Abstract
Kinetic estimation of glomerular filtration rate (KeGFR) has proved its utility in predicting acute kidney injury (AKI) in both adults and children. Our objective is to assess the clinical utility of KeGFR in predicting AKI severity and progression to acute kidney disease (AKD) in patients already diagnosed with AKI and to examine major adverse kidney events at 30 days (MAKE30). We retrospectively calculated the KeGFR within the first 24 h of identified AKI (KeGFR1) and in the 24 h prior to AKD (KeGFR2) in all admitted children under 18 years old. The cohort consisted of 803 patients with AKI. We proposed a new classification of KeGFR stages, from 1 to 5, and assessed the predictive value of KeGFR stages for AKD development and MAKE30. AKI severity was associated with lower KeGFRs. KeGFR1 and KeGFR2 predicted AKD with AUC values between 0.777 and 0.841 respectively, p < 0.001. KeGFR2 had the best performance in predicting MAKE30 (AUC of 0.819) with a sensitivity of 66.67% and specificity 87.7%. KeGFR1 stage 3, 4 and 5 increased the risk of AKD by 3.07, 6.56 and 28.07 times, respectively, while KeGFR2 stage 2, 3, 4 and 5 increased the risk of AKD 2.79, 3.58, 32.75 and 80.14 times. Stage 5 KeGFR1 and KeGFR2 stages 3, 4 and 5 increased the risk of MAKE30 by 7.77, 4.23. 5.89 and 69.42 times in the adjusted models. KeGFR proved to be a useful tool in AKI settings. KeGFR dynamics can predict AKI severity, duration and outcomes.
1. Introduction
Acute kidney injury (AKI) is a global health problem with a reported incidence of 26% in hospitalized children [1]. AKI is the reflection of the underlying disease severity. Some steps towards improving the outcomes for AKI are early identification of the individual risk factors, proper management and rapid intervention. The operational framework period for AKI that persists longer than 7 days provides time for intervention but at the cost of time-dependent nephron loss. Acute kidney disease (AKD) is defined as the link between AKI and chronic kidney disease (CKD), lasting between 7 to 90 days; thus AKD is a sequel of acute kidney injury (AKI) [2]. Current pediatric studies report AKD as an independent risk factor for progression to CKD [3,4,5]. The importance of AKD in clinical settings is poorly explored in the pediatric field [3,4,6,7,8]. AKD development and recovery are currently assessed using filtration markers, such as serum creatinine [2].
Kinetic estimated glomerular filtration rate (KeGFR) has been used in acute settings as part of a multidimensional approach for AKI prediction [9,10,11,12]. Kinetic modeling of eGFR can rapidly identify changes in kidney function any time during the first 24 h, proving its utility in intensive care unit settings [9,10,11,12]. In cardiac surgery and cardiac transplantation patients respectively, KeGFR proved to be correlated with AKI severity and mortality [9,10]. In one study in critically ill patients, the authors reported the AKI classification system and KeGFR to be complementary to each other, with a significant impact on RRT necessity and long-term survival [11]. Current studies have found KeGFR highly sensitive in predicting mostly severe AKI [10,12]. Although 10 years have passed since Chen introduced the KeGFR for cases where the plasma creatinine changes rapidly [13], only a few studies have addressed the utility of this tool in the clinical pediatric settings, and all of them were performed in intensive care units (ICUs) [9,12,14]. Also, there are no studies reporting on the importance of KeGFR in predicting AKD.
KeGFR is an adequate tool in assessing the risk of AKI in children, as the formula derives from the initial serum creatinine level, the distribution volume, the creatinine production rate and the given time differences of the two serum creatinine values [9]. Based on current data, we performed a retrospective study to assess the utility of KeGFR in predicting AKD and MAKE30 in pediatric AKI settings using personalized KeGFR for age- and height-corrected distribution volume of creatinine.
2. Material and Methods
A retrospective observational study was conducted in a tertiary care teaching and research hospital in western Romania from 1 of July 2014 to 31 December 2022. All patients under 18 years of age were screened based on changes in serum creatinine (SCr) via The Laboratory Information System and Hospital Information System. SCr measurement was performed using the Jaffe Abbott method. AKI was defined and staged using the 2012 Kidney Disease Improving Global Outcomes (KDIGO) SCr criteria [15]. AKD was defined and staged according to the 2017 ADQI consensus statement [2] on the 8th day of persistent AKI based on the SCr level. AKD is the persistence of AKI beyond day 7, regardless of AKI stage and AKI cause. AKD was classified based on the SCr value on the 8th day as follows: stage 1 AKD as a persistence of 1.5–1.9 times higher than baseline SCr; stage 2 as a persistence of 2–2.9 times higher; and stage 3 as persistence more than three times higher than baseline SCr or the necessity of renal replacement therapy (RRT). Chronic kidney disease (CKD) was defined according to the KDIGO CKD guidelines from 2012 [16]. AKI and AKD had been diagnosed based on an increase in SCr at a given time during hospitalization. Severe AKI was considered KDIGO stages 2 and 3. Severe AKD were all patients with stage 2 or 3 AKD. Exclusion criteria were a single measurement of SCr, end-stage kidney disease, patients who did not have their height and weight recorded, or a lack of two consecutive SCr measurements. Demographics, clinical characteristics, and outcomes were retrieved from data at the time of admission (day 1). Urine output measurement was not available for the entire cohort; thus we could not include urine output in our analysis.
Estimated glomerular filtration rate (eGFR) was calculated using the Schwartz formula [17] as follows:
where k = 0.33 in preterm infants, k = 0.45 in infants at term to 1 year old, k = 0.55 in children over 1 year and under 13 years old, k = 0.55 for adolescent females 13–18 years old, and k = 0.7 for adolescent males 13–18 years old. KeGFR was calculated using Chen’s formula [13],
Baseline SCr was defined as the lowest SCr in the 3 months prior to AKI diagnosis, or the minimum inpatient SCr value during follow-up. Mean SCr was calculated from two consecutive SCr measurements. ΔSCr was the difference between the two SCr values, and Δt (h) was the interval in hours between two SCr measurements (maximum of 24 h). The maximum ΔSCr/day was defined as the change (increase) in SCr that occurred in a state of no kidney function dependent on volume of distribution (VD). The VD of creatinine is close to total body water, and it was estimated using Morgenstern et al.’s recalculation of Mellits and Cheek’s equation [18] based on height and sex as follows (Table 1):

Table 1.
Total body water formula adjusted for age and sex.
After adjusting all data for age and sex, KeGFR was calculated twice, using two separate SCr set of values. KeGFR1 was calculated using the SCr levels on days 1 and 2 of identified AKI, and KeGFR2 was calculated from two SCr values in days 6 and 7, before progression to AKD. The cut-off values of KeGFR were chosen based on KDIGO CKD classification [13] as stage 1 (KeGFR > 90 mL/min/1.73 sm), stage 2 (KeGFR between 60–90 mL/min/1.73 sm), stage 3 (KeGFR between 30–60 mL/min/1.73 sm), stage 4 (KeGFR between 15–30 mL/min/1.73 sm) or stage 5 (KeGFR < 15 mL/min/1.73 sm).
Outcomes of AKI were evaluated via MAKE30 (major adverse kidney events within 30 days). MAKE was defined as death, necessity of RRT or the presence of AKD at day 30 after AKI documentation. The study was approved by the hospital’s Medical Ethics Committee in accordance with the Ethics Code of the World Medical Association and informed consent was waived.
Statistical Analysis
Data are presented as median and percentage for non-normally distributed continuous variables and categorical ones, respectively. All continuous variables were tested for normality using the Shapiro–Wilk test. For normally distributed variables, we used independent t-test or ANOVA. For non-normally distributed continuous variables, the median and interquartile ranges (IQR) were reported, and groups were compared using the Wilcoxon Signed Ranks test. Discrete variables were analyzed with the chi-square test. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated. In order to assess the independent factors that predict the risk of AKD and MAKE30 in our cohort, we employed a backward multivariable logistic regression model. An Akaike information criterion (AIC) was used in order to determine the best model. Receiver-operating characteristic curves (ROCs) were plotted to determine the prognostic values of KeGFR, and area under the curve (AUC) values were generated to determine the predictive values of KeGFR1 and KeGFR2. The following values were used to describe AUC-ROCs: 0.6–0.69, poor; 0.7–0.79, fair; and 0.8–0.89, good. In this study, a p-value of 0.05 was considered the threshold for statistical significance. Data were analyzed using MedCalc Statistical software version 22.009. MedCalc® Statistical Software version 22.009 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; accessed on 25 August 2023)
3. Results
The final cohort consisted of 803 patients with AKI that met the inclusion criteria. We divided the cases into two groups: the AKD group (219 patients) and the non-AKD group (585 patients). Baseline characteristics are presented in Table 2.

Table 2.
Baseline characteristics of the entire cohort.
There were no differences between sexes. Patients that progressed to AKD were younger, namely, 3 days versus 365 days (p < 0.0001). The prevalence of severe AKI was higher in the AKD group (88.5%) as compared to the no AKD group. Patients that developed AKD maintained the same trend, with severe AKD in 72.8% of the patients (stage 2 or 3 AKD). Prerenal AKI was the main cause of AKI. Patients with intrinsic AKI were more prone to developing AKD as compared to other AKI etiologies. Intrinsic AKI could be traced in 28% of AKD patients and only in 15.6% of the non-AKD ones. As expected, baseline SCr was higher and eGFR lower in the AKD group (p < 0.0001). KeGFR1 and KeGFR2 were 3.57 times and 4.99 times lower, respectively, in the AKD group—see Figure 1. Out of 803 patients, only 16 required RRT (2%), with the highest rate of RRT in the AKD group (12 patients). Non-AKD patients expressed lower rates of pre-existent CKD. The overall mortality was 6.6% (53 patients) without significant statistical differences between AKD and non-AKD groups.
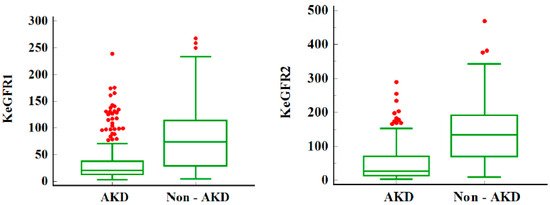
Figure 1.
KeGFR in children with AKD and non-AKD. Legend: Median and IQR for KeGFR in children with AKD and non-AKD; KeGFR1 and KeGFR2 are lower in the AKD group; AKD = acute kidney disease; KeGFR = kinetic estimated glomerular filtration rate in mL/min/1.73 s. The red dots represent the outliers—data points that are located outside the whiskers of the box plot.
We identified a strong correlation between KeGFR1 staging and AKI severity and causes—see Table 3 (p < 0.0001). KeGFR was classified in KeGFR stages as described in Methods. Within the first 24 h of identified AKI, almost half the cases (45%) were stage 1 or 2 (KeGFR > 60 mL/min/1.73 sm). Regarding AKI stages, AKI stage 1 had the highest KeGFR (stages 1 and 2) as compared to severe AKI (stages 2 and 3). As severity of AKI increased, KeGFR stage increased. In patients that progressed to AKD (217 patients out of 803), severe AKD was also associated with higher KeGFR stages.

Table 3.
KeGFR1 (derived from days 1 and 2 SCr values) staging and distribution in AKI and AKD stages and AKI causes.
After evaluating KeGFR within the first 24 h of identified AKI, we performed the second analysis of KeGFR2 on day 7 using SCr levels from days 6 and 7 in Table 4. KeGFR2 was calculated for 310 patients out of whom 151 children developed AKD (48.7%). The KeGFR2 distribution was similar to KeGFR1, with half the patients having a KeGFR > 60 mL/min/1.73 sm (stages 1 and 2). This analysis once again confirms that AKI severity is associated with higher stages of KeGFR2. Also, AKD severity is associated with decreased levels of KeGFR2. In the subgroup of patients with KeGFR2, we evaluated the distribution of AKI causes. As previously mentioned, a prerenal cause of AKI was the leading one in all KeGFR groups. The results from KeGFR1 and KeGFR2 showed that more than 60% of patients with intrinsic AKI had a KeGFR > 60 mL/min/1.73 sm.

Table 4.
KeGFR2 (derived from days 6 and 7 SCr values) staging and distribution in AKI and AKD stages and AKI causes.
We performed a logistic regression model with AKD-dependent variables and KeGFR1 stages as independent ones (Supplemental Table S1). The unadjusted model proved to be a good one (Nagelkerke R2 = 0.27), with an AUC of 0.763. KeGFR1 stages 3, 4 and 5 increased the risk of AKD development by 2.76, 5.33 and 27.23 times, respectively. After adjusting the regression model for sex, AKI stage and AKI causes, the model improved (Nagelkerke R2 = 0.32, AUC = 0.801), with KeGFR1 increasing the incidence of AKD by 3.07, 6.56 and 28.07 times in stages 3, 4 and 5, respectively.
The logistic regression models with AKD-dependent variables and KeGFR2 stages as independent ones proved to be a good fit in both unadjusted and adjusted (Nagerkerke R2 0.43 and 0.44, AUC = 0.809 and 0.827, respectively). In the unadjusted model, KeGFR2 stages 2, 3, 4 and 5 increased the risk of AKD by 2.65, 3.03, 28.72 and 70.09 times, respectively, and in the adjusted one by 2.79, 3.58, 32.75 and 80.14, respectively (Supplemental Table S2).
MAKE30 of the entire cohort revealed 111 events (13.8%): 53 deaths, 11 patients requiring RRT and 47 children with AKD. Logistic regression with MAKE30 as a dependent variable and KeGFR1 stages proved to be a good fit in both unadjusted and adjusted models (Nagerkerke R2 = 0.18 and 0.23, AUC = 0.675 and 0.764, respectively). Only stage 5 KeGFR1 increased the risk of MAKE30 by 10.42 times in the unadjusted model and by 7.77 times in the adjusted one (Supplemental Table S1). KeGFR2 stages 3, 4 and 5 increased the risk of MAKE30 by 2.8, 4.48 and 43.17, respectively, in unadjusted model and by 4.23, 5.89 and 69.42, respectively, in the adjusted one (Nagelkerke R2 0.37 and 0.43, AUC = 0.809 and 0.837, respectively) (Supplemental Table S2).
KeGFR calculation within the first 24 h of documented AKI, as well as 24 h prior to AKD, predicted AKD development and MAKE30—see Figure 2. KeGFR1 and KeGFR2 predicted AKD with AUC values between 0.777 (95% CI 0.747–0.806) and 0.841 (95% CI 0.795–0.88), p < 0.001. KeGFR2 had the best performance in predicting AKD, with 66.45% and specificity 91.14% with associated criterion ≤ 40.41 mL.min/1.73 sm, as well as in predicting MAKE30 with an AUC of 0.819 (95% CI 0.772–0.861) with a sensitivity of 66.67% and specificity 87.7% with associated criterion ≤ 25.69 mL/min/1.73 sm, p < 0.001. It should be mentioned that the ROC-AUC of KeGFR2 included 310 patients. KeGFR1 had a fair performance in predicting AKD AUC of 0.777 with a sensitivity of 76.15% and specificity of 68.55%, with associated criterion ≤ 39.18 mL/min/1.73 sm. KeGFR1 included all patients (N = 803). KeGFR1 had an AUC of 0.7 (95% CI 0.667–0.731) with an associated criterion of ≤21.28 mL/min/1.73 sm (p < 0.001), a 55.86% sensitivity and 81.65% specificity in predicting MAKE 30.
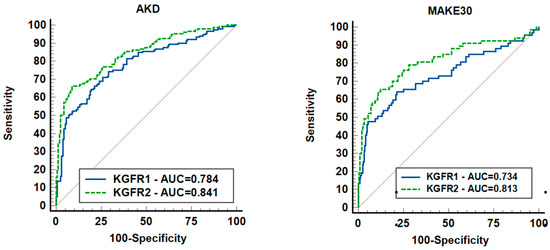
Figure 2.
ROCs showing the ability of KeGFR to predict AKD and MAKE30. Legend: AKD = acute kidney disease; KeGFR = kinetic estimated glomerular filtration rate; MAKE30 = major adverse kidney events; 310 patients were included.
4. Discussion
In this study, KeGFR was analyzed for the first time as a predictor of AKD in children. To best of our knowledge, there are no studies that used KeGFR for AKD prediction in pediatric or adult settings. KeGFR has been used in predicting AKI in high-risk populations [9,10,11,12,14,19,20,21] with a high sensitivity and specificity. Until now, KeGFR has been used as a predictor of AKI; thus, it was calculated prior to AKI occurrence. The retrospective nature of our study allowed us to calculate KeGFR within the first 24 h of identified AKI as well as 24 h prior to AKD using the patient’s baseline SCr measurements. For better estimation of KeGFR, we employed Morgenstern et al.’s recalculation of Mellits and Cheek’s [18] formula to calculate the total body water. As the distribution volume of creatinine is close to total body water, we individualized this equation in order to eliminate any bias. Unlike in adults, any changes in total body water in children will influence SCr levels. Also, to reduce bias, we used Chen’s formula without the correction factor of 1.5 mg/dL corresponding to the maximum 24 h SCr increments in anuric patients with the estimation of maximum 24 h ΔSCr adjusted for individual distribution volume.
Our results showed that 1 in 4 children progressed to AKD (27.14%), hence the importance of predicting AKD. Progression to AKD is a cumulative result of medical intervention and underlying disease severity. As previously reported by KDIGO guidelines in 2012 and the ADQI consensus report, AKD is a sequel of AKI with increased morbidity and mortality with or without progression to CKD [2,15]. With a high progression rate from AKI to AKD, there is an imperative need for new and cheap tools to predict AKD occurrence. In our cohort, children from the AKD group were younger, with higher SCr levels from day 1 to day 7 of identified AKI reflected in lower KeGFR during the documented AKI episode. Our study showed that half the patients had a KeGFR < 60 mL/min/1.73 sm, reflecting AKI severity and AKI duration, consistent with previously reported pediatric data [9,12]. Besides reflecting AKI severity, reduced KeGFR levels were associated with AKD severity. Our results emphasize the importance of KeGFR1 calculation as a predictor of AKI persistence, as 70% of the children that developed AKD had a KeGFR below 60 mL/min/1.73 sm. AKI evolution is dependent on medical intervention (fluid resuscitation, nephrotoxic medication, fluid overload and need for mechanical ventilation) that in most cases leads to renal recovery. However, more than 25% of our patients had a prolonged AKI episode that further contributed to kidney injury. KeGFR1 proved there is a link between reduced day 1 and 2 renal function and AKD development as an independent variable. As expected, KeGFR2 had a higher prediction rate of AKD compared to KeGFR1. Given the medical intervention during the timeframe between the first 24 h of identified AKI and the 24 h prior to AKD development, KeGFR2 proved once again to be an independent risk factor for AKD. For example, a patient with KeGFR1 stage 5 (<15 mL/min/1.73 sm) has a 28-fold higher risk of progressing to AKD; hence, one could use KeGFR2 in dynamic for predicting the severity of AKD as well as for the risk of MAKE30. KeGFR2 is a better predictor for AKD and MAKE30 as a result of prolonged kidney injury. More than 70% of the patients with AKD had a KeGFR2 below 60 mL/min/1.73 sm, reflecting the transition from AKI state to AKD. The risk of AKD and MAKE30 starts from KeGFR2 stage 3 (<60 mL/min/1.73 sm) as opposed to stage 5 KeGFR1 (<15 mL/min/1.73 sm). With these results, we highlight the utility of repeated kinetic measurements. We suggest careful renal function monitoring in all AKI patients, especially in those with KeGFR1 stage 5 (<15 mL/min/1.73 sm) and KeGFR2 stages 3, 4 and 5 (<60 mL/min/1.73 sm), using repeated SCr measurements.
In clinical settings, several markers proved to be effective in predicting AKI, including KeGFR. For instance, Dewitte et al. [22] showed that KeGFR had a better performance in predicting AKI compared to plasma neutrophil gelatinase-associated lipocalin (NGAL), renal resistive index and product between tissue inhibitor of metalloproteinase-2 and urine insulin-like growth factor-binding protein 7. Hekmat et al. found KeGFR a better AKI predictor compared to other creatinine-based formulas with a strong correlation between KeGFR and NGAL [23]. While it is counterproductive to use specific and expensive biomarkers of kidney injury in clinical practice, in this study, we underline the utility of KeGFR as a cheap and handy tool.
Current studies have failed to find a significant relationship between reduced KeGFR and 30-day mortality [11,24], while Dewitte showed a good prediction of MAKE using KeGFR based model [22] in ICU settings. Our results showed there is a link between reduced KeGFR and a high risk of MAKE30 development. The KeGFR2 model proved to be superior compared to KeGFR1, proving the utility of KeGFR calculation once again in predicting worse renal outcomes. AKI evolution is marked by several outcomes: rapid renal recovery with return to baseline SCr, delayed recovery (up to 90 days), and progression towards CKD or death. When renal recovery does not occur during hospitalization, with progression to AKD, the second KeGFR calculation (days 6 and 7 after AKI diagnosis) proved to be a better predictor of worse renal outcome (AKD development, mortality and AKD at 30 days).
The KeGFR1 model proved to be fair in predicting MAKE30, with an AUC of 0.7, while the KeGFR2 model proved to be good, with an AUC of 0.819. For instance, Dewitte found a similar predictive value of KeGFR for MAKE in the adult population, with an AUC of 0.81 [22].
In our opinion, when SCr increases rapidly, kinetic modeling of GFR proves to be a better predictor of AKI outcomes, AKD development and worst renal outcomes at 30 days. One should mention the clinical usefulness of KeGFR, regardless the time of calculation, in both AKI-identified patients and in at-risk AKI patients, as previously reported (i.e., in ICU settings) [9,12,14].
The strength of this study is supported by the high number of AKI cases from a mixed pediatric population, both critically ill and non-critically ill, including neonates. Our electronic data system allowed us to identify baseline SCr, making it the first study to include baseline SCr and baseline eGFR in kinetic estimation formula in children, and to include all patients with consecutive SCr measurements within the first 24 h of identified AKI and the 24 h prior to AKD. In addition, we were able to adjust the formula of KeGFR to the maximum daily creatinine production using Morgenstern et al.’s recalculation of Mellits and Cheek’s equation based on height and sex. The strongest point was the use of this tool in order to predict AKD and MAKE30. The limitations were the lack of urine output criteria, single center and retrospective design.
Kinetic modeling of eGFR can be used as a complementary tool in AKI settings. KeGFR has already been validated as a severe AKI predictor providing a good prognostic and diagnostic approach. There is an imperative need for prospective studies in the pediatric population in order to validate our results. We suggest that KeGFR measurements could be employed in pediatric intensive care units for risk stratification in patients with or without AKI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12196314/s1, Table S1. Logistic regression with AKD and MAKE30 dependant variable with KeGFR1 stages; Table S2. Logistic regression with AKD and MAKE30 dependant variable with KeGFR2 stages.
Author Contributions
F.C. and M.G. contributed equally to this article. Conceptualization, F.C. and M.G.; Methodology, F.C., M.G. and R.S.; Software L.C.; Validation, A.S.; Formal Analysis, F.C., M.G. and L.C.; Investigation, F.C. and M.G.; Resources, F.C., M.G. and R.S.; Data Curation, L.C.; Writing—Original Draft Preparation, F.C. and M.G.; Writing—Review and Editing, R.S. and A.S.; Visualization, R.S.; Supervision, A.S.; Project Administration, F.C. and M.G.; Funding Acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of “Louis Turcanu” Clinical Emergency Hospital for Children from Timisoara, Romania (protocol code 6055, date of approval: 24 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data collected for this study will be available for others, at request directly to the corresponding author. The data that will be available is represented by deidentified participant data. The inform consent form and statistical analysis plan will be available at request. The data will be available with publication. The data will be available at request at the e–mail address chisavulazar@gmail.com. The data will be shared after direct request and after approval of the proposal by all the authors.
Acknowledgments
The authors would like to thank ‘Louis Turcanu’ Hospital’s Medical Ethics Committee and the Laboratory Information System for the invaluable advice and support during statistical analysis. Current use of KeGFR proved to be useful in non-steady states. We demonstrate the potential utility of KeGFR in predicting AKD and MAKE30.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meena, J.; Mathew, G.; Kumar, J.; Chanchlani, R. Incidence of Acute Kidney Injury in Hospitalized Children: A Meta-analysis. Pediatrics 2023, 151, e2022058823. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Heipertz, A.; Joyce, E.; Kellum, J.A.; Horvat, C.; Squires, J.E.; West, S.C.; Priyanka, P.; Fuhrman, D.Y. Acute kidney disease predicts chronic kidney disease in pediatric non-kidney solid organ transplant patients. Pediatr. Transplant. 2022, 26, e14172. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Hornik, C.; Diamantidis, C.; Selewski, D.T.; Gbadegesin, R. Patient level factors increase risk of acute kidney disease in hospitalized children with acute kidney injury. Pediatr. Nephrol. 2023, 38, 3465–3474. [Google Scholar] [CrossRef]
- Murdeshwar, A.; Krishnamurthy, S.; Parameswaran, N.; Rajappa, M.; Deepthi, B.; Krishnasamy, S.; Ganapathy, S.; Karunakar, P. Etiology and outcomes of acute kidney disease in children: A cohort study. Clin. Exp. Nephrol. 2023, 27, 548–556. [Google Scholar] [CrossRef]
- Deng, Y.H.; Yan, P.; Zhang, N.Y.; Luo, X.Q.; Wang, X.F.; Duan, S.B. Acute Kidney Disease in Hospitalized Pediatric Patients With Acute Kidney Injury in China. Front. Pediatr. 2022, 10, 885055. [Google Scholar] [CrossRef]
- LoBasso, M.; Schneider, J.; Sanchez-Pinto, L.N.; Del Castillo, S.; Kim, G.; Flynn, A.; Sethna, C.B. Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr. Nephrol. 2022, 37, 659–665. [Google Scholar] [CrossRef]
- Daraskevicius, J.; Azukaitis, K.; Dziugeviciute-Tupko, J.; Peciulyte, M.; Planciunaite, R.; Vaitkeviciene, G.; Rascon, J.; Jankauskiene, A. Phenotypes and Baseline Risk Factors of Acute Kidney Injury in Children After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2020, 8, 499. [Google Scholar] [CrossRef]
- Dasgupta, M.N.; Montez-Rath, M.E.; Hollander, S.A.; Sutherland, S.M. Using kinetic eGFR to identify acute kidney injury risk in children undergoing cardiac transplantation. Pediatr. Res. 2021, 90, 632–636. [Google Scholar] [CrossRef]
- Seelhammer, T.G.; Maile, M.D.; Heung, M.; Haft, J.W.; Jewell, E.S.; Engoren, M. Kinetic estimated glomerular filtration rate and acute kidney injury in cardiac surgery patients. J. Crit. Care 2016, 31, 249–254. [Google Scholar] [CrossRef]
- de Oliveira Marques, F.; Oliveira, S.A.; de Lima e Souza, P.F.; Nojoza, W.G.; da Silva Sena, M.; Ferreira, T.M.; Costa, B.G.; Libório, A.B. Kinetic estimated glomerular filtration rate in critically ill patients: Beyond the acute kidney injury severity classification system. Crit. Care 2017, 21, 280. [Google Scholar] [CrossRef]
- Menon, S.; Basu, R.K.; Barhight, M.F.; Goldstein, S.L.; Gist, K.M. Utility of Kinetic GFR for Predicting Severe Persistent AKI in Critically Ill Children and Young Adults. Kidney360 2021, 2, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Retooling the Creatinine Clearance Equation to Estimate Kinetic GFR when the Plasma Creatinine Is Changing Acutely. J. Am. Soc. Nephrol. 2013, 24, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Latha, A.V.; Rameshkumar, R.; Bhowmick, R.; Rehman, T. Kinetic Estimated Glomerular Filtration Rate and Severity of Acute Kidney Injury in Critically Ill Children. Indian J. Pediatr. 2020, 87, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Schwartz, G.J.; Haycock, G.B.; Edelmann, C.M., Jr.; Spitzer, A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976, 58, 259–263. [Google Scholar] [CrossRef]
- Morgenstern, B.; Mahoney, D.; Warady, B. Estimating total body water in children on the basis of height and weight: A reevaluation of the formulas of Mellits and Cheek. J. Am. Soc. Nephrol. 2002, 13, 1884–1888. [Google Scholar] [CrossRef]
- Libório, A.B.; Macedo, E.; Bayas de Queiroz, R.E.; Leite, T.T.; Queiroz Rocha, I.C.; Freitas, I.A.; Correa, L.C.; Bessa Campelo, C.P.; Araújo, F.S.; de Albuquerque, C.A.; et al. Kidney Disease Improving Global Outcomes or creatinine kinetics criteria in acute kidney injury: A proof of concept study. Nephrol. Dial. Transplant. 2013, 28, 2779–2787. [Google Scholar] [CrossRef][Green Version]
- Waikar, S.S.; Bonventre, J.V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 672–679. [Google Scholar] [CrossRef]
- Kwong, Y.D.; Chen, S.; Bouajram, R.; Li, F.; Matthay, M.A.; Mehta, K.M.; Glidden, D.V.; Liu, K.D. The value of kinetic glomerular filtration rate estimation on medication dosing in acute kidney injury. PLoS ONE 2019, 14, e0225601. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Joannes-Boyau, O.; Sidobre, C.; Fleureau, C.; Bats, M.L.; Derache, P.; Leuillet, S.; Ripoche, J.; Combe, C.; Ouattara, A. Kinetic eGFR and Novel AKI Biomarkers to Predict Renal Recovery. Clin. J. Am. Soc. Nephrol. 2015, 10, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, R.; Eshraghi, H.; Esmailpour, M.; Hassankhani, G.G. Kinetic Glomerular Filtration Rate Estimation Compared With Other Formulas for Evaluating Acute Kidney Injury Stage Early After Kidney Donation. Exp. Clin. Transplant. 2017, 15 (Suppl. 1), 104–109. [Google Scholar] [PubMed]
- O’Sullivan, E.D.; Doyle, A. The clinical utility of kinetic glomerular filtration rate. Clin. Kidney J. 2017, 10, 202–208. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).