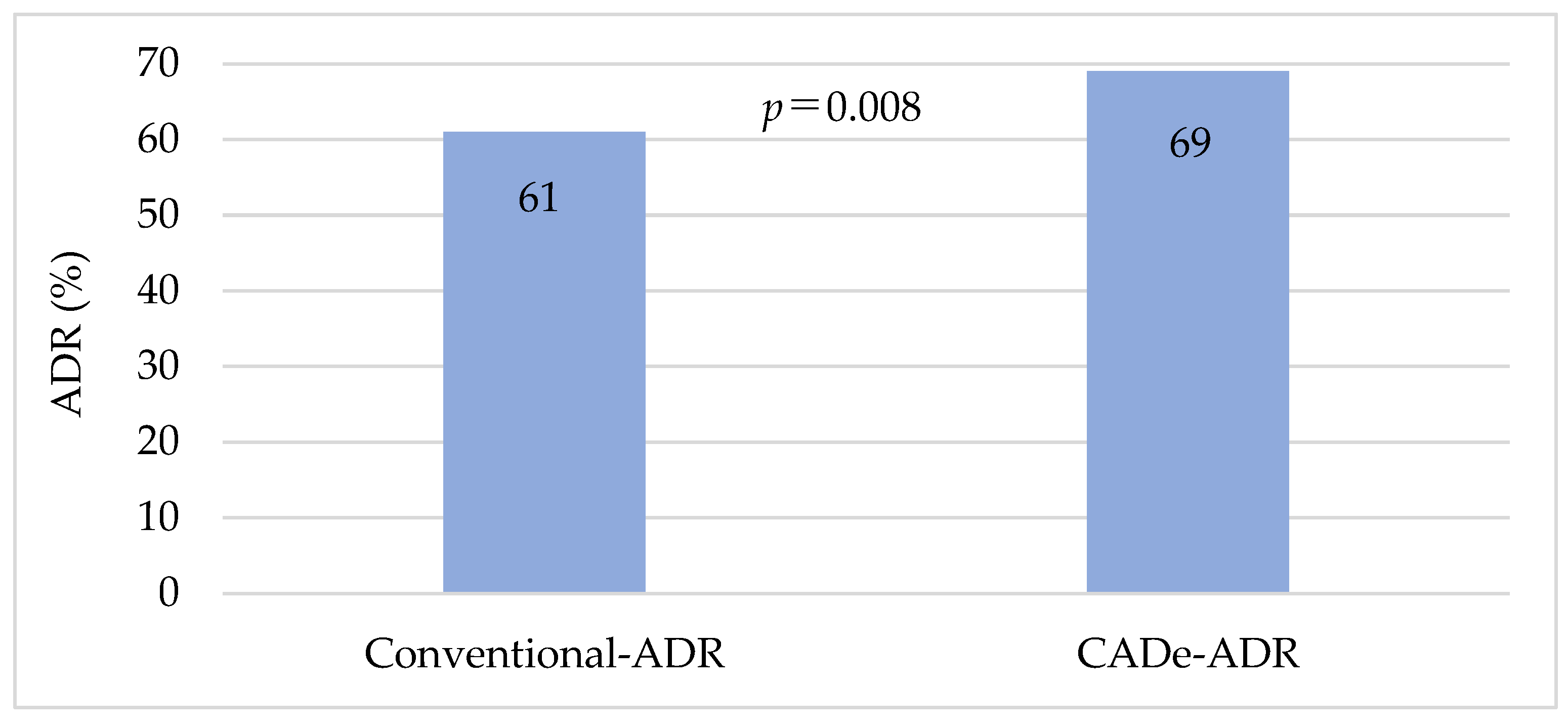
| Conventional Group | CADe Group | p-Value | ||
|---|---|---|---|---|
| ADR | ||||
| Total | 61% | 69% | 0.013 a | |
| Expert | 66% | 68.1% | 1 a | |
| Non-expert | 56.6% | 69.5% | 0.023 a | |
| MAP | ||||
| Total | 1.29 | 1.7 | <0.001 b | |
| Expert | 1.45 | 1.7 | 0.034 b | |
| Non-expert | 1.11 | 1.66 | <0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizukami, K.; Fushimi, E.; Sagami, R.; Abe, T.; Sato, T.; Terashi, S.; Fukuda, M.; Nishikiori, H.; Nagai, T.; Kodama, M.; et al. Usefulness of AI-Equipped Endoscopy for Detecting Colorectal Adenoma during Colonoscopy Screening: Confirm That Colon Neoplasm Finely Can Be Identified by AI without Overlooking Study (Confidential Study). J. Clin. Med. 2023, 12, 6332. https://doi.org/10.3390/jcm12196332
Mizukami K, Fushimi E, Sagami R, Abe T, Sato T, Terashi S, Fukuda M, Nishikiori H, Nagai T, Kodama M, et al. Usefulness of AI-Equipped Endoscopy for Detecting Colorectal Adenoma during Colonoscopy Screening: Confirm That Colon Neoplasm Finely Can Be Identified by AI without Overlooking Study (Confidential Study). Journal of Clinical Medicine. 2023; 12(19):6332. https://doi.org/10.3390/jcm12196332
Chicago/Turabian StyleMizukami, Kazuhiro, Erina Fushimi, Ryota Sagami, Takashi Abe, Takao Sato, Shohei Terashi, Masahide Fukuda, Hidefumi Nishikiori, Takayuki Nagai, Masaaki Kodama, and et al. 2023. "Usefulness of AI-Equipped Endoscopy for Detecting Colorectal Adenoma during Colonoscopy Screening: Confirm That Colon Neoplasm Finely Can Be Identified by AI without Overlooking Study (Confidential Study)" Journal of Clinical Medicine 12, no. 19: 6332. https://doi.org/10.3390/jcm12196332
APA StyleMizukami, K., Fushimi, E., Sagami, R., Abe, T., Sato, T., Terashi, S., Fukuda, M., Nishikiori, H., Nagai, T., Kodama, M., & Murakami, K. (2023). Usefulness of AI-Equipped Endoscopy for Detecting Colorectal Adenoma during Colonoscopy Screening: Confirm That Colon Neoplasm Finely Can Be Identified by AI without Overlooking Study (Confidential Study). Journal of Clinical Medicine, 12(19), 6332. https://doi.org/10.3390/jcm12196332








