Utility of Covered Self-Expanding Metal Stents for Biliary Drainage during Neoadjuvant Chemotherapy in Patients with Borderline Resectable Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Strategy of Biliary Drainage
2.3. Neoadjuvant Chemotherapy
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Complications Related to Biliary Stent Placement
3.3. Clinical Course during Neoadjuvant Chemotherapy
3.4. Outcomes of Surgery and Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Editorial Board of the Cancer Statistics in Japan. Cancer Registry and Statistics. Cancer Information Service NCCJ. Cancer Statics in Japan 2019. Foundation for Promotion of Cancer Research (FPCR). 2020. Available online: https://ganjoho.jp/en/professional/statistics/brochure/2019_en.html (accessed on 12 November 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology—Pancreatic Adenocarcinoma. Version 1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 15 November 2022).
- Kato, H.; Usui, M.; Isaji, S.; Nagakawa, T.; Wada, K.; Unno, M.; Nakao, A.; Miyakawa, S.; Ohta, T. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: A multi-institutional survey by the Japanese Society of Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2013, 20, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: A synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.F.; Fremgen, A.; Menck, H.R.; Winchester, D.P. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J. Am. Coll. Surg. 1999, 189, 1–7. [Google Scholar] [CrossRef]
- Isayama, H.; Yasuda, I.; Ryozawa, S.; Maguchi, H.; Igarashi, Y.; Matsuyama, Y.; Katanuma, A.; Hasebe, O.; Irisawa, A.; Itoi, T.; et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered Wallstent versus DoubleLayer stent. Dig. Endosc. 2011, 23, 310–315. [Google Scholar] [CrossRef]
- Kubota, K.; Sato, T.; Watanabe, S.; Hosono, K.; Kobayashi, N.; Mori, R.; Taniguchi, K.; Matsuyama, R.; Endo, I.; Nakajima, A. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig. Endosc. 2014, 26, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Sasaki, T.; Serikawa, M.; Ishii, Y.; Mouri, T.; Shimizu, A.; Kurihara, K.; Tatsukawa, Y.; Miyaki, E.; Kawamura, R.; et al. Preoperative biliary drainage in cases of borderline resectable pancreatic cancer treated with neoadjuvant chemotherapy and surgery. Gastroenterol. Res. Pract. 2016, 2016, 7968201. [Google Scholar] [CrossRef] [PubMed]
- Kuwatani, M.; Nakamura, T.; Hayashi, T.; Kimura, Y.; Ono, M.; Motoya, M.; Imai, K.; Yamakita, K.; Goto, T.; Takahashi, K.; et al. Clinical Outcomes of Biliary Drainage during a neoadjuvant Therapy for Pancreatic Cancer: Metal versus Plastic Stents. Gut Liver 2020, 14, 269–273. [Google Scholar] [CrossRef]
- Tamura, T.; Itonaga, M.; Ashida, R.; Yamashita, Y.; Hatamaru, K.; Kawaji, Y.; Emori, T.; Kitahata, Y.; Miyazawa, M.; Hirono, S.; et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patient receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: Prospective randomized study. Dig. Endosc. 2021, 33, 1170–1178. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobara, H.; Kamada, H.; Kohno, T.; Namima, D.; Fujita, N.; Yamana, H.; Fujihara, S.; Okano, K.; Masaki, T. Comparison of plastic stent versus metal stent in preoperative biliary drainage for pancreatic head cancer with neoadjuvant chemoradiotherapy. J. Hepatobiliary Pancreat. Sci. 2021, 28, 856–863. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kubota, K.; Yagi, S.; Kurita, Y.; Sato, T.; Hosono, K.; Matsuyama, R.; Endo, I.; Kobayashi, N.; Nakajima, A. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2021, 28, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Vehviläinen, S.; Seppänen, H.; Nurmi, A.; Haglund, C.; Mustonen, H.; Udd, M.; Kylänpää, L. Use of self-expandable metallic stents for endoscopic biliary decompression decreases stent complications in pancreatic cancer patients receiving chemotherapy. Surg. Endosc. 2022, 36, 614–620. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Takahashi, S.; Sueda, T. Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother. Pharmacol. 2017, 79, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Sumiyoshi, T.; Okada, K.; Seo, S.; Otsuka, H.; Murakami, Y.; Takahashi, S. A phase II study of gemcitabine/nab-paclitaxel/S-1 combination neoadjuvant chemotherapy for patients with borderline resectable pancreatic cancer with arterial contact. Eur. J. Cancer 2021, 159, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Hamada, T.; Yasuda, I.; Itoi, T.; Ryozawa, S.; Nakai, Y.; Kogure, H.; Koike, K. Tokyo criteria 2014 for transpapillary biliary stenting. Dig. Endosc. 2015, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.-Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: A prospective, randomized, open-label, multicenter phase 2/3 trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.; Besselink, M.; Bonsing, B.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: Results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Katz, M.H.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; Marsh, R.W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Yokoyama, Y.; Fujii, T.; Yamada, S.; Takami, H.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Maeda, O.; Ogawa, H.; et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann. Surg. 2022, 275, 1043–1049. [Google Scholar] [CrossRef]
- Ielpo, B.; Duran, H.; Diaz, E.; Fabra, I.; Caruso, R.; Ferri, V.; Malavé, L.; Hidalgo, M.; Alvarez, R.; Plaza, C.; et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur. J. Surg. Oncol. 2016, 42, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Janssen, Q.P.; van Dam, J.L.; Bonsing, B.A.; Bos, H.; Bosscha, K.P.; Coene, P.P.L.O.; van Eijck, C.H.J.; de Hingh, I.H.J.T.; Karsten, T.M.; van der Kolk, M.B.; et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): Study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer 2021, 21, 300. [Google Scholar]
- Yamada, D.; Kobayashi, S.; Takahashi, H.; Akita, H.; Yamada, T.; Asaoka, T.; Shimizu, J.; Takeda, Y.; Yokoyama, S.; Tsujie, M.; et al. Randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine and nanoparticle albumin-bound paclitaxel combination therapy as neoadjuvant chemotherapy for resectable/borderline resectable pancreatic ductal adenocarcinoma (PDAC-GS/GA-rP2, CSGO-HBP-015). Trials 2021, 22, 568. [Google Scholar] [PubMed]
- Xia, M.X.; Zhou, Y.F.; Zhang, M.; Wang, W.; Wu, J.; Wang, T.T.; Zhang, X.; Hu, B. Influence of fully covered metal stenting on the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis: A large multicenter study. J. Gastroenterol. Hepatol. 2020, 35, 2256–2263. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, Y.; Yang, H.; Hong, J. Prevention of pancreatitis after stent implantation for distal malignant biliary strictures: Systematic review and meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2022, 16, 141–154. [Google Scholar] [CrossRef]
- Artifon, E.L.; Sakai, P.; Ishioka, S.; Marques, S.B.; Lino, A.S.; Cunha, J.E.M.; Jukemura, J.; Cecconello, I.; Carrilho, F.J.; Opitz, E.; et al. Endoscopic sphincterotomy before deployment of covered metal stent is associated with greater complication rate: A prospective randomized control trial. J. Clin. Gastroenterol. 2008, 42, 815–819. [Google Scholar] [CrossRef]
- Hayashi, T.; Kawakami, H.; Osanai, M.; Ishiwatari, H.; Naruse, H.; Hisai, H.; Yanagawa, N.; Kaneto, H.; Koizumi, K.; Sakurai, T.; et al. No benefit of endoscopic sphincterotomy before biliary placement of self-expandable metal stents for unresectable pancreatic cancer. Clin. Gastroenterol. Hepatol. 2015, 13, 1151–1158.e2. [Google Scholar] [CrossRef]
- Nakahara, K.; Michikawa, Y.; Morita, R.; Suetani, K.; Morita, N.; Sato, J.; Tsuji, K.; Ikeda, H.; Matsunaga, K.; Watanabe, T.; et al. Endoscopic sphincterotomy before fully covered metal stent placement is not required for distal malignant biliary stricture due to a pancreatic head tumor. Gastroenterol. Res. Pract. 2019, 9675347. [Google Scholar] [CrossRef]
- Jang, S.; Stevens, T.; Parsi, M.; Lopez, R.; Zuccaro, G.; Dumot, J.; Vargo, J.J. Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest. Endosc. 2018, 87, 1061–1070. [Google Scholar] [CrossRef]
- Seo, D.W.; Sherman, S.; Dua, K.S.; Slivka, A.; Roy, A.; Costamagna, G.; Deviere, J.; Peetermans, J.; Rousseau, M.; Nakai, Y.; et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: A randomized trial. Gastrointest. Endosc. 2019, 90, 602–612.e4. [Google Scholar] [CrossRef]
- Cavell, L.K.; Allen, P.J.; Vinoya, C.; Eaton, A.A.; Gonen, M.; Gerdes, H.; Mendelsohn, R.B.; D’Angelica, M.I.; Kingham, P.T.; Fong, Y.; et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am. J. Gastroenterol. 2013, 108, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | CSEMS Group (n = 21) | PS Group (n = 19) | p Value |
|---|---|---|---|
| Median age (years) | 63 (44–82) | 61 (41–90) | 0.456 |
| Sex (male to female ratio) | 12:9 | 8:11 | 0.527 |
| Tumor size (mm) | 30 (20–50) | 30 (18–40) | 0.345 |
| Tumor location (head/body/tail) | 20/1/0 | 19/0/0 | 1.000 |
| Classification (BRPC-A/BRPC-PV) | 13/8 | 12/7 | 0.935 |
| Serological findings before drainage | |||
| T-Bil (mg/dL) | 2.7 (0.3–23.3) | 5.3 (0.5–26.4) | 0.704 |
| AST (IU/L) | 128 (13–818) | 160 (18–321) | 0.650 |
| ALT (IU/L) | 232 (12–1212) | 296 (14–560) | 0.884 |
| ALP (IU/L) | 1251 (84–3577) | 609 (117–3857) | 0.180 |
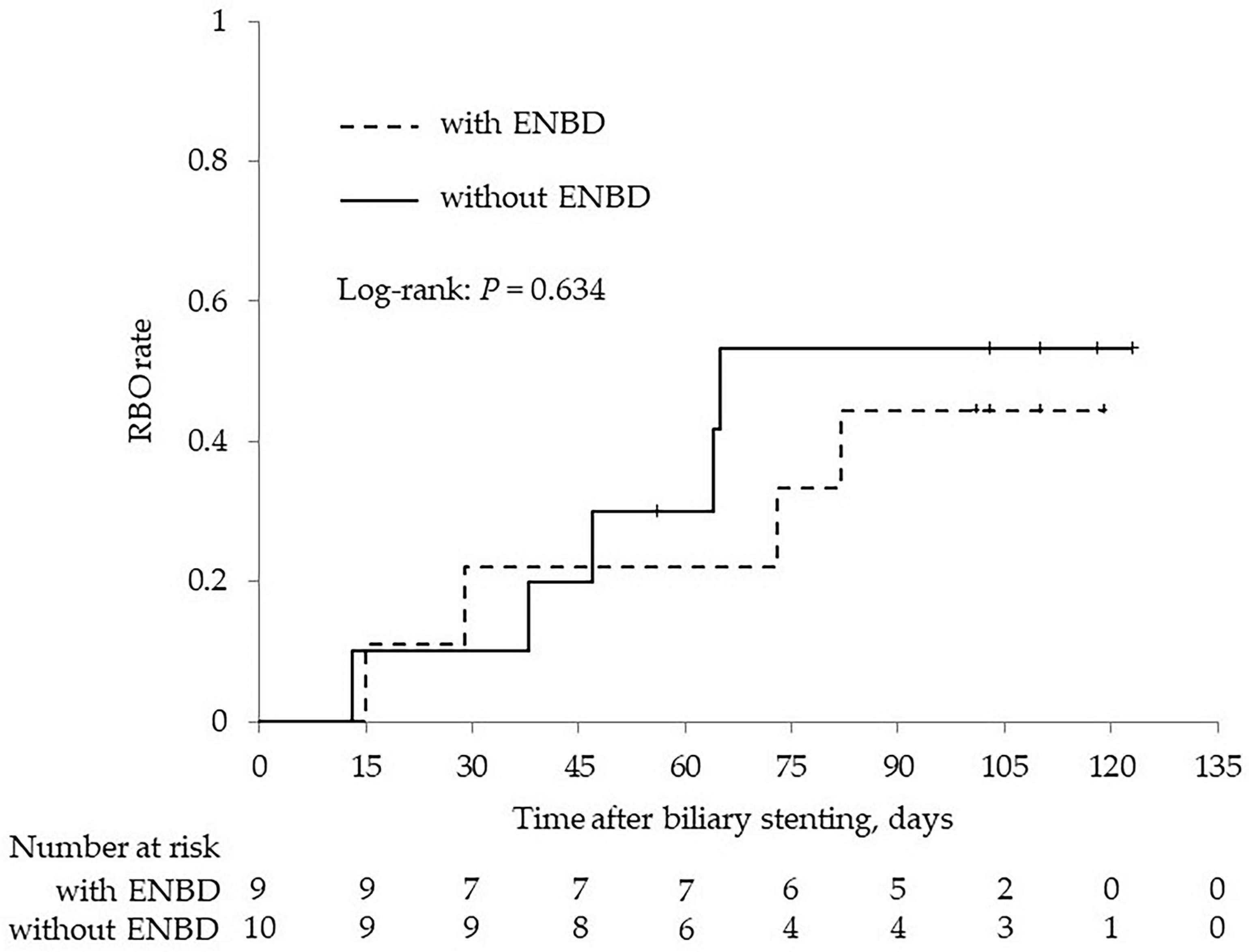
| ENBD before stenting | 21 (100) | 9 (47.4) | <0.001 |
| Neoadjuvant chemotherapy regimen | 0.554 | ||
| Gemcitabine plus S-1 | 8 (38.1) | 9 (47.4) | |
| Gemcitabine, nab-paclitaxel plus S-1 | 13 (61.9) | 10 (52.6) |
| CSEMS Group (n = 21) | PS Group (n = 19) | p Value | |
|---|---|---|---|
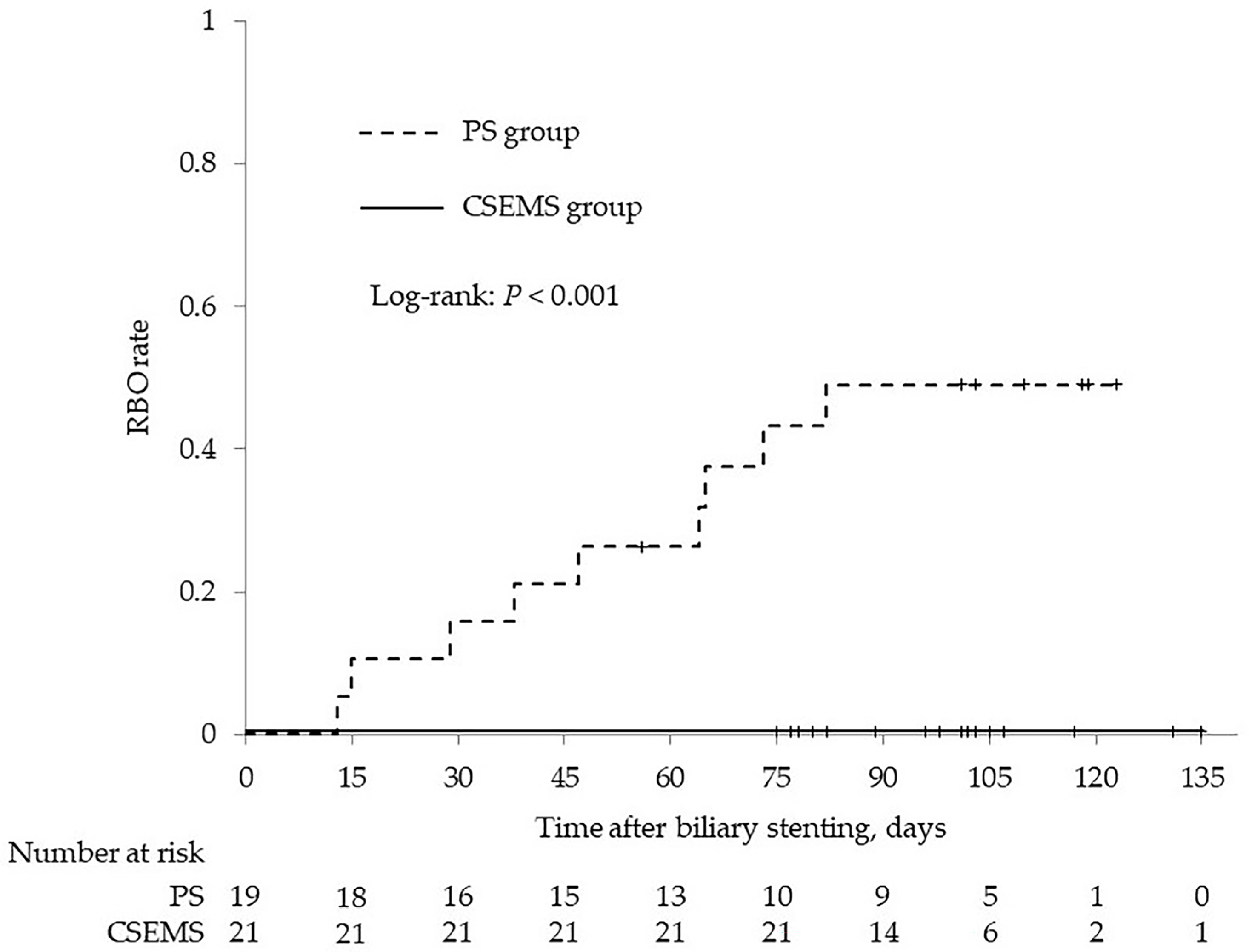
| Recurrent biliary obstruction | 0 | 9 (47.4) | <0.001 |
| Stent migration | 0 | 3 (15.8) | 0.098 |
| Proximal side | 0 | 1 (5.3) | 0.475 |
| Distal side | 0 | 2 (10.5) | 0.219 |
| Stent occlusion | 0 | 6 (31.6) | 0.007 |
| Sludge | 0 | 6 (31.6) | 0.007 |
| Tumor ingrowth or overgrowth | 0 | 0 | - |
| Biliary bleeding | 0 | 0 | - |
| Food impaction | 0 | 0 | - |
| Other complications | 4 (19.1) | 4 (21.1) | 1.000 |
| Non-occlusive cholangitis | 3 (14.3) | 4 (21.1) | 0.689 |
| Cholecystitis | 2 (9.5) | 0 | 0.489 |
| Pancreatitis | 0 | 0 | - |
| Gastrointestinal bleeding | 0 | 0 | - |
| CSEMS Group (n = 21) | PS Group (n = 19) | p Value | |
|---|---|---|---|
| Time from the initiation of neoadjuvant chemotherapy to surgery (days) | 98 (70–110) | 99 (54–118) | 0.407 |
| Gemcitabine + S-1 | 75.5 (70–107) | 87 (54–116) | 0.564 |
| Gemcitabine + nab-paclitaxel + S-1 | 99 (92–110) | 99 (92–118) | 0.412 |
| Delay in chemotherapy schedule, n (%) | 4/21 (19.1) | 12/19 (63.2) | 0.009 |
| Gemcitabine + S-1 | 2/8 (25.0) | 8/9 (88.9) | 0.015 |
| Gemcitabine + nab-paclitaxel + S-1 | 2/13 (15.4) | 4/10 (40.0) | 0.341 |
| Delay in chemotherapy schedule due to stent-related complications, n (%) | 1/21 (4.8) | 10/19 (52.6) | 0.001 |
| Gemcitabine + S-1 | 0/8 (0) | 6/9 (66.7) | 0.009 |
| Gemcitabine + nab-paclitaxel + S-1 | 1/13 (7.7) | 4/10 (40.0) | 0.127 |
| CSEMS Group (n = 21) | PS Group (n = 19) | p Value | |
|---|---|---|---|
| Pancreatectomy | 21 (100) | 17 (89.5) | 0.524 |
| Surgical procedure | 0.354 | ||
| PD | 18 (85.7) | 13 (76.5) | |
| PD + hepatic artery resection | 2 (9.5) | 4 (23.5) | |
| TP | 1 (4.8) | 0 | |
| Arterial resection | 2 (9.5) | 5 (29.4) | 0.207 |
| PV/SMV resection | 15 (71.4) | 11 (64.7) | 0.734 |
| Operation time (min) | 358 (238–686) | 455 (238–676) | 0.081 |
| Blood loss (mL) | 819 (45–6672) | 682 (158–2830) | 0.965 |
| Postoperative complications | 6 (28.6) | 5 (26.3) | 0.955 |
| Biliary fistula | 0 | 0 | - |
| Pancreatic fistula | 0 | 2 (11.8) | 0.194 |
| Cholangitis | 2 (9.5) | 1 (5.9) | 1.000 |
| Gastrointestinal bleeding | 0 | 0 | - |
| Delayed gastric emptying | 1 (4.8) | 2 (11.8) | 0.577 |
| In-hospital mortality | 1 (4.8) | 0 | 1.000 |
| Others | 3 (14.3) | 2 (11.8) | 1.000 |
| Length of hospital stay (days) | 19 (15–285) | 19 (14–63) | 0.802 |
| Study | Stents | Patients | Resectability | Neoadjuvant Therapy | RBO | Other Complications |
|---|---|---|---|---|---|---|
| Kubota [8], 2014 | PS | 21 | 21 BR | 11 NAC (G, GS) 10 NACRT (GS) | 86% | NR |
| CSEMS | 17 | 17 BR | 17 NACRT (GS) | 24% | NR | |
| Tsuboi [9], 2016 | PS | 11 | 11 BR | 11 NAC (GS) | 63.6% | 9.1% |
| CSEMS | 9 | 9 BR | 9 NAC (GS) | 0% | 0% | |
| Kuwatani [10], 2020 | PS | 12 | 6 R, 6 BR | 6 NAC (S-1), 6 NACRT (S-1) | 83% | 0% |
| CSEMS | 17 | 8 R, 9 BR | 8 NAC (S-1) 9 NACRT (S-1) | 6% | 5.8% | |
| Tamura [11], 2021 | PS | 11 | 11 BR | 11 NAC (GnP) | 64% | NR |
| CSEMS | 11 | 11 BR | 11 NAC (GnP) | 18% | NR | |
| Kobayashi [12], 2021 | PS | 22 | 15 R, 7 BR | 22 NACRT (S-1) | 95.4% | 0% |
| CSEMS | 21 | 13 R, 8 BR | 21 NACRT (S-1) | 4.8% | 4.8% | |
| Hasegawa [13], 2021 | PS | 40 | 1 R, 32 BR, 7 UR | 37 NACRT 3 NAC | 97% | 13% |
| CSEMS | 27 | 3 R, 19 BR, 5 UR | 25 NACRT 2 NAC | 15% | 11% | |
| Vehviläinen [14], 2022 | PS | 91 | NR | NAC/NACRT | 21% | NR |
| CSEMS | 15 | NR | NAC/NACRT | 3% | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, M.; Ishii, Y.; Serikawa, M.; Tsuboi, T.; Tatsukawa, Y.; Hirano, T.; Nakamura, S.; Ikemoto, J.; Kiyoshita, Y.; Saeki, S.; et al. Utility of Covered Self-Expanding Metal Stents for Biliary Drainage during Neoadjuvant Chemotherapy in Patients with Borderline Resectable Pancreatic Cancer. J. Clin. Med. 2023, 12, 6245. https://doi.org/10.3390/jcm12196245
Furukawa M, Ishii Y, Serikawa M, Tsuboi T, Tatsukawa Y, Hirano T, Nakamura S, Ikemoto J, Kiyoshita Y, Saeki S, et al. Utility of Covered Self-Expanding Metal Stents for Biliary Drainage during Neoadjuvant Chemotherapy in Patients with Borderline Resectable Pancreatic Cancer. Journal of Clinical Medicine. 2023; 12(19):6245. https://doi.org/10.3390/jcm12196245
Chicago/Turabian StyleFurukawa, Masaru, Yasutaka Ishii, Masahiro Serikawa, Tomofumi Tsuboi, Yumiko Tatsukawa, Tetsuro Hirano, Shinya Nakamura, Juri Ikemoto, Yusuke Kiyoshita, Sho Saeki, and et al. 2023. "Utility of Covered Self-Expanding Metal Stents for Biliary Drainage during Neoadjuvant Chemotherapy in Patients with Borderline Resectable Pancreatic Cancer" Journal of Clinical Medicine 12, no. 19: 6245. https://doi.org/10.3390/jcm12196245
APA StyleFurukawa, M., Ishii, Y., Serikawa, M., Tsuboi, T., Tatsukawa, Y., Hirano, T., Nakamura, S., Ikemoto, J., Kiyoshita, Y., Saeki, S., Tamura, Y., Miyamoto, S., Nakamura, K., Yamashita, Y., Iijima, N., Uemura, K., & Oka, S. (2023). Utility of Covered Self-Expanding Metal Stents for Biliary Drainage during Neoadjuvant Chemotherapy in Patients with Borderline Resectable Pancreatic Cancer. Journal of Clinical Medicine, 12(19), 6245. https://doi.org/10.3390/jcm12196245







