Rationale and Design of Heart Failure Prevalence and Evolution of Heart Failure in Diabetes Mellitus Type II Patients at High Risk (HF-LanDMark Study)
Abstract
:1. Introduction
2. Methods
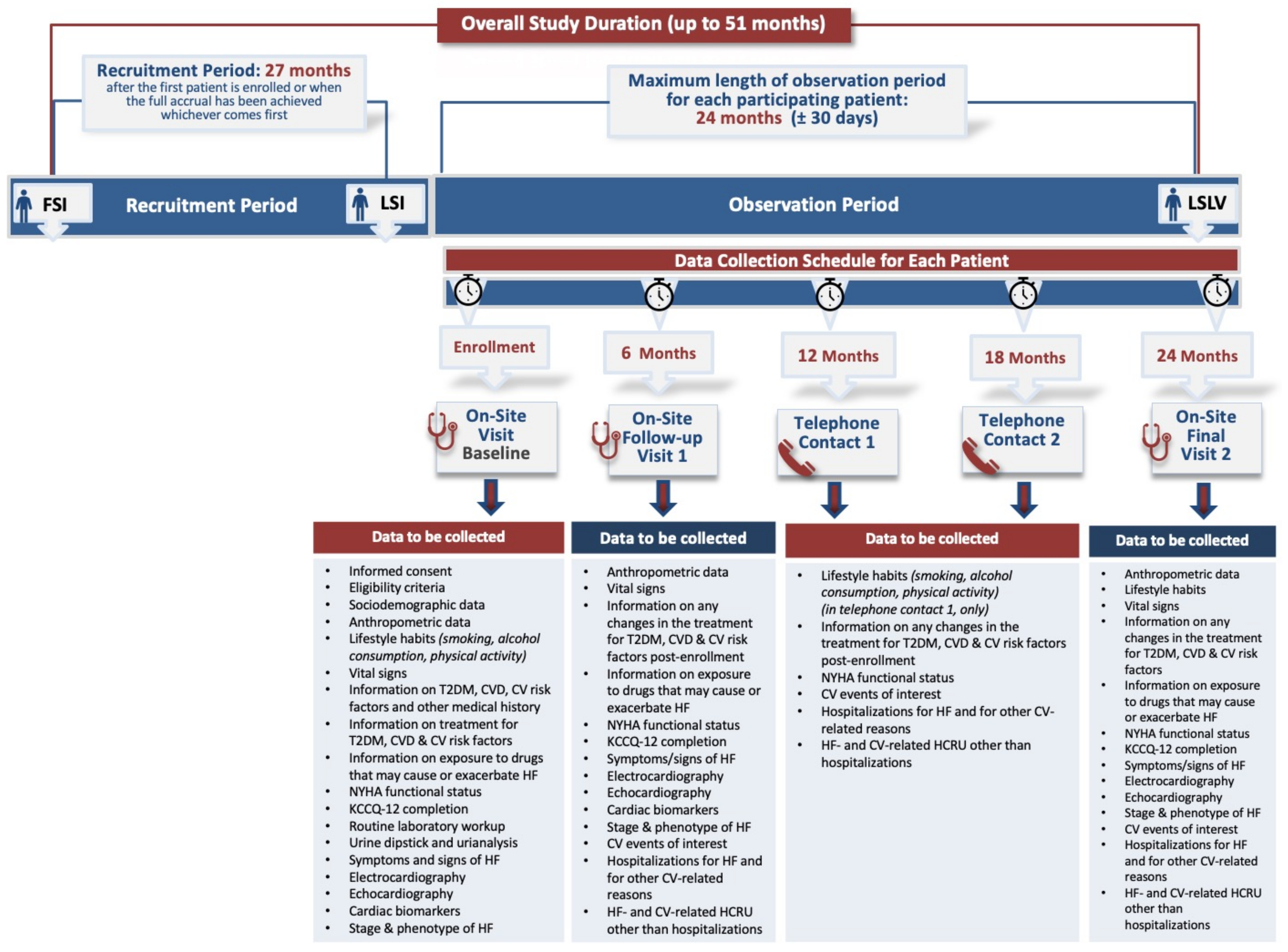
2.1. Study Design
2.2. Patient Population
2.3. Study Methods
2.4. Study Variables
2.5. Study Objectives
2.6. Statistical Analysis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and mortality following acute coronary syndromes. JAMA 2007, 298, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Braunwald, E.; Raz, I.; Cavender, M.A.; Morrow, D.A.; Jarolim, P.; Udell, J.A.; Mosenzon, O.; Im, K.; Umez-Eronini, A.A.; et al. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014, 130, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Orna, J.A.; Rodriguez-Padial, L.; Anguita-Sanchez, M.; Barrios, V.; Muniz, J.; Perez, A. Association of the KDIGO Risk Classification with the Prevalence of Heart Failure in Patients with Type 2 Diabetes. J. Clin. Med. 2021, 10, 4634. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.A.; Read, S.H.; Kerssens, J.; Livingstone, S.; McGurnaghan, S.; Jhund, P.; Petrie, J.; Sattar, N.; Fischbacher, C.; Kristensen, S.L.; et al. Incidence of Hospitalization for Heart Failure and Case-Fatality Among 3.25 Million People With and Without Diabetes Mellitus. Circulation 2018, 138, 2774–2786. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, F.S.; Kistorp, C.; Kumler, T.; Hassager, C.; Tonder, N.; Kober, L.; Hansen, P.M.; Kamstrup, P.R.; Faber, J.; Iversen, K.K.; et al. Prevalence of early stages of heart failure in an elderly risk population: The Copenhagen Heart Failure Risk Study. Open Heart 2019, 6, e000840. [Google Scholar] [CrossRef]
- Boonman-de Winter, L.J.; Rutten, F.H.; Cramer, M.J.; Landman, M.J.; Liem, A.H.; Rutten, G.E.; Hoes, A.W. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012, 55, 2154–2162. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Erqou, S.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail 2016, 4, 237–248. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Huynh, Q.; Nolan, M.; Negishi, K.; Marwick, T.H. Diagnosis of Nonischemic Stage B Heart Failure in Type 2 Diabetes Mellitus: Optimal Parameters for Prediction of Heart Failure. JACC Cardiovasc. Imaging 2018, 11, 1390–1400. [Google Scholar] [CrossRef]
- Koniari, I.; Velissaris, D.; Kounis, N.G.; Koufou, E.; Artopoulou, E.; de Gregorio, C.; Mplani, V.; Paraskevas, T.; Tsigkas, G.; Hung, M.Y.; et al. Anti-Diabetic Therapy, Heart Failure and Oxidative Stress: An Update. J. Clin. Med. 2022, 11, 4660. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rohatgi, A.; Ayers, C.R.; Patel, P.C.; Matulevicius, S.A.; Peshock, R.M.; Markham, D.W.; de Lemos, J.A.; Berry, J.D.; Drazner, M.H. Risk scores versus natriuretic peptides for identifying prevalent stage B heart failure. Am. Heart J. 2011, 161, 923–930.e2. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vaduganathan, M.; Patel, K.V.; Ayers, C.; Ballantyne, C.M.; Kosiborod, M.N.; Carnethon, M.; DeFilippi, C.; McGuire, D.K.; Khan, S.S.; et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail 2021, 9, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Liatis, S.; Dafoulas, G.E.; Kani, C.; Politi, A.; Litsa, P.; Sfikakis, P.P.; Makrilakis, K. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res. Clin. Pract. 2016, 118, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Atlas, 9th Edition. Available online: https://idf.org/our-network/regions-members/europe/members/137-greece.html (accessed on 20 September 2023).
- Van der Leeuw, J.; Beulens, J.W.; van Dieren, S.; Schalkwijk, C.G.; Glatz, J.F.; Hofker, M.H.; Verschuren, W.M.; Boer, J.M.; van der Graaf, Y.; Visseren, F.L.; et al. Novel Biomarkers to Improve the Prediction of Cardiovascular Event Risk in Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2016, 5, e003048. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: Prevalence, incidence, and risk factors. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.F.; Campbell, D.J.; Prior, D.L. Noninvasive Cardiac Imaging and the Prediction of Heart Failure Progression in Preclinical Stage A/B Subjects. JACC Cardiovasc. Imaging 2017, 10, 1504–1519. [Google Scholar] [CrossRef]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.; Kaluzna-Oleksy, M.; Lesiak, M.; Straburzynska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure-A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Soga, F.; Tatsumi, K.; Mochizuki, Y.; Sano, H.; Toki, H.; Matsumoto, K.; Shite, J.; Takaoka, H.; Doi, T.; et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc. Diabetol. 2020, 19, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parissis, J.; Georgiou, C.; Bistola, V.; Karavidas, A.; Vassilikos, V.P.; Kanakakis, J.; Davlouros, P.; Tziakas, D.N.; Alexanian, I.P.; Kochiadakis, G.; et al. Rationale and Design of Heart Failure Prevalence and Evolution of Heart Failure in Diabetes Mellitus Type II Patients at High Risk (HF-LanDMark Study). J. Clin. Med. 2023, 12, 6319. https://doi.org/10.3390/jcm12196319
Parissis J, Georgiou C, Bistola V, Karavidas A, Vassilikos VP, Kanakakis J, Davlouros P, Tziakas DN, Alexanian IP, Kochiadakis G, et al. Rationale and Design of Heart Failure Prevalence and Evolution of Heart Failure in Diabetes Mellitus Type II Patients at High Risk (HF-LanDMark Study). Journal of Clinical Medicine. 2023; 12(19):6319. https://doi.org/10.3390/jcm12196319
Chicago/Turabian StyleParissis, John, Christos Georgiou, Vasiliki Bistola, Apostolos Karavidas, Vassilios P. Vassilikos, John Kanakakis, Periklis Davlouros, Dimitrios N. Tziakas, Ioannis P. Alexanian, George Kochiadakis, and et al. 2023. "Rationale and Design of Heart Failure Prevalence and Evolution of Heart Failure in Diabetes Mellitus Type II Patients at High Risk (HF-LanDMark Study)" Journal of Clinical Medicine 12, no. 19: 6319. https://doi.org/10.3390/jcm12196319
APA StyleParissis, J., Georgiou, C., Bistola, V., Karavidas, A., Vassilikos, V. P., Kanakakis, J., Davlouros, P., Tziakas, D. N., Alexanian, I. P., Kochiadakis, G., Triposkiadis, F., Karvounis, H., Gourlis, D., Papoutsidakis, N., Polyzogopoulou, E., & Vlachopoulos, C. (2023). Rationale and Design of Heart Failure Prevalence and Evolution of Heart Failure in Diabetes Mellitus Type II Patients at High Risk (HF-LanDMark Study). Journal of Clinical Medicine, 12(19), 6319. https://doi.org/10.3390/jcm12196319







