Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
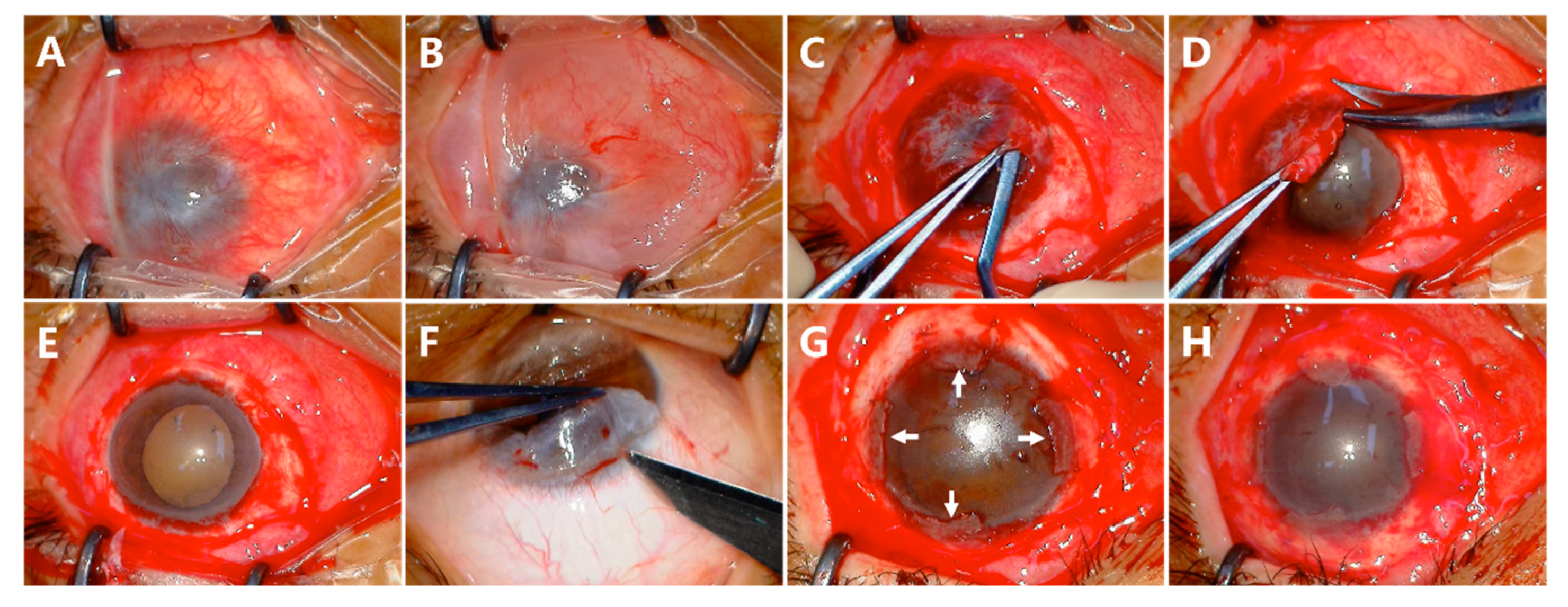
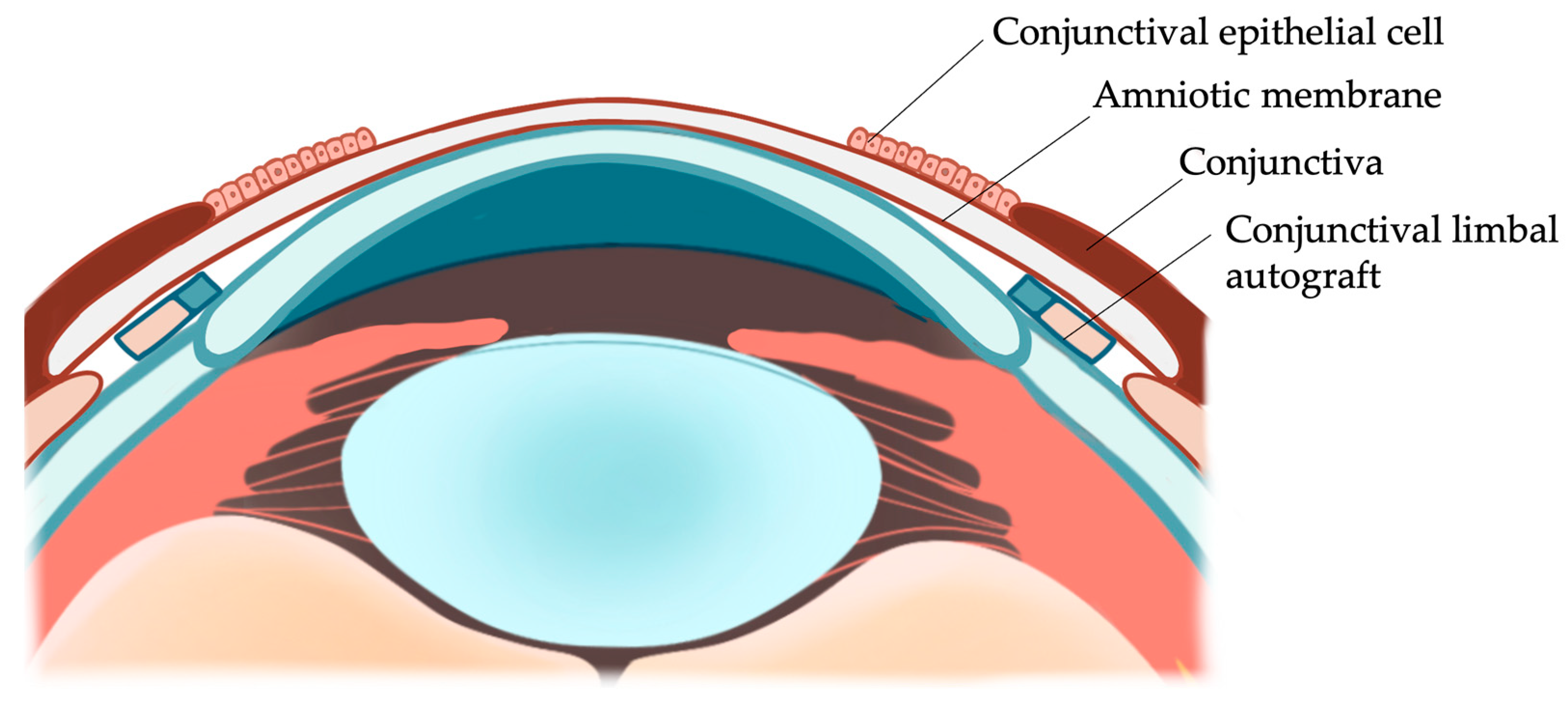
2.2. Surgical Technique
2.3. Postoperative Treatment and Follow-Up
2.4. Outcome Measures
3. Results
3.1. Pretreatment Characteristics
3.2. Conjunctival Epithelial Redirection
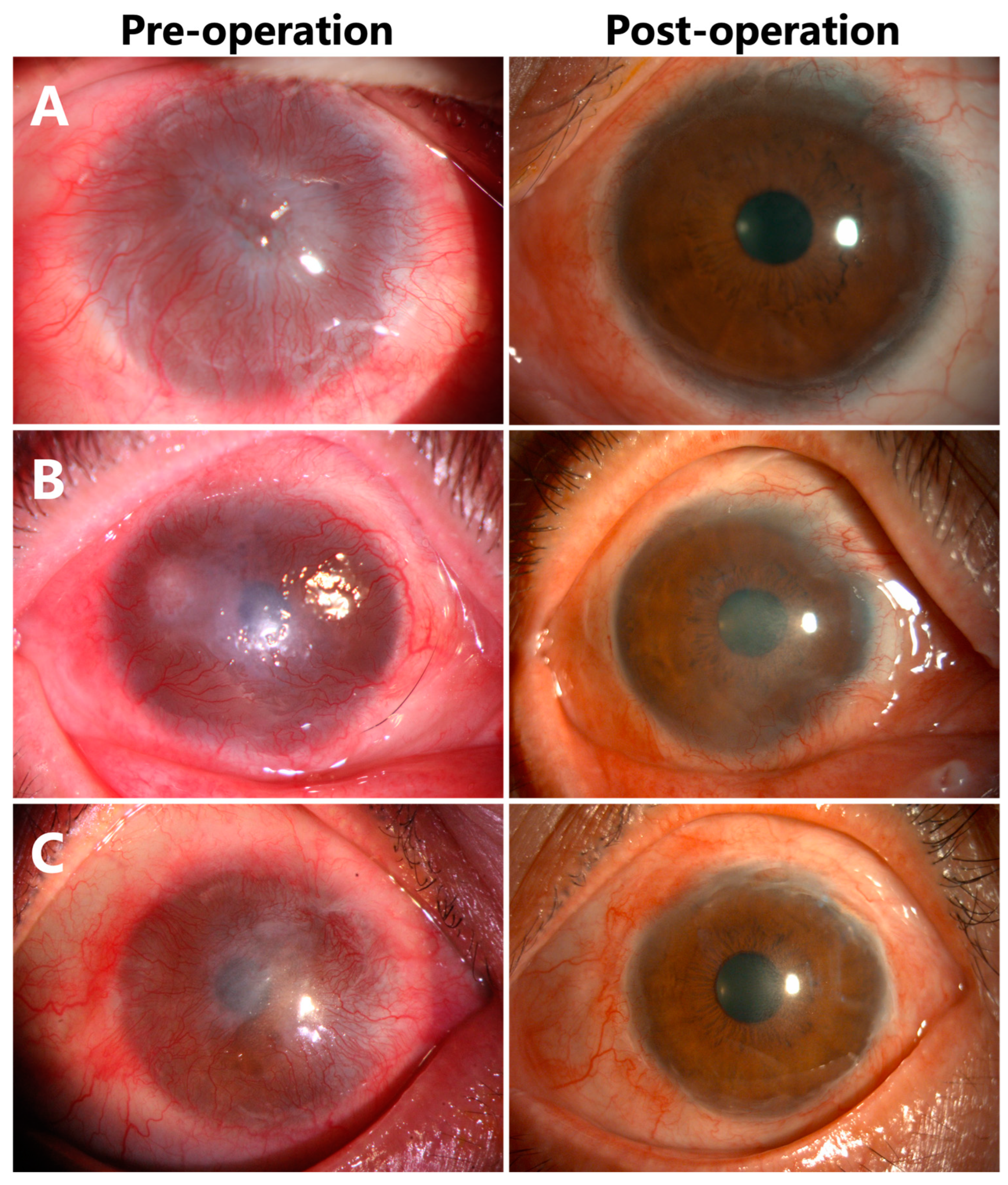
3.3. Corneal Epithelialization in Patients without Symblepharon
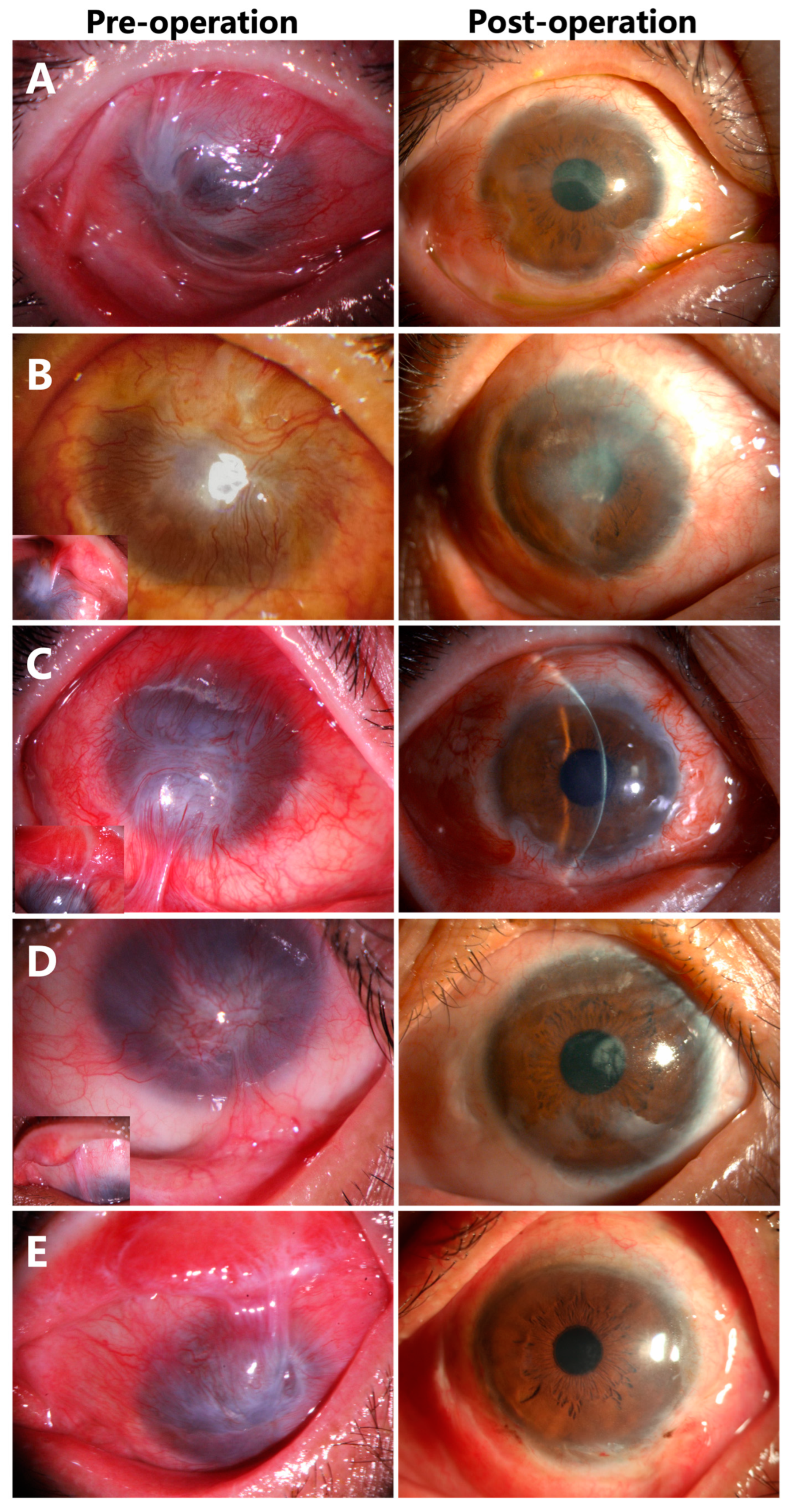
3.4. Corneal Epithelialization in Patients with Symblepharon
3.5. Visual Acuity
3.6. Complications
- (1)
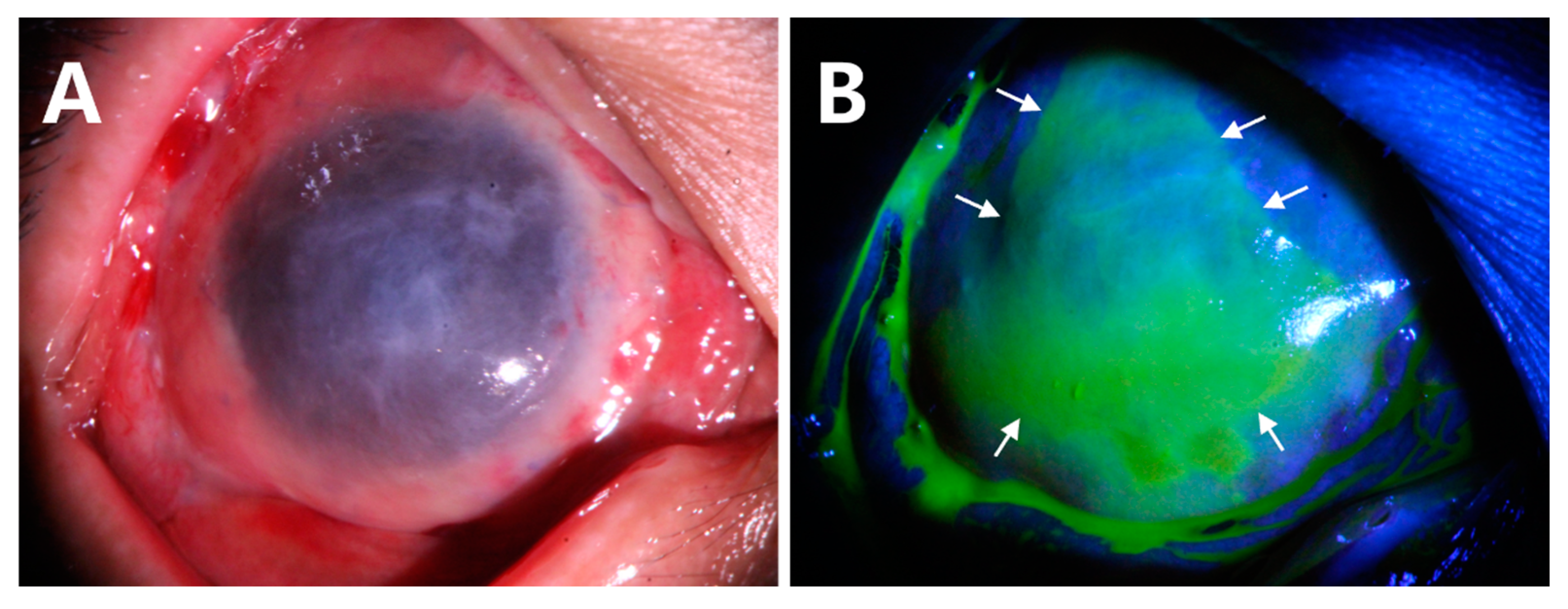
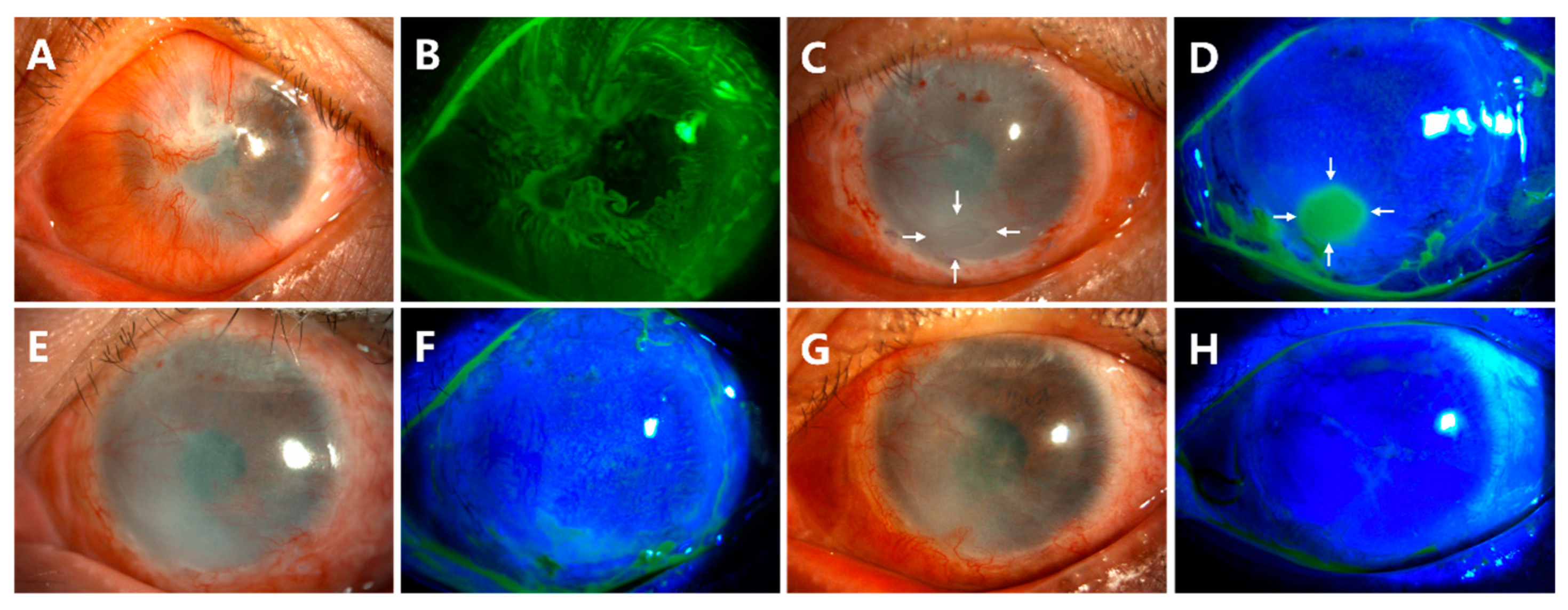
- Delayed corneal epithelial healing occurred in Case 10 (Figure 5). The preoperative anterior segment photograph showed that the pannus had grown into the cornea from 360 degrees (Figure 5A,B). At the follow-up of week 4 after surgery, there was a corneal epithelial defect of approximately 2 × 3 mm2 (Figure 5C,D, white arrows). The patient was treated with soft contact bandage lenses. The corneal epithelization was completed 2 weeks later (Figure 5E,F). The ocular surface remained smooth and stable at the last follow-up of month 18 (Figure 5G,H).
- (2)
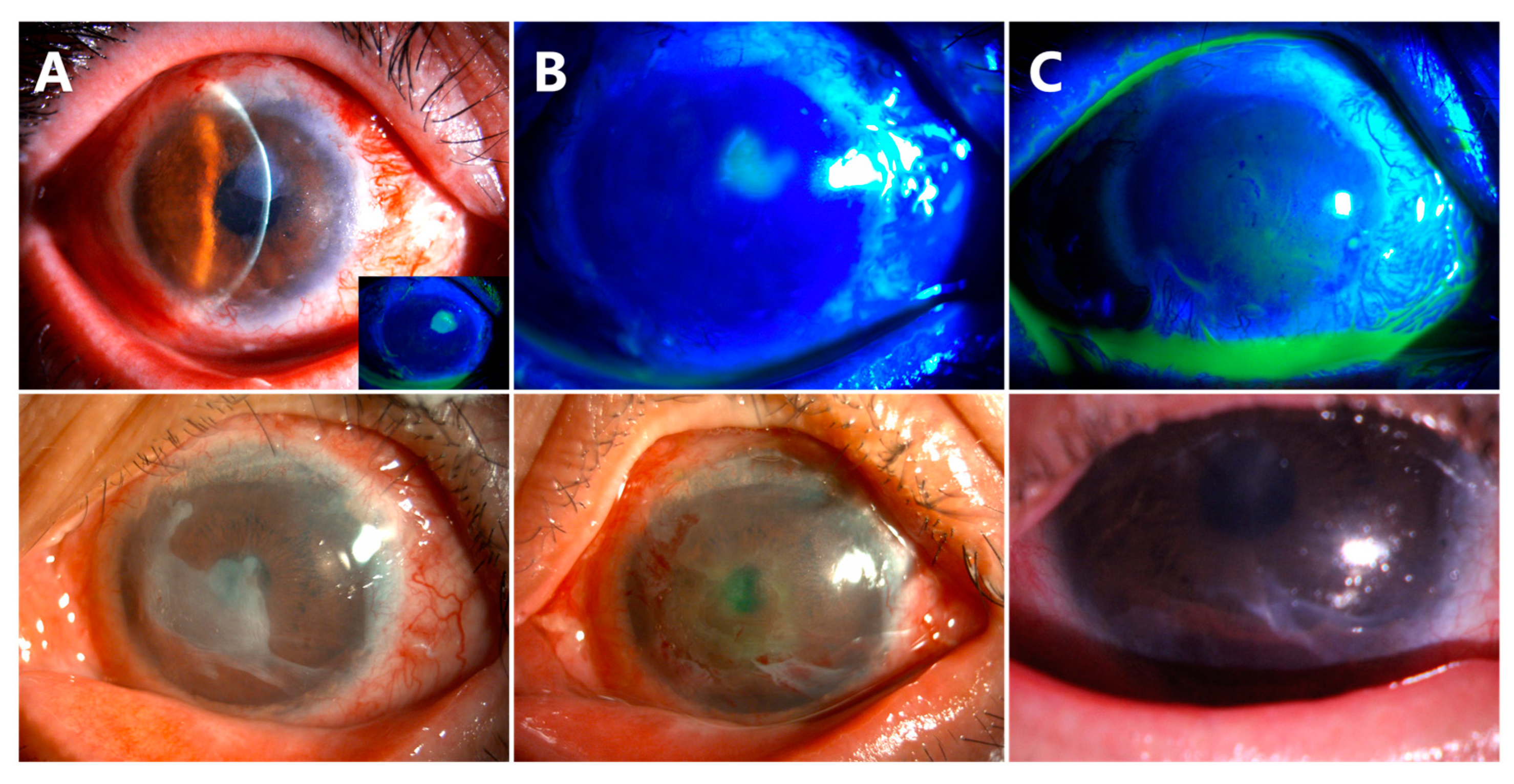
- Delayed AM dissolution and detachment occurred in Cases 6 (Figure 6, the first row) and 7 (Figure 6, the second row). At the follow-up visit of week 8, a small piece of AM was still attached on the corneal surface (Figure 6A). The residual AM was scraped off in both patients (Figure 6B). The central corneas were smooth and transparent at the follow-up visit of week 10 (Figure 6C).
- (3)
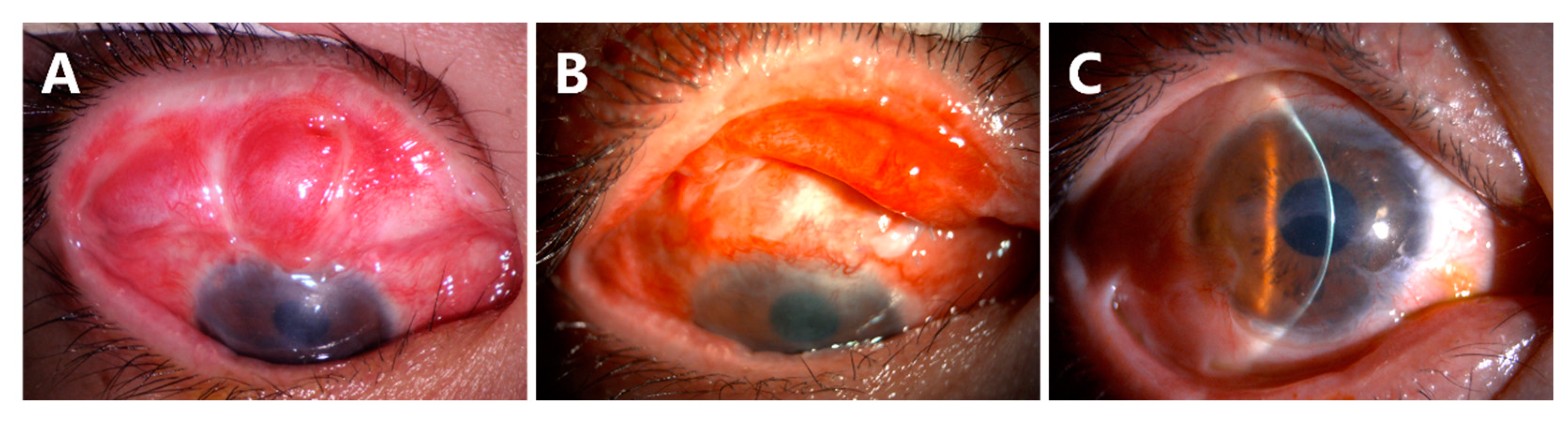
- Recurrence of symblepharon occurred in Case 3 (Figure 7). This patient had symblepharon before surgery. Eight weeks after CLAU combined with the ACER procedure, the corneal epithelialization was completed, but symblepharon recurred as presented by the superior conjunctival fornix disappearance (Figure 7A), and trichiasis was also complicated. Entropion, trichiasis, and symblepharon correction were performed. AM transplantation was used to form the conjunctival fornix. The postoperative follow-up of 2 months showed that the superior fornix was well formed (Figure 7B). The ocular surface was stable and smooth without any complications at the last follow-up of 13 months (Figure 7C).
- (4)
- For all 10 subjects undergoing biopsy, the donor eye healed without complications, and the visual acuity returned to baseline within 4 weeks.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tseng, S.C.; He, H.; Zhang, S.; Chen, S.Y. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul. Surf. 2016, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, A.A.; Ting, D.S.J.; Figueiredo, F.C. Epidemiology, economic and humanistic burdens of Ocular Surface Chemical Injury: A narrative review. Ocul. Surf. 2021, 20, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Deng, S.X.; Kruse, F.; Gomes, J.A.P.; Chan, C.C.; Daya, S.; Dana, R.; Figueiredo, F.C.; Kinoshita, S.; Rama, P.; Sangwan, V.; et al. Global Consensus on the Management of Limbal Stem Cell Deficiency. Cornea 2020, 39, 1291–1302. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Patel, C.N.; Goyal, R.; Donthineni, P.R.; Singh, V.; Basu, S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian. J. Ophthalmol. 2019, 67, 1265–1277. [Google Scholar]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Yin, J.; Johns, L.K.; Li, S.; Negre, H.; Shaw, K.L.; Samarakoon, L.; Ayala, A.R.; Kheirkhah, A.; Katikireddy, K.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation: Development of manufacturing process and clinical evaluation of feasibility and safety. Sci. Adv. 2023, 9, eadg6470. [Google Scholar] [CrossRef]
- Dua, H.S.; Miri, A.; Elalfy, M.S.; Lencova, A.; Said, D.G. Amnion-assisted conjunctival epithelial redirection in limbal stem cell grafting. Br. J. Ophthalmol. 2017, 101, 913–919. [Google Scholar] [CrossRef]
- Dua, H.S.; Ting, D.S.J.; AlSaadi, A.; Said, D.G. Management of limbal stem cell deficiency by amnion-assisted conjunctival epithelial redirection using vacuum-dried amniotic membrane and fibrin glue. Br. J. Ophthalmol. 2023, 107, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; King, A.J.; Joseph, A. A new classification of ocular surface burns. Br. J. Ophthalmol. 2001, 85, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C. Concept and application of limbal stem cells. Eye 1989, 3 Pt 2, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S. The conjunctiva in corneal epithelial wound healing. Br. J. Ophthalmol. 1998, 82, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.P.; Tanioka, H.; Kawasaki, S.; Yamasaki, K.; Do, T.P.; Thein, Z.M.; Koizumi, N.; Nakamura, T.; Yokoi, N.; Komuro, A.; et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Investig. Ophthalmol. Vis. Sci. 2010, 51, 758–764. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Kaneda, H.; Fukushima, M.; Kinoshita, S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014, 92, e447–e453. [Google Scholar] [CrossRef]
- Rao, S.K.; Rajagopal, R.; Sitalakshmi, G.; Padmanabhan, P. Limbal autografting: Comparison of results in the acute and chronic phases of ocular surface burns. Cornea 1999, 18, 164–171. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef]
- Cheng, J.; Zhai, H.; Wang, J.; Duan, H.; Zhou, Q. Long-term outcome of allogeneic cultivated limbal epithelial transplantation for symblepharon caused by severe ocular burns. BMC Ophthalmol. 2017, 17, 8. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Raju, V.K.; Tseng, S.C. Minimal conjunctival limbal autograft for total limbal stem cell deficiency. Cornea 2008, 27, 730–733. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Casas, V.; Raju, V.K.; Tseng, S.C. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am. J. Ophthalmol. 2008, 145, 787–794. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Features | Data |
|---|---|
| Age (Year) | |
| Mean | 40.9 ± 9.63 |
| Range | 26–55 |
| Gender | |
| Male | 9 (90%) |
| Female | 1 (10%) |
| Eye | |
| Right | 6 (60%) |
| Left | 4 (40%) |
| Chemical substances | |
| Alkali | 8 (80%) |
| Nitric acid | 1 (10%) |
| Hair dye | 1 (10%) |
| Symblepharon | 7 (70%) |
| Duration between injury and reconstruction (months) | |
| Mean | 7.67 ± 3.97 |
| Range | 4–18 |
| Case Number | Symblepharon | Best Corrected Visual Acuity | Corneal Opacity | Complications | Other Surgeries | Follow-Up (Months) | |
|---|---|---|---|---|---|---|---|
| Pre- Operation | Last Visit | ||||||
| 1 | No | HM/30 cm | 1.0 | No | No | No | 21 |
| 2 | No | CF/10 cm | 0.3 | Mild | No | No | 19 |
| 3 | Yes | HM/20 cm | 0.15 | Mild | Recurrence of symblepharon | Entropion correction and reconstruction of fornix | 13 |
| 4 | Yes | HM/10 cm | 0.1 | Severe | No | No | 12 |
| 5 | No | CF/20 cm | 1.0 | No | No | No | 7 |
| 6 | Yes | HM/20 cm | 0.6 | No | Delayed AM dissolution | AM scraping off | 11 |
| 7 | Yes | HM/10 cm | 0.8 | No | Delayed AM dissolution | AM scraping off | 10 |
| 8 | Yes | HM/10 cm | 0.6 | No | No | No | 6 |
| 9 | Yes | HM/30 cm | 0.1 | Severe | No | No | 6 |
| 10 | Yes | HM/10 cm | 0.1 | Severe | Delay of corneal epithelization | No | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.-Y.; Wang, J.-S.; Geng, W.; Xie, H.-T.; Zhang, M.-C. Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn. J. Clin. Med. 2023, 12, 6235. https://doi.org/10.3390/jcm12196235
Yao T-Y, Wang J-S, Geng W, Xie H-T, Zhang M-C. Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn. Journal of Clinical Medicine. 2023; 12(19):6235. https://doi.org/10.3390/jcm12196235
Chicago/Turabian StyleYao, Tian-Yu, Jia-Song Wang, Wen Geng, Hua-Tao Xie, and Ming-Chang Zhang. 2023. "Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn" Journal of Clinical Medicine 12, no. 19: 6235. https://doi.org/10.3390/jcm12196235
APA StyleYao, T.-Y., Wang, J.-S., Geng, W., Xie, H.-T., & Zhang, M.-C. (2023). Conjunctival Limbal Autograft Combined with Amnion-Assisted Conjunctival Epithelial Redirection for Unilateral Total Limbal Stem Cell Deficiency after Severe Chemical Burn. Journal of Clinical Medicine, 12(19), 6235. https://doi.org/10.3390/jcm12196235






