Inflammatory Markers as Predictors of Atrial Fibrillation Recurrence: Exploring the C-Reactive Protein to Albumin Ratio in Cryoablation Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analyses
2.3. Follow up and Outcome
2.4. Cryoballoon Ablation Procedure
2.5. Medical Treatment
2.6. Statistical Analysis
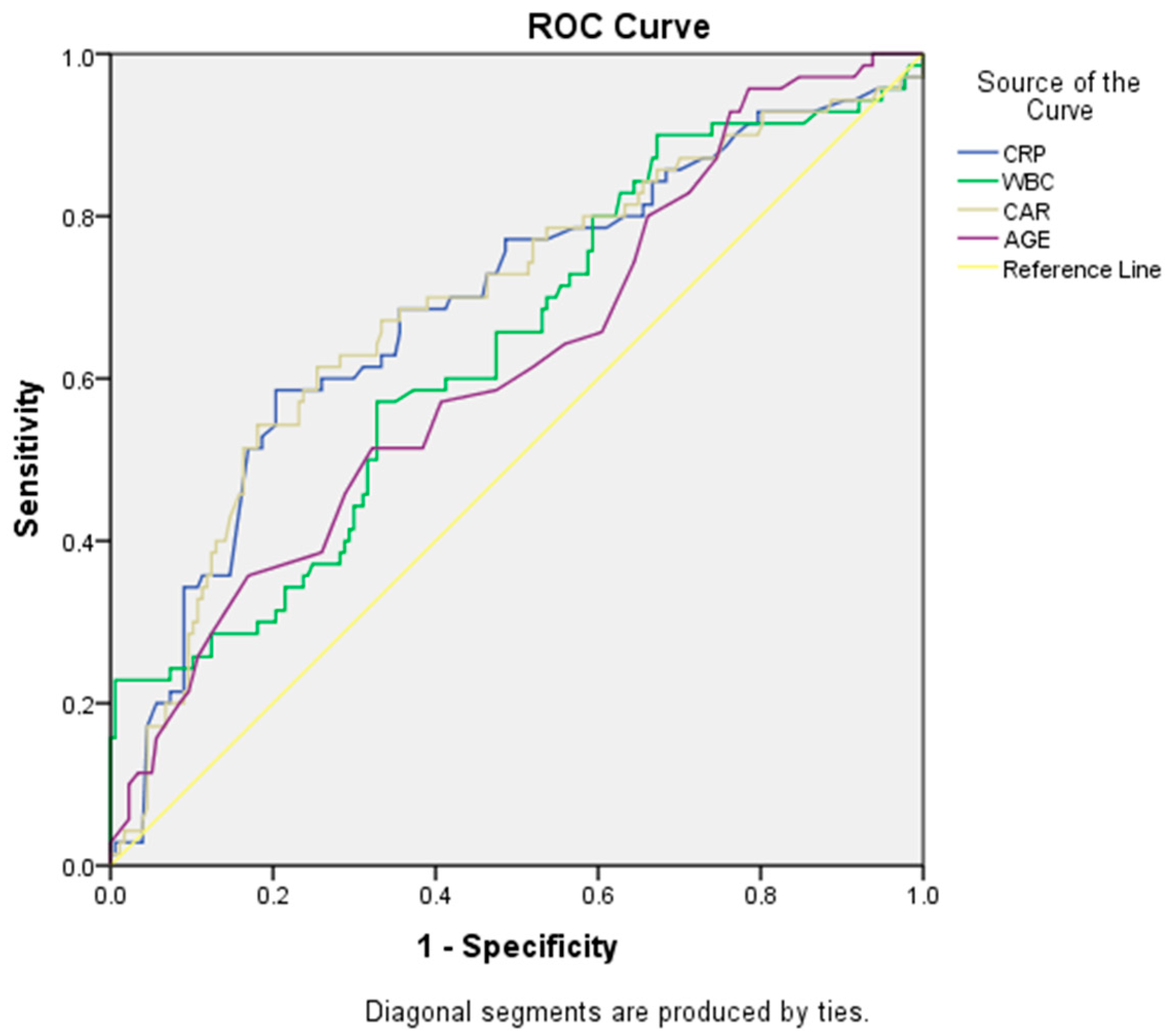
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The Clinical Profile and Pathophysiology of Atrial Fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Aguilar, M.; Atzema, C.; Bell, A.; Cairns, J.A.; Cheung, C.C.; Cox, J.L.; Dorian, P.; Gladstone, D.J.; Healey, J.S.; et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can. J. Cardiol. 2020, 36, 1847–1948. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Margulescu, A.D.; Mont, L. Persistent atrial fibrillation vs paroxysmal atrial fibrillation: Differences in management. Expert Rev. Cardiovasc. Ther. 2017, 15, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef]
- Sheinenzon, A.; Shehadeh, M.; Regina, M.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, E.; Cairns, E.; Hamilton, J.; Kelly, C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 2009, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y.; Miyazaki, K.; Yoshikawa, M.; Yamada, S.; Saito, Y.; Ikemoto, T.; Imura, S.; Morine, Y.; Shimada, M. Value of the CRP–albumin ratio in patients with resectable pancreatic cancer. J. Med. Investig. 2021, 68, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Tu, J.; Liu, L.; Luo, L.; Wu, J.; Tao, L.; Zhang, C.; Geng, X.; Chen, X.; Ai, X.; et al. Serum Albumin and C-Reactive Protein/Albumin Ratio Are Useful Biomarkers of Crohn’s Disease Activity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4393–4400. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Zampieri, F.G.; Forte, D.N.; Azevedo, L.C.P.; Park, M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS ONE 2013, 8, e59321. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Okamoto, T. ASO Author Reflections: The C-Reactive Protein (CRP)-Albumin Ratio May Be Useful as the Most Prognostic Index Among the Immuno-nutritional Parameters Using CRP and Albumin for Resected NSCLC. Ann. Surg. Oncol. 2021, 28, 3055–3056. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lip, G.Y.; Apostolakis, S. Inflammation in Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lip, G.Y.; Apostolakis, S. Inflammatory Biomarkers and Atrial Fibrillation: Potential Role of Inflammatory Pathways in the Pathogenesis of Atrial Fibrillation-induced Thromboembolism. Curr. Vasc. Pharmacol. 2015, 13, 192–201. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Aksoy, F.; Uysal, D.; Ibrişim, E. Relationship between c-reactive protein/albumin ratio and new-onset atrial fibrillation after coronary artery bypass grafting. Rev. Assoc. Medica Bras. 1992 2020, 66, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Karavelioğlu, Y.; Karapınar, H.; Yüksel, M.; Memiç, K.; Sarak, T.; Kurt, R.; Yilmaz, A. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin. Appl. Thromb. Off. J. Int. Acad. Clin. Appl. Thromb. 2015, 21, 5–9. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Bahnson, T.D.; Giczewska, A.; Mark, D.B.; Russo, A.M.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Poole, J.E.; Lee, K.L.; Packer, D.L.; et al. Association Between Age and Outcomes of Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: Results From the CABANA Trial. Circulation 2022, 145, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Liu, P.; Zhang, F.; Hui, J.; He, L. Predicting Values of Neutrophil-to-Lymphocyte Ratio (NLR), High-Sensitivity C-Reactive Protein (hs-CRP), and Left Atrial Diameter (LAD) in Patients with Nonvalvular Atrial Fibrillation Recurrence After Radiofrequency Ablation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 27, e934569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kwon, O.-S.; Shim, J.; Lee, J.; Han, H.-J.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; et al. Left Atrial Wall Stress and the Long-Term Outcome of Catheter Ablation of Atrial Fibrillation: An Artificial Intelligence-Based Prediction of Atrial Wall Stress. Front. Physiol. 2021, 12, 686507. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Rosanio, S.; Augello, G.; Gallus, G.; Vicedomini, G.; Mazzone, P.; Gulletta, S.; Gugliotta, F.; Pappone, A.; Santinelli, V.; et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: Outcomes from a controlled nonrandomized long-term study. J. Am. Coll. Cardiol. 2003, 42, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Buist, T.J.; Zipes, D.P.; Elvan, A. Atrial fibrillation ablation strategies and technologies: Past, present, and future. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, T.; Kofune, M.; Nagashima, K.; Mano, H.; Sonoda, K.; Kasamaki, Y.; et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: Importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J. Cardiovasc. Electrophysiol. 2011, 22, 987–993. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Jaroonpipatkul, S.; Trongtorsak, A.; Kewcharoen, J.; Thangjui, S.; Pokawattana, A.; Navaravong, L. High sensitivity C reactive protein levels and atrial fibrillation recurrence after catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J. Arrhythmia 2023, 39, 515–522. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Xiong, B.; Li, D.; Wang, J.; Gyawali, L.; Jing, J.; Su, L. The Effect of Catheter Ablation on Left Atrial Size and Function for Patients with Atrial Fibrillation: An Updated Meta-Analysis. PLoS ONE 2015, 10, e0129274. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Lin, X. Serum albumin level for prediction of all-cause mortality in acute coronary syndrome patients: A meta-analysis. Biosci. Rep. 2020, 40, BSR20190881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, P.; Xiao, Q.; Li, J.; Liu, X.; Tan, J.; Zhang, X. Relationship Between Serum Albumin and Risk of Atrial Fibrillation: A Dose-Response Meta-Analysis. Front. Nutr. 2021, 8, 728353. [Google Scholar] [CrossRef] [PubMed]
- Joles, J.A.; Willekes-Koolschijn, N.; Koomans, H.A. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997, 52, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Çinier, G.; Bakırcı, E.M.; Duman, H.; Şimşek, Z.; Hamur, H.; Değirmenci, H.; Emlek, N. Relationship Between C-Reactive Protein to Albumin Ratio and Thrombus Burden in Patients With Acute Coronary Syndrome. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029618824418. [Google Scholar] [CrossRef] [PubMed]
- Çınar, T.; Çağdaş, M.; Rencüzoğulları, I.; Karakoyun, S.; Karabağ, Y.; Yesin, M.; Çağdaş, S.; Tanboğa, H. Prognostic efficacy of C-reactive protein/albumin ratio in ST elevation myocardial infarction. Scand. Cardiovasc. J. 2019, 53, 83–90. [Google Scholar] [CrossRef]
| No Recurrence Group | AF Recurrence Group | p Value | |
|---|---|---|---|
| n = 180 | n = 69 | ||
| Age (year) | 57.2 ± 9.9 | 62.5 ± 8.4 | 0.001 |
| Male | 88 (48%) | 30 (44%) | 0.401 |
| Hypertension | 74 (41%) | 38 (55%) | 0.102 |
| DM | 34 (18%) | 16 (23%) | 0.601 |
| CAD | 42 (23%) | 13 (19%) | 0.399 |
| Thyroid disorder | 4 (2.2%) | 2 (2.8%) | 0.753 |
| Smokers | 14 (7%) | 6 (8%) | 0.912 |
| BMI (kg/m2) | 24.4 ± 2.7 | 25.9 ± 2.3 | 0.463 |
| CHA2DS2-VASc score | 1.84 | 1.85 | 0.851 |
| GFR mL/min/1.73 m2 | 94.2 ± 20.5 | 93.1 ± 20.5 | 0.756 |
| Albumin g/L | 4.3 ± 0.3 | 4.3 ± 0.4 | 0.464 |
| Triglyceride (mg/dL) | 111.3 ± 54.5 | 119.7 ± 68.1 | 0.197 |
| LDL (mg/dL) | 119.3 ± 35.4 | 118.6 ± 35.3 | 0.857 |
| HDL (mg/dL) | 36.5 ± 10.8 | 37.1 ± 9.1 | 0.553 |
| Total cholesterol (mg/dL) | 178.5 ± 45.2 | 185.1 ± 45.3 | 0.113 |
| Serum glucose (mg/dL) | 195.4 ± 89.6 | 183.6 ± 62.0 | 0.301 |
| Hemoglobin (g/L) | 14.1 ± 1.9 | 14.2 ± 2.0 | 0.933 |
| Platelet (/mm3) | 239.5 ± 62.5 | 263.7 ± 69.7 | 0.011 |
| WBC (103/uL) | 7.8 ± 2.3 | 9.4 ± 3.9 | 0.002 |
| Neutrophil (%) | 5.1 ± 2.2 | 6.7 ± 3.6 | 0.001 |
| Lymphocyte (%) | 2.1 ± 0.9 | 1.9 ± 0.8 | 0.132 |
| Hs-CRP mg/L | 5.2 ± 1.3 | 9.4 ± 2.8 | <0.001 |
| CAR | 1.213 (1.07–1.443) | 2.238 (1.792–2.765) | <0.001 |
| Lowest temperature achieved during ablation (°C) | |||
| LSPV | −44 ± 4 | −46 ± 2 | 0.322 |
| LIPV | −45 ± 3 | −44 ± 3 | 0.843 |
| RSPV | −50 ± 2 | −49 ± 4 | 0.334 |
| RIPV | −48 ± 2 | −46 ± 4 | 0.344 |
| Echocardiographic findings | |||
| LVEF (%) | 53.9 ± 7.4 | 54.6 ± 8.7 | 0.248 |
| LA diameter (cm) | 4.0 ± 0.5 | 4.2 ± 0.7 | 0.001 |
| Variables | Unadjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age | 1.058 | 1.024–1.093 | 0.001 | 1.051 | 1.005–1.100 | 0.029 |
| BMI | 0.937 | 0.867–1.014 | 0.104 | |||
| Male | 0.641 | 0.401–1.027 | 0.064 | |||
| CAD | 0.456 | 0.391–1.524 | 0.456 | |||
| Hypertension | 1.224 | 0.816–1.835 | 0.328 | |||
| Smoking | 1.140 | 0.765–1.701 | 0.519 | |||
| WBC | 1.201 | 1.092–1.322 | <0.001 | 1.163 | 0.787–1.719 | 0.449 |
| Neutrophil | 1.239 | 1.114–1.378 | 0.001 | 1.069 | 0.709–1.611 | 0.751 |
| CAR | 1.409 | 1.183–1.678 | <0.001 | 1.661 | 1.304–2.116 | <0.001 |
| LA diameters on admission | 0.968 | 0.948–0.989 | 0.002 | 0.990 | 0.965–1.015 | 0.414 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, E.; Elcik, D.; Barutcu, S.; Kelesoglu, S.; Alp, M.E.; Ozan, R.; Capar, G.; Turkmen, O.; Cinier, G.; Polat, V.; et al. Inflammatory Markers as Predictors of Atrial Fibrillation Recurrence: Exploring the C-Reactive Protein to Albumin Ratio in Cryoablation Patients. J. Clin. Med. 2023, 12, 6313. https://doi.org/10.3390/jcm12196313
Ozkan E, Elcik D, Barutcu S, Kelesoglu S, Alp ME, Ozan R, Capar G, Turkmen O, Cinier G, Polat V, et al. Inflammatory Markers as Predictors of Atrial Fibrillation Recurrence: Exploring the C-Reactive Protein to Albumin Ratio in Cryoablation Patients. Journal of Clinical Medicine. 2023; 12(19):6313. https://doi.org/10.3390/jcm12196313
Chicago/Turabian StyleOzkan, Eyup, Deniz Elcik, Suleyman Barutcu, Saban Kelesoglu, Murat Erdem Alp, Ramazan Ozan, Gazi Capar, Omer Turkmen, Goksel Cinier, Veli Polat, and et al. 2023. "Inflammatory Markers as Predictors of Atrial Fibrillation Recurrence: Exploring the C-Reactive Protein to Albumin Ratio in Cryoablation Patients" Journal of Clinical Medicine 12, no. 19: 6313. https://doi.org/10.3390/jcm12196313
APA StyleOzkan, E., Elcik, D., Barutcu, S., Kelesoglu, S., Alp, M. E., Ozan, R., Capar, G., Turkmen, O., Cinier, G., Polat, V., Inanc, M. T., Kepez, A., & Akgun, T. (2023). Inflammatory Markers as Predictors of Atrial Fibrillation Recurrence: Exploring the C-Reactive Protein to Albumin Ratio in Cryoablation Patients. Journal of Clinical Medicine, 12(19), 6313. https://doi.org/10.3390/jcm12196313





