No More Venous Ulcers—What More Can We Do?
Abstract
1. Introduction
1.1. Definition of Venous Leg Ulcer
1.2. Epidemiology and Socioeconomic Burden
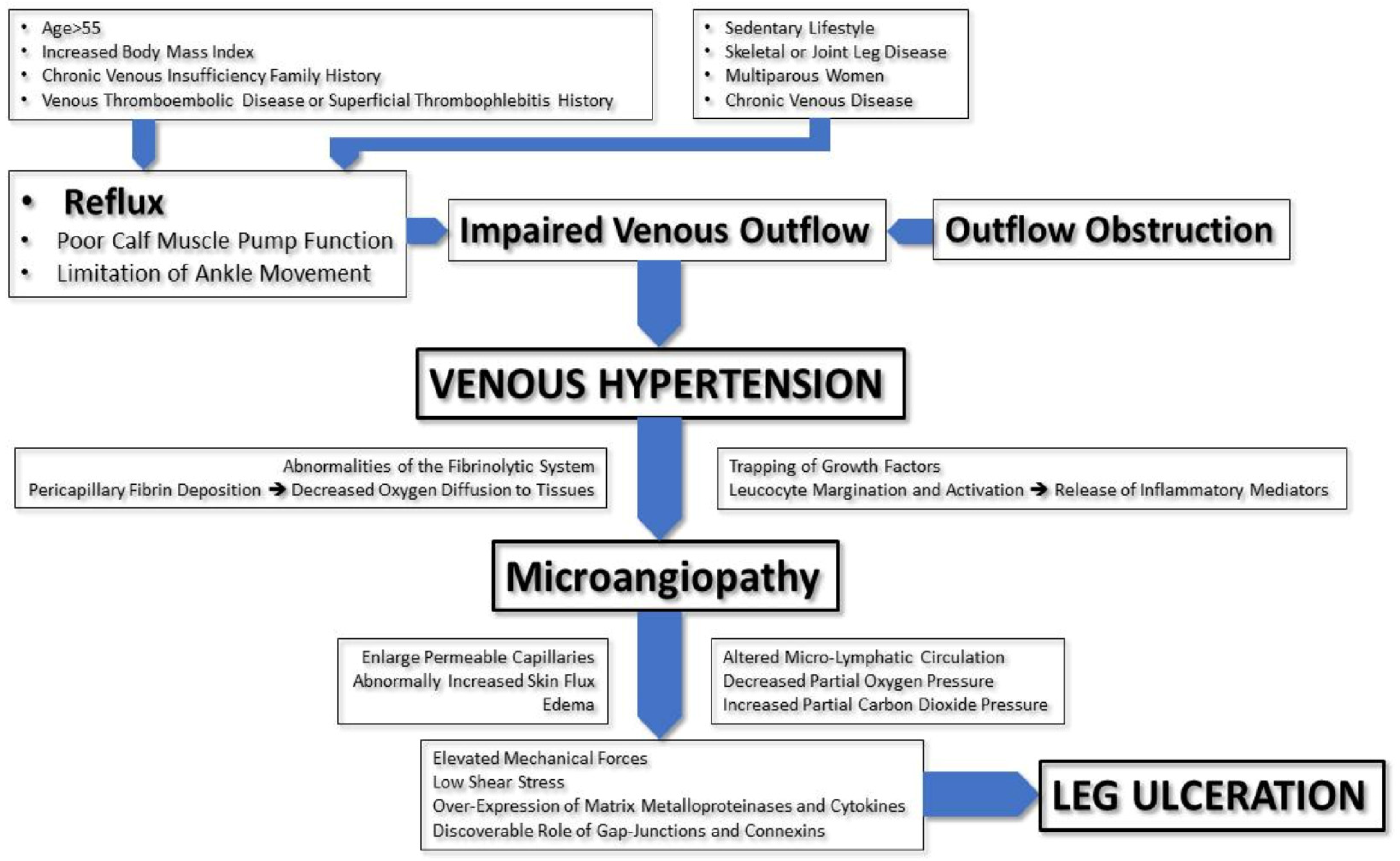
2. Pathophysiology of Venous Ulcers
3. Diagnosis of Venous Ulcers
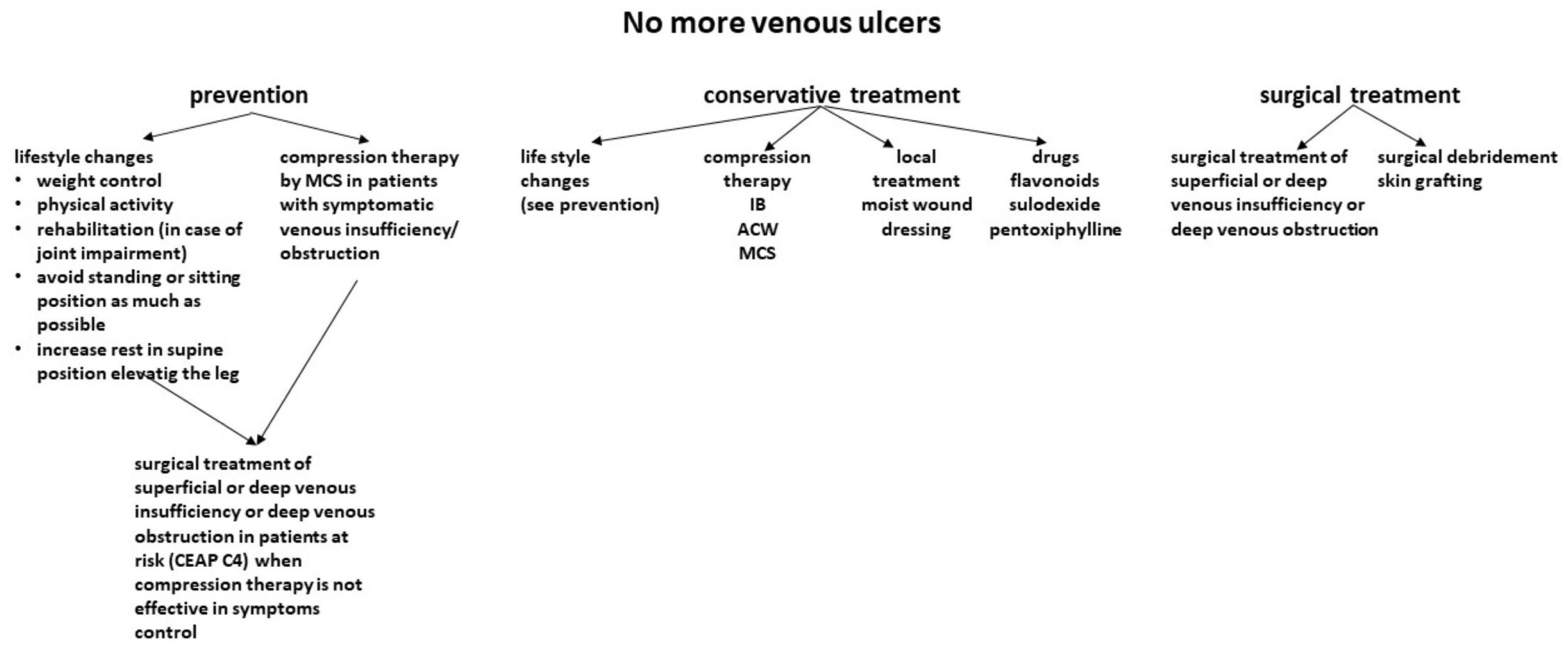
4. Current Treatment of Venous Ulcers
4.1. Conservative Treatment
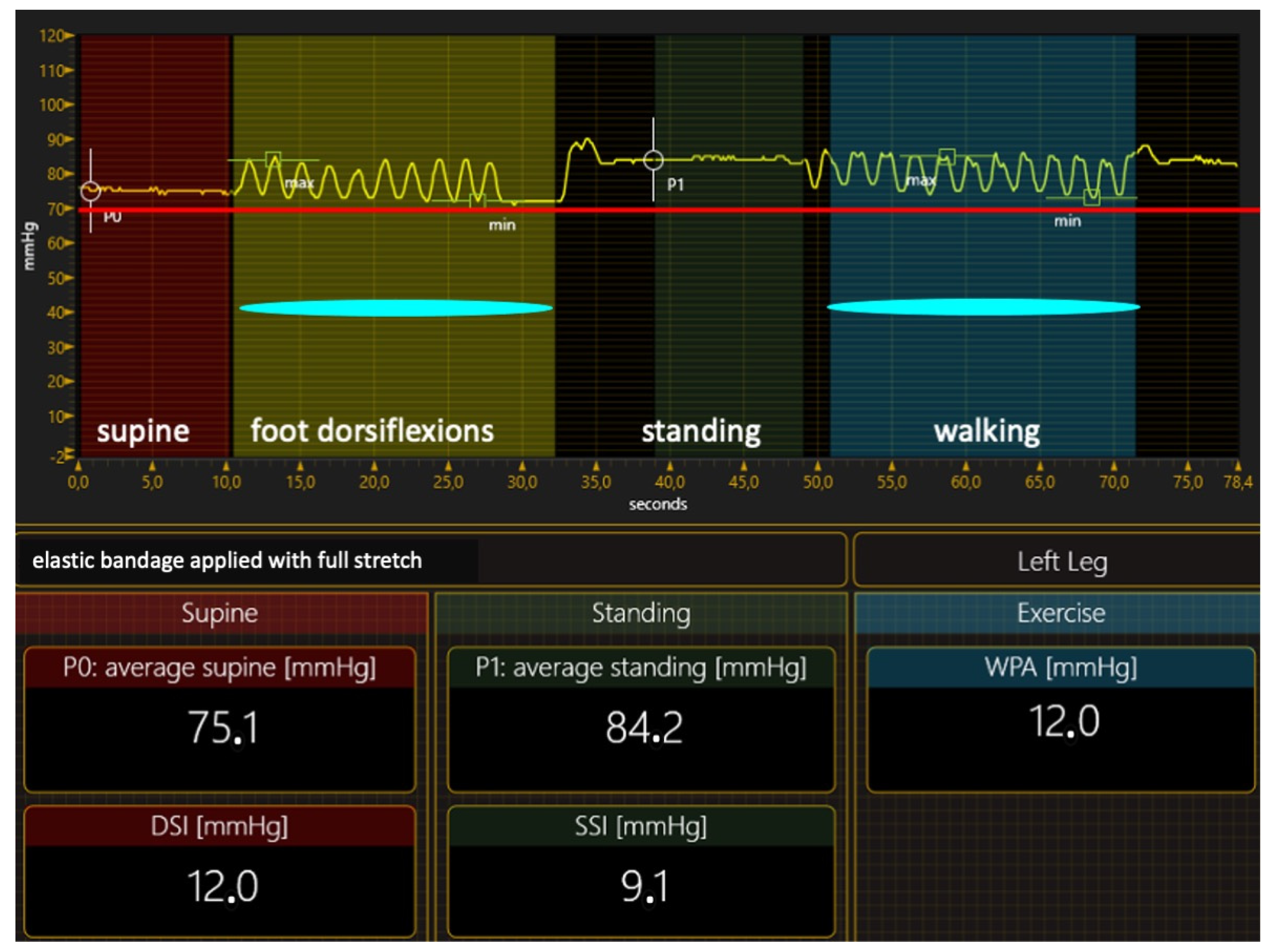
4.1.1. Compression Therapy
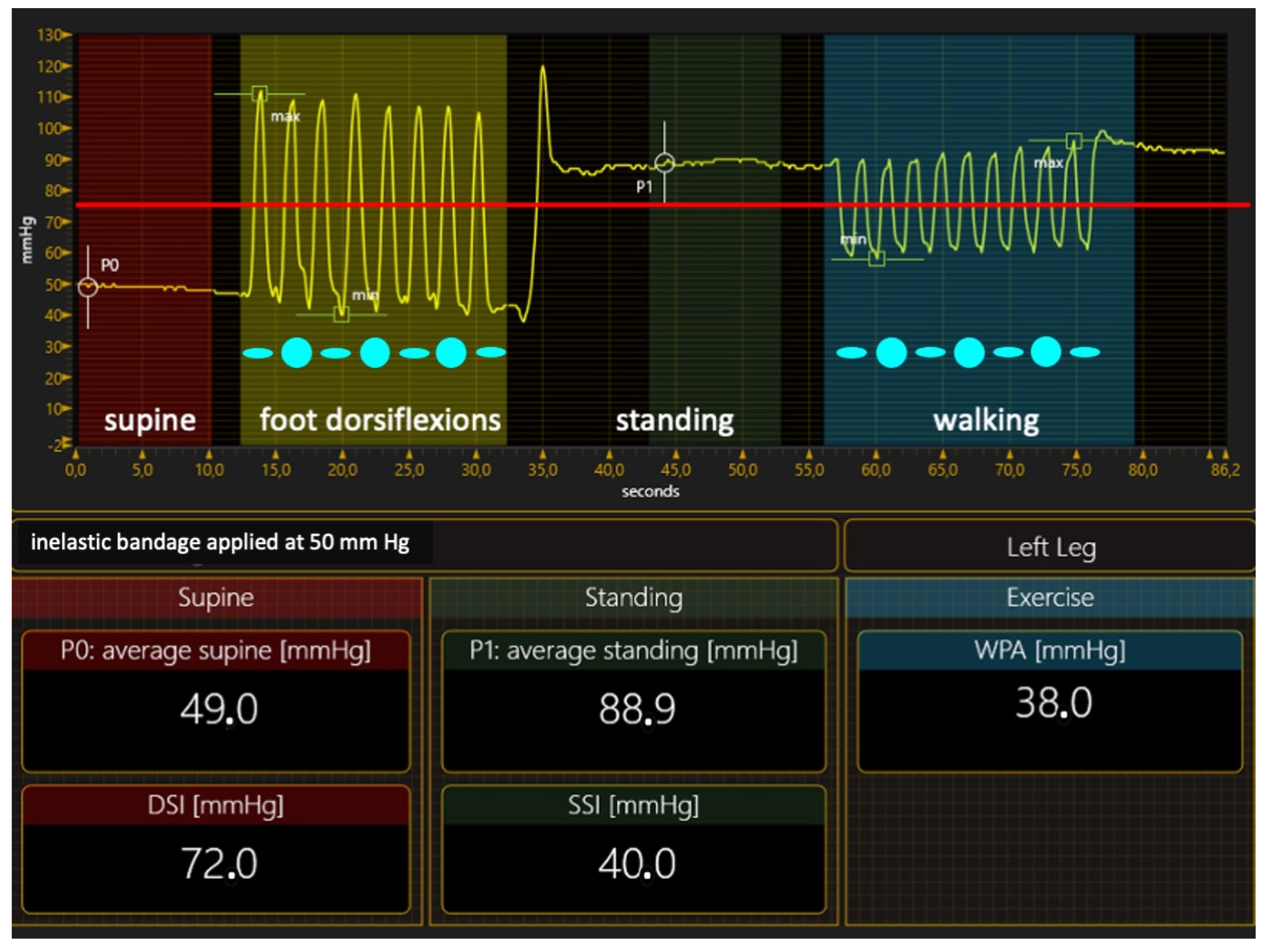
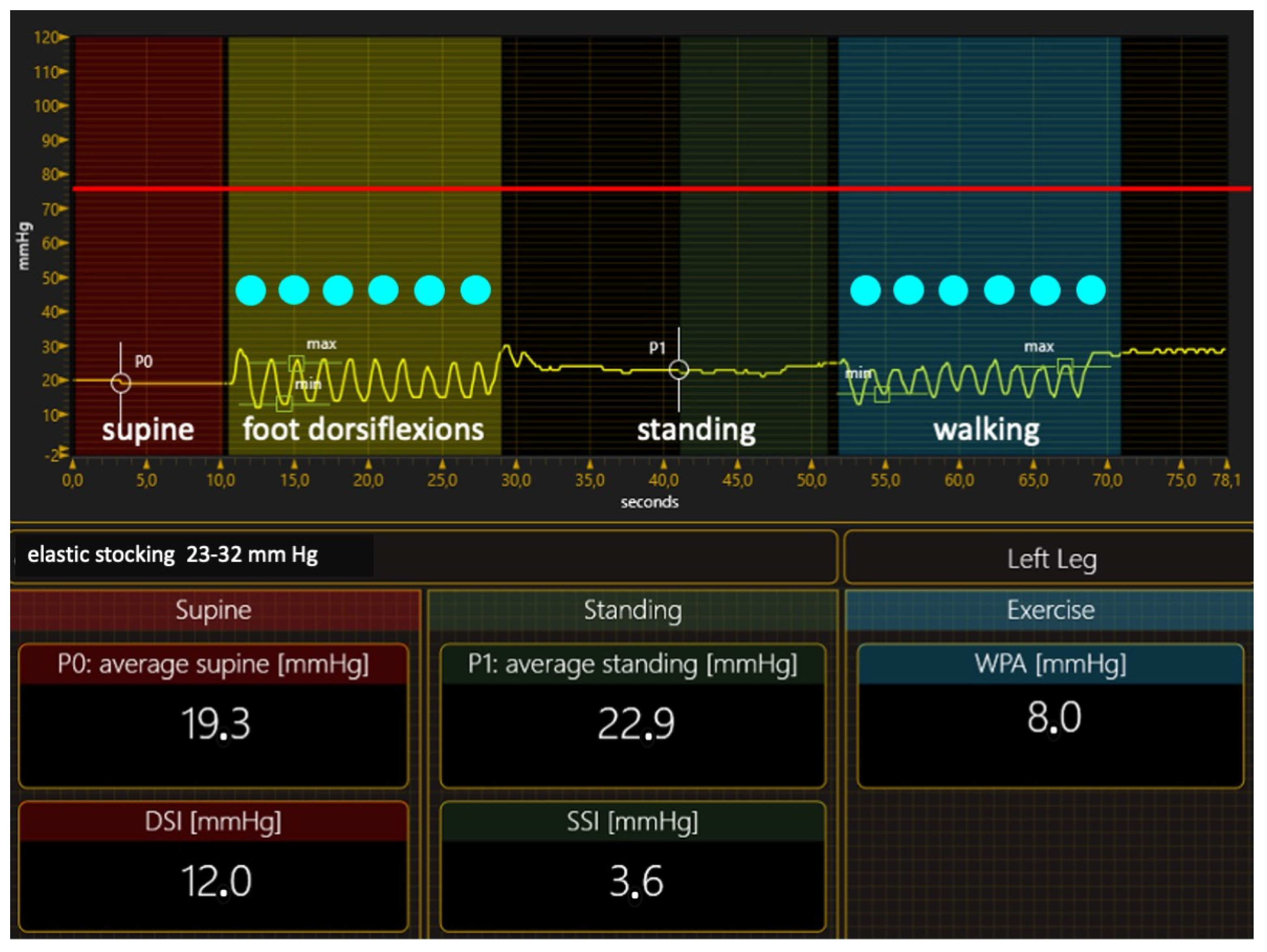
Compression Materials and Their Hemodynamic Effects
- Inelastic materials
- 2.
- Elastic materials
- 3.
- What material for ulcer treatment?
- Inelastic bandages. When correctly applied to exert strong pressure, inelastic bandages can achieve an ulcer healing rate close to 100% in three months of treatment [68]. Therefore, preferring inelastic material in VLU treatment is the right option from a logical point of view, but we need ultimate proof that this is correct at present. In contrast, many papers claim the greater effectiveness of elastic stockings or bandages than inelastic material in achieving ulcer healing [69,70,71,72,73,74,75,76,77,78,79,80,81,82]. However, studies comparing elastic and inelastic devices have so many flaws that their conclusions are hard to believe [83]. Two significant deficiencies in papers on compression therapy must be highlighted: the lack of compression pressure measurement and stiffness assessment, and the lack of healthcare professionals’ expertise in applying inelastic bandages. The compression pressure, the dosage of the compression therapy, was seldom measured in the papers comparing different materials, even though compression pressure measurements are easy to perform [84]. Not measuring the compression pressure and not knowing the level of expertise of healthcare professionals [58] make it impossible to tell whether the bandages were correctly applied, making any conclusion challenging to trust. Inelastic bandages could also be so poorly applied that their compression pressure is lower than that exerted by an elastic kit [80]. In addition, not measuring the pressure or assessing the SSI produced a notable mistake in almost all studies comparing elastic and inelastic bandages. In these studies [69,70,71,72,73,74,75,76], the prototype of elastic material was the so-called four-layer bandage, which was dogmatically considered elastic, as it comprises four different elastic components. Nevertheless, measuring the supine and standing pressure of the final bandage and calculating its SSI showed that the SSI is in the inelastic range [85]. In conclusion, all these studies compared two different inelastic bandages, and the conclusions must be critically reviewed;
- Adjustable compression wraps (ACWs). These devices are quite inelastic and offer the advantages of inelastic material, already reported, in terms of improving the venous hemodynamics [86,87]. At the same time, they are straightforward to use and can be applied and re-adjusted by the patients themselves after a very short wear and education time [64,65,66]. ACWs have been proven more effective at achieving healing in patients with VLUs when compared with Unna boot bandages [88], four-layer bandages [89], or two-layer bandages [90]. Even though more studies must confirm these results, their outcomes seem promising;
- Elastic stockings. Is there any role for MCSs? As already reported [40], MCSs are very effective in preventing ulcer recurrence after ulcer healing, but they also have a role in the active VLU treatment, especially when using the so-called elastic kits of two superimposed stockings and exerting pressure at about 40 mm Hg. The elastic kits were effective in small ulcers of recent onset. They achieved ulcer healing in 36–96% of patients with these VLU characteristics [77,78,79,80,81]. As ACWs, elastic kits do not require expert personnel to be applied and allow self-management.
4.1.2. Lifestyle and Physical Exercise
4.1.3. Local Treatment
4.1.4. Pharmacologic Treatment
4.2. Invasive Treatment
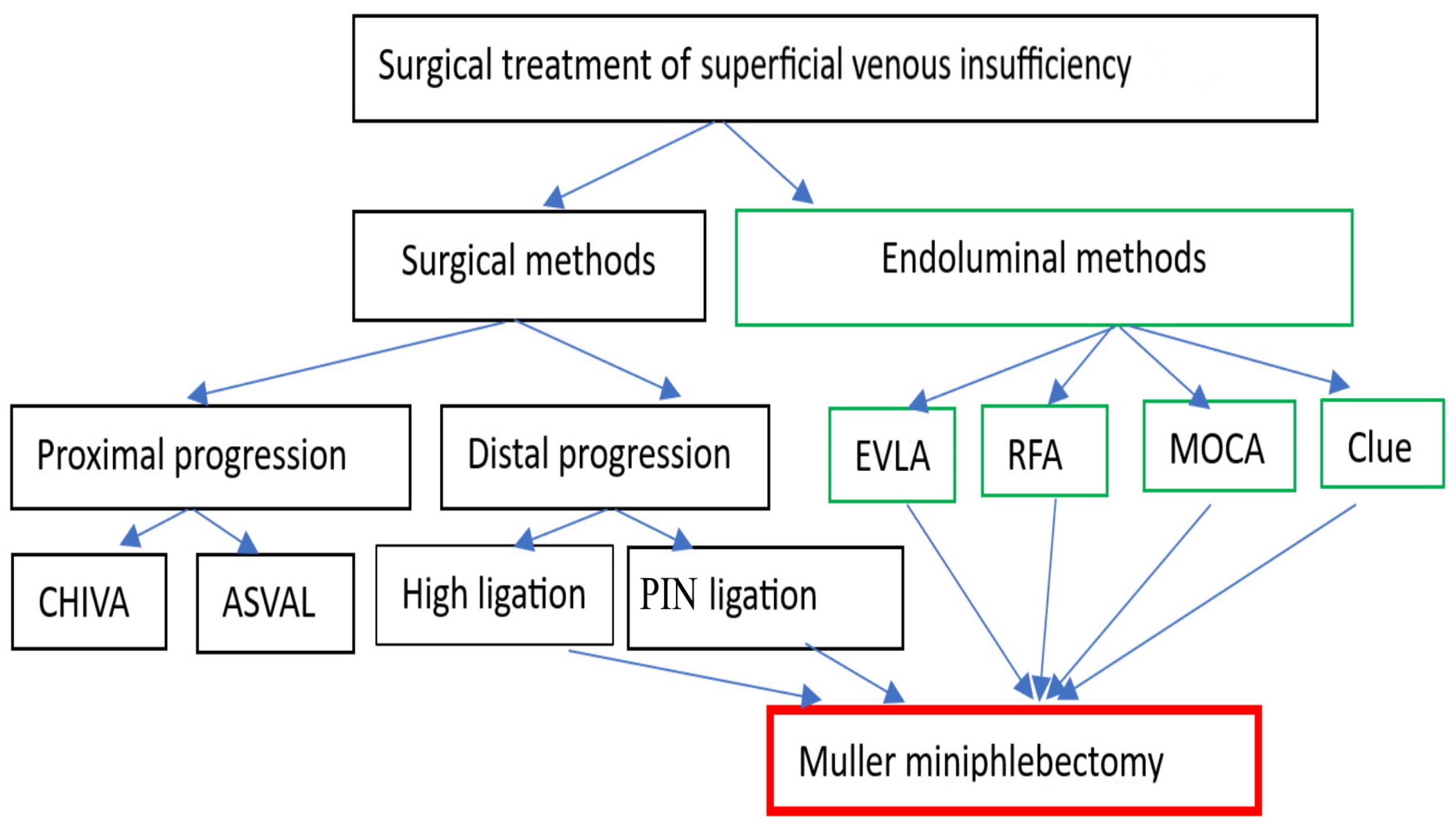
4.2.1. Surgical and Endovenous Treatment of Superficial Venous Insufficiency
4.2.2. Surgical and Endovenous Treatment of Deep-Vein Occlusion
4.2.3. Surgical Debridement and Skin Grafting
4.3. Challenges in the Treatment of Mixed Arterial–Venous Leg Ulcers
5. Focus on Prevention: What More Can We Do?
5.1. Primary Prevention and Risk Factors
5.2. Recurrence Prevention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef]
- Raffetto, J.D. The definition of the venous ulcer. J. Vasc. Surg. 2010, 52 (Suppl. 5), 46S–49S. [Google Scholar] [CrossRef]
- Kahle, B.; Hermanns, H.J.; Gallenkemper, G. Evidence-based treatment of chronic leg ulcers. Dtsch. Arztebl. Int. 2011, 108, 231–237. [Google Scholar] [CrossRef]
- Wipke-Tevis, D.D.; Rantz, M.J.; Mehr, D.R.; Popejoy, L.; Petroski, G.; Madsen, R.; Conn, V.S.; Grando, V.T.; Porter, R.; Maas, M. Prevalence, incidence, management, and predictors of venous ulcers in the long-term-care population using the MDS. Adv. Skin Wound Care 2000, 13, 218–224. [Google Scholar] [PubMed]
- Kantor, J.; Margolis, D.J. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br. J. Dermatol. 2000, 142, 960–964. [Google Scholar] [CrossRef]
- Finlayson, K.; Wu, M.L.; Edwards, H.E. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: A longitudinal study. Int. J. Nurs. Stud. 2015, 52, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.T. Venous Ulcer Assessment and Management: Using the Updated CEAP Classification System. Adv. Skin Wound Care 2020, 33, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, C.; Nilsson, A.; Fagerström, C. Leg ulcers in older people: A national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr. 2016, 16, 25. [Google Scholar] [CrossRef]
- Kolluri, R.; Lugli, M.; Villalba, L.; Varcoe, R.; Maleti, O.; Gallardo, F.; Black, S.; Forgues, F.; Lichtenberg, M.; Hinahara, J.; et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc. Med. 2022, 27, 63–72. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.; Nelson, E.A.; Dumville, J.C. Compression for venous leg ulcers. Cochrane Database Syst. Rev. 2012, 11, CD000265. [Google Scholar] [CrossRef]
- Lal, B.K. Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens. Semin. Vasc. Surg. 2015, 28, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Berszakiewicz, A.; Kasperczyk, J.; Sieroń, A.; Krasiński, Z.; Cholewka, A.; Stanek, A. The effect of compression therapy on quality of life in patients with chronic venous disease: A comparative 6-month study. Postepy Dermatol. Alergol. 2021, 38, 389–395. [Google Scholar] [CrossRef]
- Jones, J.E.; Robinson, J.; Barr, W.; Carlisle, C. Impact of exudate and odour from chronic venous leg ulceration. Nurs. Stand. 2008, 22, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Scottish Leg Ulcer Participants. Effect of National Community Intervention Program on healing rates of chronic leg ulcer: Randomised controlled trial. Phlebology 2002, 17, 47–53. [Google Scholar] [CrossRef]
- Phillips, C.J.; Humphreys, I.; Thayer, D.; Elmessary, M.; Collins, H.; Roberts, C.; Naik, G.; Harding, K. Cost of managing patients with venous leg ulcers. Int. Wound J. 2020, 17, 1074–1082. [Google Scholar] [CrossRef]
- Lo, Z.J.; Lim, X.; Eng, D.; Car, J.; Hong, Q.; Yong, E.; Zhang, L.; Chandrasekar, S.; Tan, G.W.L.; Chan, Y.M.; et al. Clinical and economic burden of wound care in the tropics: A 5-year institutional population health review. Int. Wound J. 2020, 17, 790–803. [Google Scholar] [CrossRef]
- Graves, N.; Zheng, H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract. Res. 2014, 22, 20–24, 26–33. [Google Scholar]
- Purwins, S.; Herberger, K.; Debus, E.S.; Rustenbach, S.J.; Pelzer, P.; Rabe, E.; Schäfer, E.; Stadler, R.; Augustin, M. Cost-of-illness of chronic leg ulcers in Germany. Int. Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that different wound types impose on the UK’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef]
- Yim, E.; Richmond, N.A.; Baquerizo, K.; Van Driessche, F.; Slade, H.B.; Pieper, B.; Kirsner, R.S. The effect of ankle range of motion on venous ulcer healing rates. Wound Repair Regen. 2014, 22, 492–496. [Google Scholar] [CrossRef]
- Vivas, A.; Lev-Tov, H.; Kirsner, R.S. Venous Leg Ulcers. Ann. Intern. Med. 2016, 165, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Browse, N.L.; Burnand, K.G. The cause of venous ulceration. Lancet 1982, 2, 243–245. [Google Scholar] [CrossRef]
- Thomas, P.R.; Nash, G.B.; Dormandy, J.A. White cell accumulation in dependent legs of patients with venous hypertension: A possible mechanism for trophic changes in the skin. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1693–1695. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W.; Takase, S.; Bergan, J.J. New advances in the understanding of the pathophysiology of chronic venous insufficiency. Angiology 2001, 52 (Suppl. 1), S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D. Inflammation in chronic venous ulcers. Phlebology 2013, 28 (Suppl. 1), 61–67. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D. Pathophysiology of Chronic Venous Disease and Venous Ulcers. Surg. Clin. N. Am. 2018, 98, 337–347. [Google Scholar] [CrossRef]
- Ghatnekar, G.S.; Grek, C.L.; Armstrong, D.G.; Desai, S.C.; Gourdie, R.G. The effect of a connexin43-based Peptide on the healing of chronic venous leg ulcers: A multicenter, randomized trial. J. Investig. Dermatol. 2015, 135, 289–298. [Google Scholar] [CrossRef]
- Spoljar, S. Osnovni dijagnosticki postupci kod bolesnika s venskim ulkusom [List of diagnostic tests and procedures in leg ulcer]. Acta Med. Croat. 2013, 67 (Suppl. 1), 21–28. [Google Scholar]
- Himanshu, V.; Ramesh, K.T. Venous ulcer. In Ulcer of the Lower Extremity; Khanna, A.K., Tiwary, S.K., Eds.; Springer: New Delhi, India, 2016; pp. 141–162. [Google Scholar] [CrossRef]
- Planinšek Ručigaj, T. Diseases of the veins and arteries (leg ulcers), chronic wounds, and their treatment. In Atlas of Dermatology, Dermatopathology and Venereology: Inflammatory Dermatoses; Smoller, B.R., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1205–1331. ISBN 9783319538044. [Google Scholar]
- Morison, M.J.; Moffat, C.J. A framework for patient assessment and care planning. In Leg Ulcers: A Problem-Based Learning Approach; Morison, M.J., Moffat, C.J., Franks, P.J., Eds.; Mosby, Elsevier: Philadelphia, PA, USA, 2007; pp. 119–139. [Google Scholar]
- Bonkemeyer Millan, S.; Gan, R.; Townsend, P.E. Venous Ulcers: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 298–305. [Google Scholar]
- Kecelj, N.; Kozak, M.; Slana, A.; Šmuc Berger, K.; Šikovec, A.; Makovec, M.; Blinc, A.; Žuran, I.; Planinšek Ručigaj, T. Recommendations of the diagnosis and treatment of chronic venous disease. Zdr. Vestn. Glas. Slov. Zdr. Društva 2017, 86, 345–361. [Google Scholar]
- Srisuwan, T.; Inmutto, N.; Kattipathanapong, T.; Rerkasem, A.; Rerkasem, K. Ultrasound Use in Diagnosis and Management of Venous Leg Ulcer. Int. J. Low. Extrem. Wounds 2020, 19, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Doliner, B.; Jaller, J.A.; Lopez, A.J.; Lev-Tov, H. Treatments to prevent primary venous ulceration after deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 260–271. [Google Scholar] [CrossRef]
- Abbade, L.P.; Lastória, S. Venous ulcer: Epidemiology, physiopathology, diagnosis and treatment. Int. J. Dermatol. 2005, 44, 449–456. [Google Scholar] [CrossRef]
- Finlayson, K.; Edwards, H.; Courtney, M. Factors associated with recurrence of venous leg ulcers: A survey and retrospective chart review. Int. J. Nurs. Stud. 2009, 46, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; Dorman, M.C. Recurrence of leg ulcers within a community ulcer service. J. Wound Care 1995, 4, 57–61. [Google Scholar] [CrossRef]
- Nelson, E.A.; Harper, D.R.; Prescott, R.J.; Gibson, B.; Brown, D.; Ruckley, C.V. Prevention of recurrence of venous ulceration: Randomized controlled trial of class 2 and class 3 elastic compression. J. Vasc. Surg. 2006, 44, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Clarke-Moloney, M.; Keane, N.; O’Connor, V.; Ryan, M.A.; Meagher, H.; Grace, P.A.; Kavanagh, E.; Walsh, S.R.; Burke, P.E. Randomised controlled trial comparing European standard class 1 to class 2 compression stockings for ulcer recurrence and patient compliance. Int. Wound J. 2014, 11, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.S.; Barwell, J.R.; Taylor, M.; Chant, T.; Foy, C.; Earnshaw, J.J.; Heather, B.P.; Mitchell, D.C.; Whyman, M.R.; Poskitt, K.R. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): Randomised controlled trial. BMJ 2007, 335, 83. [Google Scholar] [CrossRef]
- Gohel, M.S.; Heatley, F.; Liu, X.; Bradbury, A.; Bulbulia, R.; Cullum, N.; Epstein, D.M.; Nyamekye, I.; Poskitt, K.R.; Renton, S.; et al. A Randomized Trial of Early Endovenous Ablation in Venous Ulceration. N. Engl. J. Med. 2018, 378, 2105–2114. [Google Scholar] [CrossRef]
- O’Donnell, T.F.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J. Vasc. Surg. 2014, 60 (Suppl. 2), 3S–59S. [Google Scholar] [CrossRef]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef]
- Mosti, G.; De Maeseneer, M.; Cavezzi, A.; Parsi, K.; Morrison, N.; Nelzén, O.; Rabe, E.; Partsch, H.; Caggiati, A.; Simka, M.; et al. Society for Vascular Surgery and American Venous Forum Guidelines on the management of venous leg ulcers: The point of view of the International Union of Phlebology. Int. Angiol. 2015, 34, 202–218. [Google Scholar]
- Franks, P.J.; Barker, J.; Collier, M.; Gethin, G.; Haesler, E.; Jawien, A.; Laeuchli, S.; Mosti, G.; Probst, S.; Weller, C. Management of Patients With Venous Leg Ulcers: Challenges and Current Best Practice. J. Wound Care 2016, 25 (Suppl. 6), S1–S67. [Google Scholar] [CrossRef] [PubMed]
- Berszakiewicz, A.; Sieroń, A.; Krasiński, Z.; Cholewka, A.; Stanek, A. Compression therapy in venous diseases: Current forms of compression materials and techniques. Postepy Dermatol. Alergol. 2020, 37, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Partsch, H. The static stiffness index: A simple method to assess the elastic property of compression material in vivo. Dermatol. Surg. 2005, 31, 625–630. [Google Scholar] [CrossRef]
- Partsch, H. The use of pressure change on standing as a surrogate measure of the stiffness of a compression bandage. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 415–421. [Google Scholar] [CrossRef]
- Partsch, B.; Partsch, H. Calf compression pressure required to achieve venous closure from supine to standing positions. J. Vasc. Surg. 2005, 42, 734–738. [Google Scholar] [CrossRef]
- Partsch, H.; Mosti, G.; Mosti, F. Narrowing of leg veins under compression demonstrated by magnetic resonance imaging (MRI). Int. Angiol. 2010, 29, 408–410. [Google Scholar] [PubMed]
- Partsch, H.; Menzinger, G.; Mostbeck, A. Inelastic leg compression is more effective to reduce deep venous refluxes than elastic bandages. Dermatol. Surg. 1999, 25, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Mosti, G.; Partsch, H. Duplex scanning to evaluate the effect of compression on venous reflux. Int. Angiol. 2010, 29, 416–420. [Google Scholar]
- Mosti, G.; Mattaliano, V.; Partsch, H. Inelastic compression increases venous ejection fraction more than elastic bandages in patients with superficial venous reflux. Phlebology 2008, 23, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mosti, G.; Partsch, H. Measuring venous pumping function by strain-gauge plethysmography. Int. Angiol. 2010, 29, 421–425. [Google Scholar]
- Partsch, B.; Mayer, W.; Partsch, H. Improvement of ambulatory venous hypertension by narrowing of the femoral vein in congenital absence of venous valves. Phlebology 1992, 7, 101–104. [Google Scholar] [CrossRef]
- Protz, K.; Heyer, K.; Dörler, M.; Stücker, M.; Hampel-Kalthoff, C.; Augustin, M. Compression therapy: Scientific background and practical applications. J. Dtsch. Dermatol. Ges. 2014, 12, 794–801. [Google Scholar] [CrossRef]
- Berszakiewicz, A.; Sieroń, A.; Krasiński, Z.; Cholewka, A.; Stanek, A. Compression therapy in venous diseases: Physical assumptions and clinical effects. Postepy Dermatol. Alergol. 2020, 37, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Müller, M.L.; Calow, T.; Kern, I.K.; Schumann, H. Bandage pressure measurement and training: Simple interventions to improve efficacy in compression bandaging. Int. Wound J. 2009, 6, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S. The impact of a bandage training programme. J. Wound Care 1999, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Ruckley, C.V.; Barbenel, J.C. Improvements in bandaging technique following training. J. Wound Care 1995, 4, 181–184. [Google Scholar] [CrossRef]
- Zarchi, K.; Jemec, G.B. Delivery of compression therapy for venous leg ulcers. JAMA Dermatol. 2014, 150, 730–736. [Google Scholar] [CrossRef]
- Partsch, H. Reliable self-application of short stretch leg compression: Pressure measurements under self-applied, adjustable compression wraps. Phlebology 2019, 34, 208–213. [Google Scholar] [CrossRef]
- Mosti, G.; Partsch, H. Self-management by firm, non-elastic adjustable compression wrap device. Veins Lymphat. 2017, 6, 7003. [Google Scholar] [CrossRef][Green Version]
- Damstra, R.J.; Partsch, H. Prospective, randomized, controlled trial comparing the effectiveness of adjustable compression Velcro wraps versus inelastic multicomponent compression bandages in the initial treatment of leg lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Milic, D.J.; Zivic, S.S.; Bogdanovic, D.C.; Jovanovic, M.M.; Jankovic, R.J.; Milosevic, Z.D.; Stamenkovic, D.M.; Trenkic, M.S. The influence of different sub-bandage pressure values on venous leg ulcers healing when treated with compression therapy. J. Vasc. Surg. 2010, 51, 655–661. [Google Scholar] [CrossRef]
- Mosti, G.; Crespi, A.; Mattaliano, V. Comparison between a New, Two-component Compression System with Zinc Paste Bandages for Leg Ulcer Healing: A Prospective, Multicenter, Randomized, Controlled Trial Monitoring Sub-bandage Pressures. Wounds 2011, 23, 126–134. [Google Scholar] [PubMed]
- Franks, P.J.; Moody, M.; Moffatt, C.J.; Martin, R.; Blewett, R.; Seymour, E.; Hildreth, A.; Hourican, C.; Collins, J.; Heron, A.; et al. Randomized trial of cohesive short-stretch versus four-layer bandaging in the management of venous ulceration. Wound Repair Regen. 2004, 12, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; McCullagh, L.; O’Connor, T.; Doherty, D.C.; Hourican, C.; Stevens, J.; Mole, T.; Franks, P.J. Randomized trial of four-layer and two-layer bandage systems in the management of chronic venous ulceration. Wound Repair Regen. 2003, 11, 166–171. [Google Scholar] [CrossRef]
- Fletcher, A.; Cullum, N.; Sheldon, T.A. A systematic review of compression treatment for venous leg ulcers. BMJ 1997, 315, 576–580. [Google Scholar] [CrossRef]
- Callam, M.J.; Harper, D.R.; Dale, J.J.; Brown, D.; Gibson, B.; Prescott, R.J.; Ruckley, C.V. Lothian and Forth Valley Leg Ulcer Healing Trial Part 1. Elastic versus non-elastic bandaging in the treatment of chronic leg ulceration. Phlebology 1992, 7, 136–141. [Google Scholar] [CrossRef]
- Duby, T.; Hoffman, D.; Cameron, J.; Doblhoff-Brown, D.; Cherry, G.; Ryan, T. A randomized trial in the treatment of venous leg ulcers comparing short stretch bandages, four-layer bandage system, and a long stretch-paste bandage system. Wounds 1993, 5, 276–279. [Google Scholar]
- Ukat, A.; Konig, M.; Vanscheidt, W.; Münter, K.C. Short-stretch versus multilayer compression for venous leg ulcers: A comparison of healing rates. J. Wound Care 2003, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Scriven, J.M.; Taylor, L.E.; Wood, A.J.; Bell, P.R.; Naylor, A.R.; London, N.J. A prospective randomised trial of four-layer versus short stretch compression bandages for the treatment of venous leg ulcers. Ann. R. Coll. Surg. Engl. 1998, 80, 215–220. [Google Scholar] [PubMed]
- Nelson, E.A.; Iglesias, C.P.; Cullum, N.; Torgerson, D.J.; VenUS I collaborators. Randomized clinical trial of four-layer and short-stretch compression bandages for venous leg ulcers (VenUS I). Br. J. Surg. 2004, 91, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Mattaliano, V.; Mosti, G.; Gasbarro, V.; Bucalossi, M.; Blättler, W. The treatment of venous leg ulcers with a specifically designed compression stocking kit. Phlebologie 2008, 37, 191–197. [Google Scholar] [CrossRef]
- Junger, M.; Partsch, H.; Ramelet, A.A.; Zuccarelli, F. Efficacy of a ready-made tubular compression device versus short stretch bandages in the treatment of venous leg ulcers. Wounds 2004, 16, 313–320. [Google Scholar]
- Jünger, M.; Wollina, U.; Kohnen, R.; Rabe, E. Efficacy and tolerability of an ulcer compression stocking for therapy of chronic venous ulcer compared with a below-knee compression bandage: Results from a prospective, randomized, multicentre trial. Curr. Med. Res. Opin. 2004, 20, 1613–1623. [Google Scholar] [CrossRef]
- Horakova, M.A.; Partsch, H. Compression stockings in treatment of lower leg venous ulcer. Wien. Med. Wochenschr. 1994, 144, 242–249. [Google Scholar]
- Brizzio, E.; Amsler, F.; Lun, B.; Blättler, W. Comparison of low-strength compression stockings with bandages for the treatment of recalcitrant venous ulcers. J. Vasc. Surg. 2010, 51, 410–416. [Google Scholar] [CrossRef]
- Amsler, F.; Willenberg, T.; Blättler, W. In search of optimal compression therapy for venous leg ulcers: A meta-analysis of studies comparing diverse [corrected] bandages with specifically designed stockings. J. Vasc. Surg. 2009, 50, 668–674. [Google Scholar] [CrossRef]
- Mosti, G. Elastic stockings versus inelastic bandages for ulcer healing: A fair comparison? Phlebology 2012, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Partsch, H.; Mosti, G. Comparison of three portable instruments to measure compression pressure. Int. Angiol. 2010, 29, 426–430. [Google Scholar] [PubMed]
- Mosti, G.; Mattaliano, V.; Partsch, H. Influence of different materials in multicomponent bandages on pressure and stiffness of the final bandage. Dermatol. Surg. 2008, 34, 631–639. [Google Scholar] [CrossRef]
- Spence, R.K.; Cahall, E. Inelastic versus elastic leg compression in chronic venous insufficiency: A comparison of limb size and venous hemodynamics. J. Vasc. Surg. 1996, 24, 783–787. [Google Scholar] [CrossRef]
- Murthy, G.; Ballard, R.E.; Breit, G.A.; Watenpaugh, D.E.; Hargens, A.R. Intramuscular pressures beneath elastic and inelastic leggings. Ann. Vasc. Surg. 1994, 8, 543–548. [Google Scholar] [CrossRef]
- DePalma, R.G.; Kowallek, D.; Spence, R.K.; Caprini, J.A.; Nehler, M.R.; Jensen, J.; Goldman, M.P. Comparison of Costs and Healing Rates of Two Forms of Compression in Treating Venous Ulcers. Vasc. Surg. 1999, 33, 683–690. [Google Scholar] [CrossRef]
- Blecken, S.R.; Villavicencio, J.L.; Kao, T.C. Comparison of elastic versus nonelastic compression in bilateral venous ulcers: A randomized trial. J. Vasc. Surg. 2005, 42, 1150–1155. [Google Scholar] [CrossRef]
- Mosti, G.; Mancini, S.; Bruni, S.; Serantoni, S.; Gazzabin, L.; Bucalossi, M.; Polignano, R.; Mariani, F.; Luca, B.; Partsch, H.; et al. Adjustable compression wrap devices are cheaper and more effective than inelastic bandages for venous leg ulcer healing. A Multicentric Italian Randomized Clinical Experience. Phlebology 2020, 35, 124–133. [Google Scholar] [CrossRef]
- Jawien, A. The influence of environmental factors in chronic venous insufficiency. Angiology 2003, 54 (Suppl. 1), S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Dix, F.P.; Reilly, B.; David, M.C.; Simon, D.; Dowding, E.; Ivers, L. Effect of leg elevation on healing, venous velocity, and ambulatory venous pressure in venous ulceration. Phlebology 2005, 20, 87–94. [Google Scholar] [CrossRef]
- Simon, D.A.; Dix, F.P.; McCollum, C.N. Management of venous leg ulcers. BMJ 2004, 328, 1358–1362. [Google Scholar] [CrossRef]
- Wittens, C.; Davies, A.H.; Bækgaard, N.; Broholm, R.; Cavezzi, A.; Chastanet, S.; de Wolf, M.; Eggen, C.; Giannoukas, A.; Gohel, M.; et al. Editor’s Choice—Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2015, 49, 678–737. [Google Scholar] [CrossRef]
- Brown, A. Life-style advice and self-care strategies for venous leg ulcer patients: What is the evidence? J. Wound Care 2012, 21, 342–350. [Google Scholar] [CrossRef]
- Kostas, T.I.; Ioannou, C.V.; Drygiannakis, I.; Georgakarakos, E.; Kounos, C.; Tsetis, D.; Katsamouris, A.N. Chronic venous disease progression and modification of predisposing factors. J. Vasc. Surg. 2010, 51, 900–907. [Google Scholar] [CrossRef]
- Roaldsen, K.S.; Rollman, O.; Torebjörk, E.; Olsson, E.; Stanghelle, J.K. Functional ability in female leg ulcer patients--a challenge for physiotherapy. Physiother. Res. Int. 2006, 11, 191–203. [Google Scholar] [CrossRef]
- Mutlak, O.; Aslam, M.; Standfield, N. The influence of exercise on ulcer healing in patients with chronic venous insufficiency. Int. Angiol. 2018, 37, 160–168. [Google Scholar] [CrossRef]
- Qiu, Y.; Osadnik, C.R.; Team, V.; Weller, C.D. Effects of physical activity as an adjunct treatment on healing outcomes and recurrence of venous leg ulcers: A scoping review. Wound Repair Regen. 2022, 30, 172–185. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Finlayson, K.; Kerr, G.; Edwards, H. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: A randomised controlled trial. Int. Wound J. 2017, 14, 130–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, L.; Song, J.L.; Wang, L. Effects of exercise in treating patients with venous leg ulcers: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 1776–1783. [Google Scholar] [CrossRef]
- Palfreyman, S.J.; Nelson, E.A.; Lochiel, R.; Michaels, J.A. Dressings for healing venous leg ulcers. Cochrane Database Syst. Rev. 2006, 3, CD001103. [Google Scholar] [CrossRef]
- Norman, G.; Westby, M.J.; Rithalia, A.D.; Stubbs, N.; Soares, M.O.; Dumville, J.C. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst. Rev. 2018, 6, CD012583. [Google Scholar] [CrossRef] [PubMed]
- Callam, M.J.; Harper, D.R.; Dale, J.J.; Brown, D.; Gibson, B.; Prescott, R.J.; Ruckley, C.V. Lothian and Forth valley leg ulcer healing trial: 2 Knitted viscose dressing versus a hydrocellular dressing in the treatment of chronic leg ulceration. Phlebology 1992, 7, 142–145. [Google Scholar] [CrossRef]
- Skog, E.; Arnesjö, B.; Troëng, T.; Gjöres, J.E.; Bergljung, L.; Gundersen, J.; Hallböök, T.; Hessman, Y.; Hillström, L.; Månsson, T.; et al. A randomized trial comparing cadexomer iodine and standard treatment in the out-patient management of chronic venous ulcers. Br. J. Dermatol. 1983, 109, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Dore, C.J.; Charlett, A.; Lewis, J.D. A randomized trial of biofilm dressing for venous leg ulcers. Phlebology 1992, 7, 108–113. [Google Scholar] [CrossRef]
- Lindholm, C. Results of cost-efficacy aspects in wound care trials. In Proceedings of the Fourth Annual Meeting of the European Tissue Repair Society Academic Centre—John Radcliffe Hospital, Oxford, UK, 25–28 August 1994; p. 199. [Google Scholar] [CrossRef]
- Meridith, K.; Gray, E. Dressed to heal. J. District Nurs. 1988, 7, 8–10. [Google Scholar]
- Nair, H.; Venkateshwaran, N.; Seetharaman, S.S.; Deng, W.; Uthaipaisanwong, A.; Galea, E. Benefits of sucrose octasulfate (TLC-NOSF) dressings in the treatment of chronic wounds: A systematic review. J. Wound Care 2021, 30 (Suppl. 4), S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, A.N. The Benefits of Micronized Purified Flavonoid Fraction (MPFF) Throughout the Progression of Chronic Venous Disease. Adv. Ther. 2020, 37 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Mansilha, A.; Sousa, J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef]
- Coleridge-Smith, P.; Lok, C.; Ramelet, A.A. Venous leg ulcer: A meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Guilhou, J.J.; Février, F.; Debure, C.; Dubeaux, D.; Gillet-Terver, M.N.; Guillot, B.; Levesque, H.; Marzin, L.; Mignot, J.; Ouvry, P.; et al. Benefit of a 2-month treatment with a micronized, purified flavonoidic fraction on venous ulcer healing. A randomized, double-blind, controlled versus placebo trial. Int. J. Microcirc. Clin. Exp. 1997, 17 (Suppl. 1), 21–26. [Google Scholar] [CrossRef]
- Glinski, W.; Chodynicka, B.; Roszkiewicz, J.; Bogdanowski, T.; Lecewicz-Torun, B.; Kaszuba, A.; Bowszyc, J.; Nowak, A.; Wnorowski, J.; Wasik, F.; et al. Efficacia della frazione flavonoica purificata micronizzata (FFPM) nell’aumentare la guarigione della ulcere agli arti inferiori. Studio multicentrico in aperto, controllato e randomizato [Effectiveness of a micronized purified flavonoid fraction (MPFF) in the healing process of lower limb ulcers. An open multicentre study, controlled and randomized]. Minerva Cardioangiol. 2001, 49, 107–114. [Google Scholar]
- Smith, P.C. Daflon 500 mg and venous leg ulcer: New results from a meta-analysis. Angiology 2005, 56 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Guilhou, J.J.; Dereure, O.; Marzin, L.; Ouvry, P.; Zuccarelli, F.; Debure, C.; Van Landuyt, H.; Gillet-Terver, M.N.; Guillot, B.; Levesque, H.; et al. Efficacy of Daflon 500 mg in venous leg ulcer healing: A double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology 1997, 48, 77–85. [Google Scholar] [CrossRef]
- Coccheri, S.; Scondotto, G.; Agnelli, G.; Aloisi, D.; Palazzini, E.; Zamboni, V. Venous arm of the SUAVIS (Sulodexide Arterial Venous Italian Study) Group. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb. Haemost. 2002, 87, 947–952. [Google Scholar] [PubMed]
- González Ochoa, A. Sulodexide and phlebotonics in the treatment of venous ulcer. Int. Angiol. 2017, 36, 82–87. [Google Scholar] [CrossRef]
- Pompilio, G.; Nicolaides, A.; Kakkos, S.K.; Integlia, D. Systematic literature review and network Meta-analysis of sulodexide and other drugs in chronic venous disease. Phlebology 2021, 36, 695–709. [Google Scholar] [CrossRef]
- Wu, B.; Lu, J.; Yang, M.; Xu, T. Sulodexide for treating venous leg ulcers. Cochrane Database Syst. Rev. 2016, 2016, CD010694. [Google Scholar] [CrossRef]
- Sullivan, G.W.; Carper, H.T.; Novick WJJr Mandell, G.L. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect. Immun. 1988, 56, 1722–1729. [Google Scholar] [CrossRef]
- Broderick, C.; Forster, R.; Abdel-Hadi, M.; Salhiyyah, K. Pentoxifylline for intermittent claudication. Cochrane Database Syst. Rev. 2020, 10, CD005262. [Google Scholar] [CrossRef]
- Ahmadi, M.; Khalili, H. Potential benefits of pentoxifylline on wound healing. Expert. Rev. Clin. Pharmacol. 2016, 9, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Nicolaides, A.N.; De Sanctis, M.T.; Incandela, L.; Geroulakos, G. Treatment of venous ulcers with pentoxifylline: A 6-month randomized, double-blind, placebo controlled trial. Angiology 2002, 53 (Suppl. 1), S45–S47. [Google Scholar]
- Falanga, V.; Fujitani, R.M.; Diaz, C.; Hunter, G.; Jorizzo, J.; Lawrence, P.F.; Lee, B.Y.; Menzoian, J.O.; Tretbar, L.L.; Holloway, G.A.; et al. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: Efficacy in a randomized, placebo-controlled trial. Wound Repair Regen. 1999, 7, 208–213. [Google Scholar] [CrossRef]
- Nelson, E.A.; Prescott, R.J.; Harper, D.R.; Gibson, B.; Brown, D.; Ruckley, C.V. A factorial, randomized trial of pentoxifylline or placebo, four-layer or single-layer compression, and knitted viscose or hydrocolloid dressings for venous ulcers. J. Vasc. Surg. 2007, 45, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Arroll, B.; Parag, V.; Waters, J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst. Rev. 2012, 12, CD001733. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Li, Y.; Gao, Y.Y.; Ran, X.W. Efficacy and Safety of Pentoxifylline for Venous Leg Ulcers: An Updated Meta-Analysis. Int. J. Low. Extrem. Wounds. 2021. [Google Scholar] [CrossRef]
- van den Bremer, J.; Moll, F.L. Historical overview of varicose vein surgery. Ann. Vasc. Surg. 2010, 24, 426–432. [Google Scholar] [CrossRef]
- Trendelenburg, F. Uber die unterbindung der vena faphena magna bei unterschendelzaricen. Berl. Klin. Chir. 1890, 7, 195. [Google Scholar]
- Winterborn, R.J.; Earnshaw, J.J. Crossectomy and great saphenous vein stripping. J. Cardiovasc. Surg. 2006, 47, 19–33. [Google Scholar]
- Scheltinga, M.R.; Wijburg, E.R.; Keulers, B.J.; de Kroon, K.E. Conventional versus invaginated stripping of the great saphenous vein: A randomized, double-blind, controlled clinical trial. World J. Surg. 2007, 31, 2236–2242. [Google Scholar] [CrossRef]
- Lacroix, H.; Nevelsteen, A.; Suy, R. Invaginating versus classic stripping of the long saphenous vein. A randomized prospective study. Acta Chir. Belg. 1999, 99, 22–25. [Google Scholar] [CrossRef]
- Goren, G.; Yellin, A.E. Minimally invasive surgery for primary varicose veins: Limited invaginal axial stripping and tributary (hook) stab avulsion. Ann. Vasc. Surg. 1995, 9, 401–414. [Google Scholar] [CrossRef]
- Sam, R.C.; Silverman, S.H.; Bradbury, A.W. Nerve injuries and varicose vein surgery. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 113–120. [Google Scholar] [CrossRef]
- Subramonia, S.; Lees, T. Sensory abnormalities and bruising after great saphenous vein stripping: Impact on short-term quality of life. J. Vasc. Surg. 2005, 42, 510–514. [Google Scholar] [CrossRef]
- Morrison, C.; Dalsing, M.C. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: Preva lence, severity, and relevance for modern practice. J. Vasc. Surg. 2003, 38, 886–890. [Google Scholar] [CrossRef]
- Oesch, A. PIN-stripping: A novel method of atraumatic stripping. Phlebology 1993, 8, 171–173. [Google Scholar] [CrossRef]
- Durkin, M.P.; Turton, E.P.; Scott, D.J.; Berridge, D.C. A prospective randomised trial of PIN versus conventional stripping in varicose vein surgery. Ann. R. Coll. Surg. Eng. 1999, 81, 171–174. [Google Scholar]
- Garde, C. Cryosurgery of varicose veins. J. Dermatol. Surg. Oncol. 1994, 20, 56–58. [Google Scholar] [CrossRef]
- Kostas, T.; Ioannou, C.; Touloupakis, E.; Daskalaki, E.; Giannoukas, A.; Tsetis, D.; Katsamouris, A. Recurrent varicose veins after surgery: A new appraisal of a common and complex problem in vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 275–282. [Google Scholar] [CrossRef]
- Hartmann, K. Endovenous (minimally invasive) procedures for treatment of varicose veins: The gentle and effective alternative to high ligation and stripping operations. Hautarzt 2020, 71 (Suppl. 2), 67–73. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.I.; Kaufman, J.; Göckeritz, O.; Chopra, P.; Evans, M.T.; Hoheim, D.F.; Makhoul, R.G.; Richards, T.; Wenzel, C.; Raines, J.K. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: A multicenter, single-blinded, randomized study (RECOVERY study). J. Vasc. Interv. Radiol. 2009, 20, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Sroka, R.; Weick, K.; Sadeghi-Azandaryani, M.; Steckmeier, B.; Schmedt, C.G. Endovenous laser therapy—application studies and latest investigations. J. Biophotonics 2010, 3, 269–276. [Google Scholar] [CrossRef]
- Sroka, R.; Schaur, P.; Rühm, A.; Pongratz, T.; Schmedt, C.G. Ex-Vivo Investigations of Innovative Fibres for Use in Endoluminal Vein Treatment Procedures. 57; DGP: Bamberg, Germany, 2015. [Google Scholar]
- Hartmann, K.; Stenger, D.; Hartmann, M.; Rafi-Stenger, L. Endochirurgie versus offene Chirugie der Varikose. Vers. Einer Wertung. Hautarzt 2017, 68, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L.; Raines, J.K. ClariVein mechanochemical ablation: Background and procedural details. Vasc. Endovasc. Surg. 2013, 47, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Terlecki, P.; Boryga, M.; Kołodziej, P.; Gołacki, K.; Stropek, Z.; Janczak, D.; Antkiewicz, M.; Zubilewicz, T. Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation. Materials 2022, 15, 2599. [Google Scholar] [CrossRef]
- Gibson, K.; Ferris, B. Cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of post-procedure compression: Initial outcomes of a post-market evaluation of the VenaSeal System (the WAVES Study). Vascular 2017, 25, 149–156. [Google Scholar] [CrossRef]
- Muller, R. Treatment of varicose veins by ambulatory phlebectomy. Phlebologie 1966, 19, 277–279. [Google Scholar] [PubMed]
- Fratila, A.; Rabe, E.; Biltz, H.; Kreysel, H.W. Stellenwert der perkutanen mikrochirurgischen Phlebextraktion nach Varady in der Varizenchirurgie [Value of Varady percutaneous microsurgical phlebologic extraction of varicose veins]. Z. Hautkr. 1990, 65, 487–491. [Google Scholar]
- Juan, J.; Escribano, J.M.; Criado, E.; Fontcuberta, F. Haemodynamic surgery for varicose veins: Surgical strategy. Phlebology 2005, 20, 2–13. [Google Scholar] [CrossRef]
- Bellmunt-Montoya, S.; Escribano, J.M.; Pantoja Bustillos, P.E.; Tello-Díaz, C.; Martinez-Zapata, M.J. CHIVA method for the treatment of chronic venous insufficiency. Cochrane Database Syst. Rev. 2021, 9, CD009648. [Google Scholar] [CrossRef]
- Solıs, J.V.; Ribe’, L.; Portero, J.L.; Rio, J. Stripping saphenectomy, CHIVA and Laser ablation for the treatment of the saphenous vein insufficiency. Amb. Surg. 2009, 15, 11–14. [Google Scholar]
- Onida, S.; Davies, A.H. CHIVA, ASVAL and related techniques—Concepts and evidence. Phlebology 2015, 30 (Suppl. 2), 42–45. [Google Scholar] [CrossRef]
- Pittaluga, P.; Chastanet, S.; Rea, B.; Barbe, R. Midterm results of the surgical treatment of varices by phlebectomy with conservation of a refluxing saphenous vein. K. Vasc. Surg. 2009, 50, 107–118. [Google Scholar] [CrossRef]
- Mavor, G.E.; Galloway, J.M. Iliofemoral venous thrombosis. Pathological considerations and surgical management. Br. J. Surg. 1969, 56, 45–59. [Google Scholar] [CrossRef]
- Douketis, J.D.; Crowther, M.A.; Foster, G.A.; Ginsberg, J.S. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am. J. Med. 2001, 110, 515–519. [Google Scholar] [CrossRef]
- May, R.; Thurner, J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957, 8, 419–427. [Google Scholar] [CrossRef]
- Thijs, W.; Rabe, K.F.; Rosendaal, F.R.; Middeldorp, S. Predominance of left-sided deep vein thrombosis and body weight. J. Thromb. Haemost. 2010, 8, 2083–2084. [Google Scholar] [CrossRef]
- Raju, S.; Hardy, J.D. Technical options in venous valve reconstruction. Am. J. Surg. 1997, 173, 301–307. [Google Scholar] [CrossRef]
- Kistner, R. Surgical repair of a venous valve. Straub. Clin. Proc. 1968, 34, 41–43. [Google Scholar]
- Ajay, K. Khanna.; Shivanshu, Singh. Postthrombotic Syndrome: Surgical Possibilities. Thrombosis 2012, 2012, 520604. [Google Scholar] [CrossRef]
- Palma, E.C.; Esperon, R. Vein transplants and grafts in the surgical treatment of post phlebitic syndrome. J. Cardiovasc. Surg. 1960, 1, 94–107. [Google Scholar]
- Dale, W.A.; Hams, J. Crossover grafts for iliac and femoral venous occlusion. Ann. Surg. 1968, 168, 319–329. [Google Scholar] [CrossRef]
- Edwards, W.S. A-V fistula after venous reconstruction. Ann. Surg. 1982, 196, 669–671. [Google Scholar] [CrossRef]
- Kölbel, T.; Gottsäter, A.; Kühme, T.; Lindh, M.; Ivancev, K. Endovascular Treatment of Venous Occlusive Disease. Ann. Vasc. Dis. 2008, 1, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Neglén, P.; Hollis, K.C.; Olivier, J.; Raju, S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 2007, 46, 979–990. [Google Scholar] [CrossRef]
- Raju, S. Best management options for chronic iliac vein stenosis and occlusion. J. Vasc. Surg. 2013, 57, 1163–1169. [Google Scholar] [CrossRef]
- Razavi, M.K.; Jaff, M.R.; Miller, L.E. Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2015, 8, e002772. [Google Scholar] [CrossRef] [PubMed]
- Marston, W.A.; Chinubhai, A.; Kao, S.; Kalbaugh, C.; Kouri, A. In vivo evaluation of safety and performance of a nitinol venous stent in an ovine iliac venous model. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 73–79. [Google Scholar] [CrossRef]
- Powell, T.; Raju, S.; Jayaraj, A. Comparison between a dedicated venous stent and standard composite Wallstent–Z stent approach to iliofemoral venous stenting: Intermediate-term outcomes. J. Vasc. Surg. Venous Lymphat. Disord. 2023, 11, 82–90. [Google Scholar] [CrossRef]
- Aurshina, A.; Chait, J.; Kibrik, P.; Ostrozhynskyy, Y.; Rajaee, S.; Marks, N.; Ascher, E. Efficacy of balloon venoplasty alone in the correction of nonthrombotic iliac vein lesions. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 665–669. [Google Scholar] [CrossRef]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; Cowman, S.; Kolbach, D.N. Debridement for venous leg ulcers. Cochrane Database Syst. Rev. 2015, 2015, CD008599. [Google Scholar] [CrossRef]
- Serra, R.; Rizzuto, A.; Rossi, A.; Perri, P.; Barbetta, A.; Abdalla, K.; Caroleo, S.; Longo, C.; Amantea, B.; Sammarco, G.; et al. Skin grafting for the treatment of chronic leg ulcers—A systematic review in evidence-based medicine. Int. Wound J. 2017, 14, 149–157. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Eaglstein, W.H.; Kerdel, F.A. Split-thickness skin grafting for lower extremity ulcerations. Dermatol. Surg. 1997, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Carson, J.G.; Chi, Y.W.; Link, D. Management of mixed arterial venous lower extremity ulceration: A review. Vasc. Med. 2015, 20, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ammermann, F.; Meinel, F.G.; Beller, E.; Busse, A.; Streckenbach, F.; Teichert, C.; Weinrich, M.; Neumann, A.; Weber, M.A.; Heller, T. Concomitant chronic venous insufficiency in patients with peripheral artery disease: Insights from MR angiography. Eur. Radiol. 2020, 30, 3908–3914. [Google Scholar] [CrossRef] [PubMed]
- Körber, A.; Klode, J.; Al-Benna, S.; Wax, C.; Schadendorf, D.; Steinstraesser, L.; Dissemond, J. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J. Dtsch. Dermatol. Ges. 2011, 9, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.E.; Harding, K.G.; Enoch, S. Venous and arterial leg ulcers. BMJ 2006, 332, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Nag, F.; De, A.; Hazra, A.; Chatterjee, G.; Ghosh, A.; Surana, T.V. Chronic venous ulceration of leg associated with peripheral arterial disease: An underappreciated entity in developing country. Int. Wound J. 2014, 11, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, M.K.; Giannoukas, A.D. The role of hemodynamic measurements in the management of venous and ischemic ulcers. Int. J. Low. Extrem. Wounds 2007, 6, 254–261. [Google Scholar] [CrossRef]
- Treiman, G.S.; Copland, S.; McNamara, R.M.; Yellin, A.E.; Schneider, P.A.; Treiman, R.L. Factors influencing ulcer healing in patients with combined arterial and venous insufficiency. J. Vasc. Surg. 2001, 33, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, A.S.; Nyamekye, I.; Grabs, A.J.; Farndon, J.R.; Poskitt, K.R. The diagnosis and management of mixed arterial/venous leg ulcers in community-based clinics. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 350–355. [Google Scholar] [CrossRef]
- Humphreys, M.L.; Stewart, A.H.; Gohel, M.S.; Taylor, M.; Whyman, M.R.; Poskitt, K.R. Management of mixed arterial and venous leg ulcers. Br. J. Surg. 2007, 94, 1104–1107. [Google Scholar] [CrossRef]
- Georgopoulos, S.; Kouvelos, G.N.; Koutsoumpelis, A.; Bakoyiannis, C.; Lymperi, M.; Klonaris, C.; Tsigris, C. The effect of revascularization procedures on healing of mixed arterial and venous leg ulcers. Int. Angiol. 2013, 32, 368–374. [Google Scholar] [PubMed]
- Lantis, J.C., 2nd; Boone, D.; Lee, L.; Mendes, D.; Benvenisty, A.; Todd, G. The effect of percutaneous intervention on wound healing in patients with mixed arterial venous disease. Ann. Vasc. Surg. 2011, 25, 79–86. [Google Scholar] [CrossRef]
- Meulendijks, A.M.; de Vries, F.M.C.; van Dooren, A.A.; Schuurmans, M.J.; Neumann, H.A.M. A systematic review on risk factors in developing a first-time Venous Leg Ulcer. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Bültemann, A.; Gerber, V.; Jäger, B.; Münter, C.; Kröger, K. Definitionen für die Wundbehandlung. Hautarzt 2016, 67, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53 (Suppl. 5), 2S–48S. [Google Scholar] [CrossRef]
- Cronenwett, J.; Johnston, K.W. (Eds.) Rutherford’s Vascular Surgery, 7th ed.; Saunders Elsevier: London, UK, 2010; Volume 1–2, 2448p. [Google Scholar]
- Robertson, L.; Lee, A.J.; Gallagher, K.; Carmichael, S.J.; Evans, C.J.; McKinstry, B.H.; Fraser, S.C.; Allan, P.L.; Weller, D.; Ruckley, C.V.; et al. Risk factors for chronic ulceration in patients with varicose veins: A case control study. J. Vasc. Surg. 2009, 49, 1490–1498. [Google Scholar] [CrossRef]
- Johnston, S.; Finlayson, K.; Bui, U.; O’Donoghue, E.; Fletcher, B.; Parker, C.N. Risk factors for the recurrence of venous leg ulcers in adults: A systematic review protocol. J. Tissue Viability 2022, 31, 804–807. [Google Scholar] [CrossRef]
- Uhl, J.F.; Cornu-Thénard, A.; Carpentier, P.H.; Widmer, M.T.; Partsch, H.; Antignani, P.L. Clinical and hemodynamic significance of corona phlebectatica in chronic venous disorders. J. Vasc. Surg. 2005, 42, 1163–1168. [Google Scholar] [CrossRef][Green Version]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Willenberg, T.; Schumacher, A.; Amann-Vesti, B.; Jacomella, V.; Thalhammer, C.; Diehm, N.; Baumgartner, I.; Husmann, M. Impact of obesity on venous hemodynamics of the lower limbs. J. Vasc. Surg. 2010, 52, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, A.M.; Franssen, W.M.A.; Schoonhoven, L.; Neumann, H.A.M. A scoping review on Chronic Venous Disease and the development of a Venous Leg Ulcer: The role of obesity and mobility. J. Tissue Viability 2020, 29, 190–196. [Google Scholar] [CrossRef]
- Finlayson, K.J.; Parker, C.N.; Miller, C.; Edwards, H.E.; Campbell, J. Decreased mobility, lack of social support, haemosiderosis and use of antidepressant medications may predict recurrent venous leg ulcers within 12 months of healing: A prospective longitudinal study. Phlebology 2022, 37, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Scholl, L.; Dörler, M.; Stücker, M. Ulkus bei Adipositas-assoziierter chronischer Veneninsuffizienz. Hautarzt 2017, 68, 560–565. [Google Scholar] [CrossRef]
- Sugerman, H.; Windsor, A.; Bessos, M.; Wolfe, L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J. Intern. Med. 1997, 241, 71–79. [Google Scholar] [CrossRef]
- Arfvidsson, B.; Eklof, B.; Balfour, J. Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc. Endovasc. Surg. 2005, 39, 505–509. [Google Scholar] [CrossRef]
- Willenberg, T.; Clemens, R.; Haegeli, L.M.; Amann-Vesti, B.; Baumgartner, I.; Husmann, M. The influence of abdominal pressure on lower extremity venous pressure and hemodynamics: A human in-vivo model simulating the effect of abdominal obesity. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 849–855. [Google Scholar] [CrossRef]
- Wiewiora, M.; Piecuch, J.; Glück, M.; Slowinska-Lozynska, L.; Sosada, K. Impact of weight loss due to sleeve gastrectomy on shear stress of the femoral vein in morbid obesity. Obes. Surg. 2014, 24, 806–812. [Google Scholar] [CrossRef][Green Version]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Rattazzi, M.; Callegari, E.; Sponchiado, A.; Galliazzo, S.; Pagliara, V.; Villalta, S.; Pauletto, P. Visceral obesity, but not metabolic syndrome, is associated with the presence of post-thrombotic syndrome. Thromb. Res. 2015, 136, 225–228. [Google Scholar] [CrossRef]
- Araujo, D.N.; Ribeiro, C.T.; Maciel, A.C.; Bruno, S.S.; Fregonezi, G.A.; Dias, F.A. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst. Rev. 2016, 12, CD010637, Update in Cochrane Database Syst. Rev. 2023, 6, CD010637. [Google Scholar] [CrossRef]
- Weller, C.D.; Buchbinder, R.; Johnston, R.V. Interventions for helping people adhere to compression treatments for venous leg ulceration. Cochrane Database Syst. Rev. 2016, 3, CD008378. [Google Scholar] [CrossRef]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Martin, R.; Blewett, R.; Ross, F. Prevalence of leg ulceration in a London population. QJM 2004, 97, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.P.; Lastória, S.; Rollo Hde, A. Venous ulcer: Clinical characteristics and risk factors. Int. J. Dermatol. 2011, 50, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, W.B.; Catarinella, F.S.; Lam, Y.L.; Nieman, F.H.; Toonder, I.M.; van der Ham, A.C.; Wittens, C.H. Conservative versus surgical treatment of venous leg ulcers: 10-year follow up of a randomized, multicenter trial. Phlebology 2015, 30 (Suppl. 1), 35–41. [Google Scholar] [CrossRef]
- Van Gent, W.B.; Hop, W.C.; van Praag, M.C.; Mackaay, A.J.; de Boer, E.M.; Wittens, C.H. Conservative versus surgical treatment of venous leg ulcers: A prospective, randomized, multicenter trial. J. Vasc. Surg. 2006, 44, 563–571. [Google Scholar] [CrossRef]
- Gohel, M.S.; Mora MSc, J.; Szigeti, M.; Epstein, D.M.; Heatley, F.; Bradbury, A.; Bulbulia, R.; Cullum, N.; Nyamekye, I.; Poskitt, K.R.; et al. Long-term Clinical and Cost-effectiveness of Early Endovenous Ablation in Venous Ulceration: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 1113–1121. [Google Scholar] [CrossRef]
- Kapp, S.; Miller, C.; Donohue, L. The clinical effectiveness of two compression stocking treatments on venous leg ulcer recurrence: A randomized controlled trial. Int. J. Low. Extrem. Wounds 2013, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kankam, H.K.N.; Lim, C.S.; Fiorentino, F.; Davies, A.H.; Gohel, M.S. A Summation Analysis of Compliance and Complications of Compression Hosiery for Patients with Chronic Venous Disease or Post-thrombotic Syndrome. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 406–416. [Google Scholar] [CrossRef]
- Bush, R.G. New technique to heal venous ulcers: Terminal interruption of the reflux source (TIRS). Perspect. Vasc. Surg. Endovasc. Ther. 2010, 22, 194–199. [Google Scholar] [CrossRef]
- Bush, R.; Bush, P. Percutaneous foam sclerotherapy for venous leg ulcers. J. Wound Care 2013, 22 (Suppl. 10), S20–S522. [Google Scholar] [CrossRef]
- Kamhawy, A.H.; Elbarbary, A.H.; Elhenidy, M.A.; Elwagih, A.M.M. Periulcer Foam Sclerotherapy Injection in Chronic Venous Leg Ulcers Using Near-Infrared Laser for Vein Visualization. Int. J. Low Extrem. Wounds 2020, 19, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Vandongen, Y.K.; Stacey, M.C. Graduated Compression Elastic Stockings Reduce Lipodermatosclerosis and Ulcer Recurrence. Phlebology 2000, 15, 33–37. [Google Scholar] [CrossRef]
- Kapp, S.; Miller, C. The experience of self-management following venous leg ulcer healing. J. Clin. Nurs. 2015, 24, 1300–1309. [Google Scholar] [CrossRef]
- Nelson, E.A.; Bell-Syer, S.E. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst. Rev. 2014, 2014, CD002303. [Google Scholar] [CrossRef]
- Moffatt, C.; Kommala, D.; Dourdin, N.; Choe, Y. Venous leg ulcers: Patient concordance with compression therapy and its impact on healing and prevention of recurrence. Int. Wound J. 2009, 6, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, K.J.; Parker, C.N.; Miller, C.; Gibb, M.; Kapp, S.; Ogrin, R.; Anderson, J.; Coleman, K.; Smith, D.; Edwards, H.E. Predicting the likelihood of venous leg ulcer recurrence: The diagnostic accuracy of a newly developed risk assessment tool. Int. Wound J. 2018, 15, 686–694. [Google Scholar] [CrossRef]
- Bar, L.; Brandis, S.; Marks, D. Improving Adherence to Wearing Compression Stockings for Chronic Venous Insufficiency and Venous Leg Ulcers: A Scoping Review. Patient Prefer. Adherence 2021, 15, 2085–2102. [Google Scholar] [CrossRef]
- Van Hecke, A.; Grypdonck, M.; Beele, H.; Vanderwee, K.; Defloor, T. Adherence to leg ulcer lifestyle advice: Qualitative and quantitative outcomes associated with a nurse-led intervention. J. Clin. Nurs. 2011, 20, 429–443. [Google Scholar] [CrossRef]
- Probst, S.; Weller, C.D.; Bobbink, P.; Saini, C.; Pugliese, M.; Skinner, M.B.; Gethin, G. Prevalence and incidence of venous leg ulcers-a protocol for a systematic review. Syst. Rev. 2021, 10, 148. [Google Scholar] [CrossRef]
- Klonizakis, M.; Tew, G.A.; Gumber, A.; Crank, H.; King, B.; Middleton, G.; Michaels, J.A. Supervised exercise training as an adjunct therapy for venous leg ulcers: A randomized controlled feasibility trial. Br. J. Dermatol. 2018, 178, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, K.; Edwards, H.; Courtney, M. Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: A prospective study. J. Adv. Nurs. 2011, 67, 2180–2190. [Google Scholar] [CrossRef] [PubMed]

| Medical History | |
| History of present symptoms and signs | Duration and presence of symptoms: Cramps, tired legs, swollen legs, heavy legs, restless legs, venous claudication, itching Pain: distribution, intensity (VAS score 0–10), duration, intermittent, during night/day, pain during dressing changes |
| Duration and presence of signs: Varicose veins: duration, uni-/bilateral, bleeding from the vein Swelling: uni-/bilateral, region: around the ankle, whole leg, relation to standing/sitting a whole day Active ulcer: spontaneous/post-traumatic, duration, dressings (type/frequency of changes), compression therapy | |
| Past signs | Previously healed/recurrent ulcers: spontaneous/post-traumatic, duration, dressings, compression therapy DVT/SVT/PE: time of occurrence, therapy Previous leg fractures Previous surgical therapy |
| Comorbidities | Diabetes mellitus, hypertension, chronic renal insufficiency, heart failures, malignancy, rheumatoid arthritis, PAD, obesity, back problems |
| Treatment | Treatment of present and past varicose veins: laser, sclerotherapy, surgical treatment, endovenous ablation (non-thermal/thermal), compression therapy (short-/long-stretch bandages, stockings, Velcro® materials) Treatment of present ulcer: dressings, therapy of surrounding skin, compression; where and by whom treatment is provided (patient/nurse/in hospital/in healthcare center); medications (anticoagulants, contraceptives, hormone replacement therapy, antidiabetics antihypertensives, immunosuppressive therapy, other) |
| Allergies | Contact/systemic drug reaction |
| Pregnancy | When, number, signs, and symptoms during pregnancy, therapy of signs/symptoms |
| Family history | Presence of varicose veins in relatives, ulcers, DVT |
| Occupation | Prolonged standing/sitting |
| Bad habits | Smoking, alcohol consumption, drug use |
| Trauma | Mechanical, chemical, radiotherapy, chemotherapy, etc. |
| Clinical Assessment | |
| Inspection and palpation | Mobility, BMI Presence of varicose veins, corona phlebectatica Limb swelling: Stemmer’s sign, non-pitting/pitting, Bisgaard sign Skin changes: hyperpigmentations/redness (whole leg/during the vein, eczema), lipodermatosclerosis/atrophie blanche Peripheral arterial pulses, capillarity refilling Groin lymph nodes Leg temperature (cold/warm) Scars after previous surgical therapy, trauma Trophic changes in nails |
| Leg ulcers: where, number, size, wound bed (necrosis, fibrin (ogen), granulation tissue, epithelial tissue, isles in wound bed), edges, surrounding skin, smell, presence of infection, wound exudate, possibility of ankle movements | |
| Functional/Diagnostics Testing | |
| Venous system | CW Doppler: S–F junction reflux Duplex US Photoplethysmography |
| Arterial system | Measurement of ABI (with CW Doppler; automatic) |
| Lymphatic system | Limb circumferences, perimetry, bioimpedance |
| Non-invasive/invasive tests | Monofilament test Capillaroscopy Venography IVUS Angiography Lymphoscintigraphy CT MR |
| Microbiological | Swab for pathogenic bacteria and fungi |
| Skin/ulcer biopsy | Pathohistological examination Direct immunofluorescence |
| Blood tests | Complete/differential blood count, C-reactive protein, erythrocyte sedimentation rate, blood glucose, HBA1c, blood lipids electrolytes, urea, creatinine, liver function tests tests of coagulations total proteins, circulating immune complex, immunoglobulins, cryoglobulins, APC resistance, protein C, S, homocysteine ANAs, ENA, anti-DNA, ANCAs, antiphospholipid antibodies, lupus antibodies, pemphigus and pemphigoid antibodies, vitamins (B12, D3, folic acid, A), trace elements (Fe, Zn, Mg, Cu) Serological tests (lues tests—TPHA, leprosis, tbc) |
| The Questions We Ask Ourselves | The Causes/Symptoms/Signs | Tests for Making the Diagnosis |
|---|---|---|
| What is the immediate cause of the wound? |
| |
| Is there any underlying pathology? |
|
|
| Does the patient have any (medical) conditions? | Diabetes mellitus, malnutrition, cardiovascular disease, anemia, renal disease, rheumatoid arthritis, cerebrovascular disease, old age, reduction in sensory perception, increasing susceptibility to trauma, therapy with immunosuppression agents, malignancies and their treatment, smoking, alcohol consumption, drug use, prolonged standing/sitting, obesity, poor mobility |
| Palpation of lower-extremity arterial pulses and calculated ABI are recommended for all patients with suspected venous leg ulcers | C |
| Duplex ultrasound sonography is recommended for patients with venous leg ulcers to assess venous reflux and/or obstruction | C |
| Biopsy is recommended for patients with venous leg ulcers if healing stalls | C |
| Biopsy is recommended for patients with ulcers if there is suspicion that the ulcer may be venous, but it has an atypical appearance | C |
| Referral to a subspecialist is recommended for patients with venous leg ulcers if healing stalls | C |
| Referral to a subspecialist is recommended for patients with ulcers if there is suspicion that the ulcer is not venous, but it is of an atypical appearance | C |
| Screening of patients using a hand-held Doppler detector makes sense only in mild involvement when only telangiectasias and venectasias are present | C |
| X-ray contrast venography, magnetic resonance, or computed tomography venography are reasonable to perform only in a small number of selected patients who have anatomical venous anomalies, and in those patients in whom surgical intervention on the deep venous system is planned. | C |
| Venous Reflux (VR) | Calf Pumping Function (CPF) | Ambulatory Venous Hypertension (AVH) | |
|---|---|---|---|
| Elastic material | ~ | ~ | ~ |
| Inelastic material | +++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, A.; Mosti, G.; Nematillaevich, T.S.; Valesky, E.M.; Planinšek Ručigaj, T.; Boucelma, M.; Marakomichelakis, G.; Liew, A.; Fazeli, B.; Catalano, M.; et al. No More Venous Ulcers—What More Can We Do? J. Clin. Med. 2023, 12, 6153. https://doi.org/10.3390/jcm12196153
Stanek A, Mosti G, Nematillaevich TS, Valesky EM, Planinšek Ručigaj T, Boucelma M, Marakomichelakis G, Liew A, Fazeli B, Catalano M, et al. No More Venous Ulcers—What More Can We Do? Journal of Clinical Medicine. 2023; 12(19):6153. https://doi.org/10.3390/jcm12196153
Chicago/Turabian StyleStanek, Agata, Giovanni Mosti, Temirov Surat Nematillaevich, Eva Maria Valesky, Tanja Planinšek Ručigaj, Malika Boucelma, George Marakomichelakis, Aaron Liew, Bahar Fazeli, Mariella Catalano, and et al. 2023. "No More Venous Ulcers—What More Can We Do?" Journal of Clinical Medicine 12, no. 19: 6153. https://doi.org/10.3390/jcm12196153
APA StyleStanek, A., Mosti, G., Nematillaevich, T. S., Valesky, E. M., Planinšek Ručigaj, T., Boucelma, M., Marakomichelakis, G., Liew, A., Fazeli, B., Catalano, M., & Patel, M. (2023). No More Venous Ulcers—What More Can We Do? Journal of Clinical Medicine, 12(19), 6153. https://doi.org/10.3390/jcm12196153








