A Pilot Nurse-Administered CBT Intervention for Insomnia in Patients with Schizophrenic Disorder: A Randomized Clinical Effectiveness Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedure
2.2.1. Sample Selection
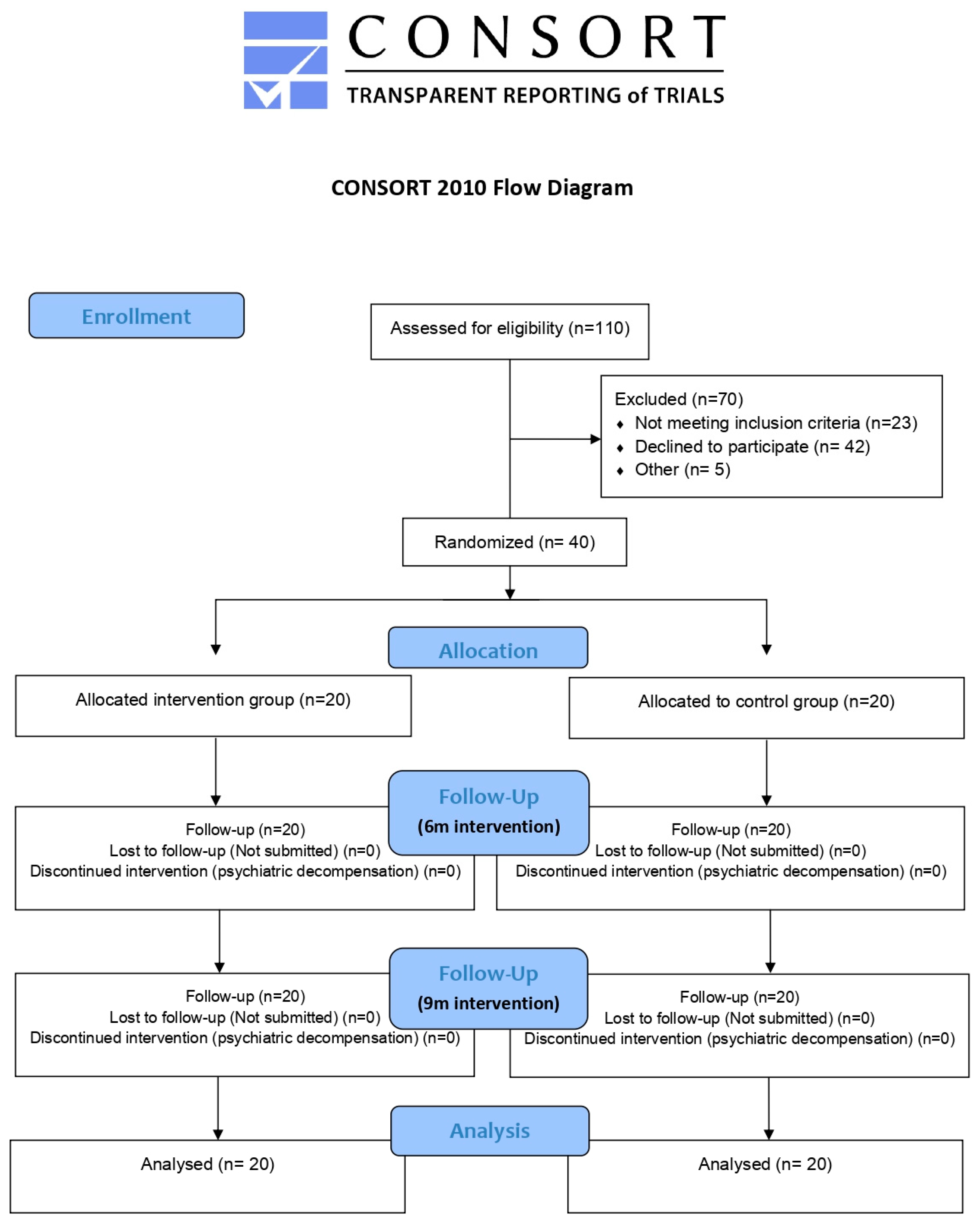
2.2.2. First Evaluation and Randomisation
2.2.3. Evaluation Follow-Up
2.3. Instruments
2.3.1. Insomnia Severity Index
2.3.2. Pittsburgh Sleep Quality Index
2.3.3. EuroQol-5D Scale
2.4. Group Treatments
2.4.1. Description of the Psychoeducational Group Intervention
2.4.2. Description of the Control Group
2.5. Analysis
Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Intervention Results
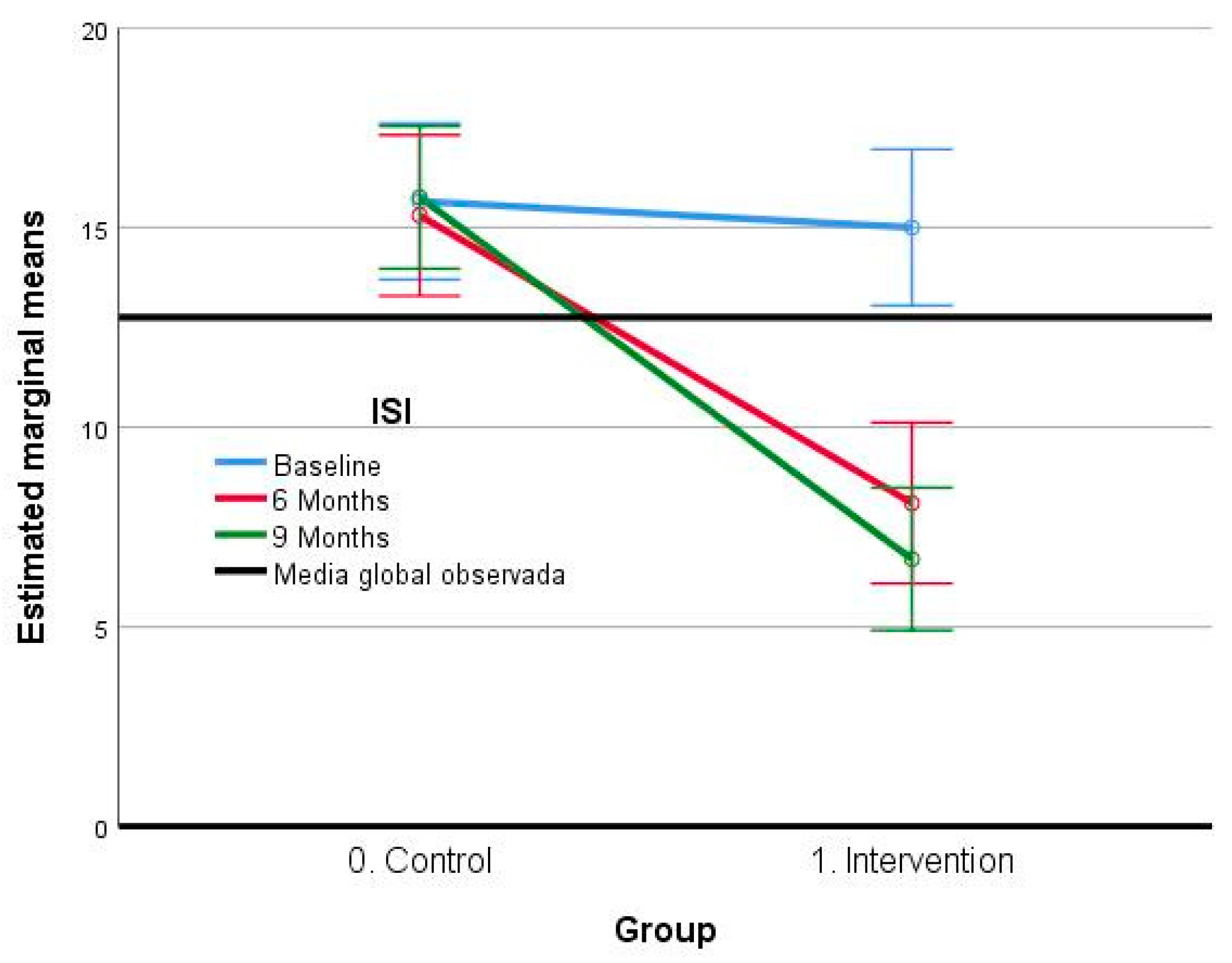
3.2.1. Insomnia Severity Index (ISI)
3.2.2. Pittsburgh Scale
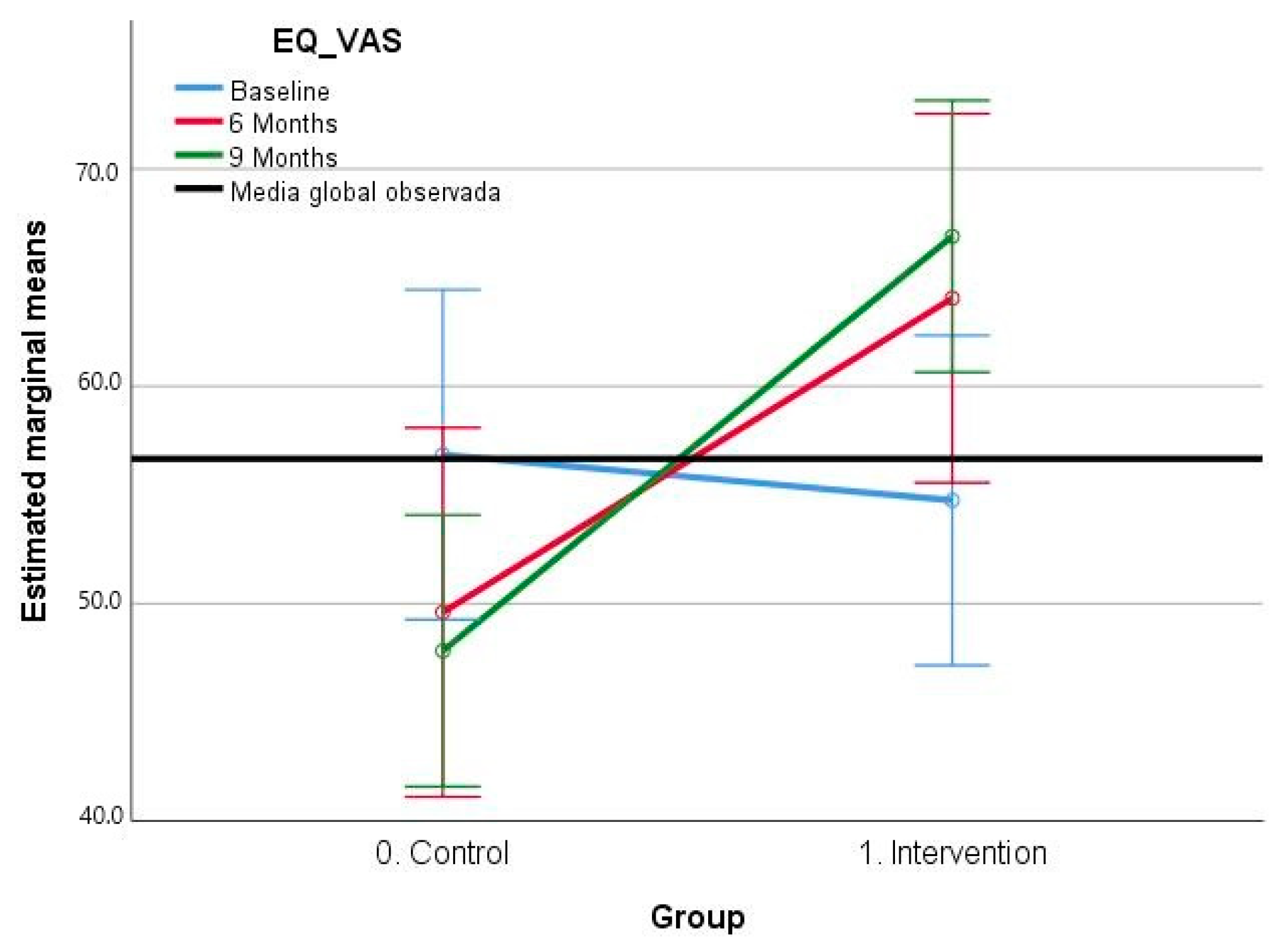
3.2.3. EQ-5D–VAS Scale Results
4. Discussion
4.1. Study Limitations
4.2. Future Implications and Future Lines of Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velayos, J.L. Medicina del Sueño: Enfoque Multidisciplinario; Editorial Médica Panamericana: Madrid, Spain, 2009. [Google Scholar]
- Miró, E.; del Cano-Lozano, M.C.; Buela-Casal, G. Sueño y calidad de vida. Rev. Colomb. Psicol. 2005, 14, 11–27. [Google Scholar]
- MedinaOrtiz, Ó.; Sánchez Mora, N.; Conejo Galindo, J.; Fraguas Herráez, D.; Arango López, C. Alteraciones del sueño en los trastornos psiquiátricos. Rev. Colomb. Psiquiatr. 2007, 36, 701–717. [Google Scholar]
- Torres, V.; Monteghirfo, R. Trastornos del sueño. Arch. Med. Interna 2011, XXXIII, 29–46. [Google Scholar]
- Hou, C.-L.; Li, Y.; Cai, M.-Y.; Ma, X.-R.; Zang, Y.; Jia, F.-J.; Lin, Y.-Q.; Ungvari, G.S.; Chiu, H.F.K.; Ng, C.H.; et al. Prevalence of Insomnia and Clinical and Quality of Life Correlates in Chinese Patients with Schizophrenia Treated in Primary Care. Perspect. Psychiatr. Care 2017, 53, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, S. Sleep Disturbances in Patients with Schizophrenia. CNS Drugs 2008, 22, 939–962. [Google Scholar] [CrossRef]
- Kaufmann, C.N.; Spira, A.P.; Rae, D.S.; West, J.C.; Mojtabai, R. Sleep Problems, Psychiatric Hospitalization, and Emergency Department Use Among Psychiatric Patients with Medicaid. Psychiatr. Serv. 2011, 62, 1101–1105. [Google Scholar] [CrossRef]
- Mondal, G.; Bajaj, V.; Goyal, B.L.; Mukherjee, N. Prevalence of sleep disorders and severity of insomnia in psychiatric outpatients attending a tertiary level mental health care facility in Punjab, India. Asian J. Psychiatr. 2018, 32, 8–13. [Google Scholar] [CrossRef]
- Hatoum, H.T.; Kong, S.X.; Kania, C.M.; Wong, J.M.; Mendelson, W.B. Insomnia, Health-Related Quality of Life and Healthcare Resource Consumption. Pharmacoeconomics 1998, 14, 629–637. [Google Scholar] [CrossRef]
- Roth, T.; Ancoli-Israel, S. Daytime consequences and correlates of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. II. Sleep 1999, 22 (Suppl. 2), S354–S358. [Google Scholar]
- Benson, K.L. Sleep in Schizophrenia: Impairments, Correlates, and Treatment. Psychiatr. Clin. N. Am. 2006, 29, 1033–1045. [Google Scholar] [CrossRef]
- Leger, D.; Poursain, B. An international survey of insomnia: Under-recognition and under-treatment of a polysymptomatic condition. Curr. Med. Res. Opin. 2005, 21, 1785–1792. [Google Scholar] [CrossRef]
- Ozminkowski, R.J.; Wang, S.; Walsh, J.K. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 2007, 30, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Bonin, E.M.; Beecham, J.; Swift, N.; Raikundalia, S.; Brown, J.S.L. Psycho-educational CBT-Insomnia workshops in the community. A cost-effectiveness analysis alongside a randomised controlled trial. Behav. Res. Ther. 2014, 55, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Alanen, Y.O.; González De Chávez, M.; Silver, A.-L.S.; Martindale, B. Abordajes Psicoterapéuticos de las Psicosis Esquizofrénicas. Historia, Desarrollo y Prespectivas; Fundación para la Investigación y el Tratamiento de la Esquizofrénia y Otros Trastornos: Madrid, Spain, 2008; ISBN 978-84-612-6634-0. [Google Scholar]
- Crowe, M.; Whitehead, L.; Wilson, L.; Carlyle, D.; O’Brien, A.; Inder, M.; Joyce, P. Disorder-specific psychosocial interventions for bipolar disorder—A systematic review of the evidence for mental health nursing practice. Int. J. Nurs. Stud. 2010, 47, 896–908. [Google Scholar] [CrossRef]
- Cuevas-Cancino, J.J.; Moreno-Pérez, N.E. Psicoeducación: Intervención de enfermería para el cuidado de la familia en su rol de cuidadora. Enfermería Univ. 2017, 14, 207–218. [Google Scholar] [CrossRef]
- Casañas, R.; Catalán, R.; del Val, J.L.; Real, J.; Valero, S.; Casas, M. Effectiveness of a psycho-educational group program for major depression in primary care: A randomized controlled trial. BMC Psychiatry 2012, 12, 230. [Google Scholar] [CrossRef]
- Cheung, J.M.Y.; Jarrin, D.C.; Ballot, O.; Bharwani, A.A.; Morin, C.M. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med. Rev. 2019, 44, 23–36. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, S.Y.; Tsai, P.S. Cognitive behavioural therapy for primary insomnia: A systematic review. J. Adv. Nurs. 2005, 50, 553–564. [Google Scholar] [CrossRef]
- Wu, J.Q.; Appleman, E.R.; Salazar, R.D.; Ong, J.C. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions a meta-analysis. JAMA Intern. Med. 2015, 175, 1461–1472. [Google Scholar] [CrossRef]
- Hwang, D.K.; Nam, M.; Lee, Y.J.G. The effect of cognitive behavioral therapy for insomnia in schizophrenia patients with sleep Disturbance: A non-randomized, assessor-blind trial. Psychiatry Res. 2019, 274, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Waite, F.; Startup, H.; Myers, E.; Lister, R.; McInerney, J.; Harvey, A.G.; Geddes, J.; Zaiwalla, Z.; Luengo-Fernandez, R.; et al. Efficacy of cognitive behavioural therapy for sleep improvement in patients with persistent delusions and hallucinations (BEST): A prospective, assessor-blind, randomised controlled pilot trial. Lancet Psychiatry 2015, 2, 975–983. [Google Scholar] [CrossRef]
- Myers, E.; Startup, H.; Freeman, D. Cognitive behavioural treatment of insomnia in individuals with persistent persecutory delusions: A pilot trial. J. Behav. Ther. Exp. Psychiatry 2011, 42, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Sheaves, B.; Freeman, D.; Isham, L.; McInerney, J.; Nickless, A.; Yu, L.M.; Rek, S.; Bradley, J.; Reeve, S.; Attard, C.; et al. Stabilising sleep for patients admitted at acute crisis to a psychiatric hospital (OWLS): An assessor-blind pilot randomised controlled trial. Psychol. Med. 2018, 48, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- de Niet, G.; Tiemens, B.; van Achterberg, T.; Hutschemaekers, G. Applicability of two brief evidence-based interventions to improve sleep quality in inpatient mental health care. Int. J. Ment. Health Nurs. 2011, 20, 319–327. [Google Scholar] [CrossRef]
- Watanabe, N.; Furukawa, T.A.; Shimodera, S.; Morokuma, I.; Katsuki, F.; Fujita, H.; Sasaki, M.; Kawamura, C.; Perlis, M.L. Brief behavioral therapy for refractory insomnia in residual depression: An assessor-blind, randomized controlled trial. J. Clin. Psychiatry 2011, 72, 1651–1658. [Google Scholar] [CrossRef]
- Faulkner, S.; Bee, P. Experiences, perspectives and priorities of people with schizophrenia spectrum disorders regarding sleep disturbance and its treatment: A qualitative study. BMC Psychiatry 2017, 17, 158. [Google Scholar] [CrossRef]
- Mijnster, T.; Boersma, G.J.; Meijer, E.; Lancel, M. Effectivity of (Personalized) Cognitive Behavioral Therapy for Insomnia in Mental Health Populations and the Elderly: An Overview. J. Pers. Med. 2022, 12, 1070. [Google Scholar] [CrossRef]
- Waite, F.; Myers, E.; Harvey, A.G.; Espie, C.A.; Startup, H.; Sheaves, B.; Freeman, D. Treating Sleep Problems in Patients with Schizophrenia. Behav. Cogn. Psychother. 2016, 44, 273–287. [Google Scholar] [CrossRef]
- Batalla-Martín, D.; Martorell-Poveda, M.-A.; Belzunegui-Eraso, A.; Miralles Garijo, E.; Del-Cuerpo Serratosa, A.; Valdearcos Pérez, J.; Montané Escobar, M.; Lopez-Ruiz, M. The Experience of Insomnia in Patients with Schizophrenic Disorder: A Qualitative Study. Front. Psychiatry 2022, 12, 805601. [Google Scholar] [CrossRef]
- Batalla-martín, D.; Belzunegui-eraso, A.; Garijo, E.M.; Martín, E.M.; Garcia, R.R.; Heras, J.S.M.; Lopez-ruiz, M.; Martorell-poveda, M.A. Insomnia in schizophrenia patients: Prevalence and quality of life. Int. J. Environ. Res. Public Health 2020, 17, 1350. [Google Scholar] [CrossRef]
- Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 2019, 13, S27–S30. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993. [Google Scholar]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Rodriguez-Muñoz, A.; Vela-Bueno, A.; Olavarrieta-Bernardino, S.; Calhoun, S.L.; Bixler, E.O.; Vgontzas, A.N. The Spanish version of the Insomnia Severity Index: A confirmatory factor analysis. Sleep Med. 2012, 13, 207–210. [Google Scholar] [CrossRef]
- Sierra, J.C.; Guillén-Serrano, V.; Santos-Iglesias, P. Insomnia Severity Index: Some indicators about its reliability and validity on an older adults sample. Rev. Neurol. 2008, 47, 566–570. [Google Scholar] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Macias Fernandez, J.A.; Royuela Rico, A. La versión española del Índice de Calidad de Sueño de Pittsburgh Clinical practice in Psychiatry View project neuropsychiatry View project. Inf. Psiquiatr. 1996, 146, 465–472. [Google Scholar]
- Jiménez-Genchi, A.; Monteverde-Maldonado, E.; Nenclares-Portocarrero, A.; Esquivel-Adame, G.; De La Vega-Pacheco, A. Confiabilidad y análisis factorial de la versión en español del índice de calidad de sueño de Pittsburgh en pacientes psiquiátricos. Gac. Med. Mex. 2008, 144, 491–496. [Google Scholar] [PubMed]
- EuroQol Group EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clin. 1999, 112 (Suppl. 1), 79–85. [Google Scholar]
- van Agt, H.M.; Essink-Bot, M.L.; Krabbe, P.F.; Bonsel, G.J. Test-retest reliability of health state valuations collected with the EuroQol questionnaire. Soc. Sci. Med. 1994, 39, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Sacristán, J.A.; Hormaechea, J.A.; Casado, A.; Badia, X.; Gómez, J.C. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr. Med. Res. Opin. 2004, 20, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.P.; Baker, C.A. Intrusive thoughts and auditory hallucinations: A comparative study of intrusions in psychosis. Behav. Res. Ther. 2000, 38, 1097–1106. [Google Scholar] [CrossRef]
- Chiu, V.W.; Harvey, R.H.; Sloan, N.B.; Ree, M.; Lin, A.; Janca, A.; Waters, F. Cognitive and behavioral factors associated with insomnia in inpatients with schizophrenia and related psychoses. J. Nerv. Ment. Dis. 2015, 203, 798–803. [Google Scholar] [CrossRef]
- Luca, M. Maladaptive Rumination as a Transdiagnostic Mediator of Vulnerability and Outcome in Psychopathology. J. Clin. Med. 2019, 8, 314. [Google Scholar] [CrossRef]
- Chiu, V.W.; Ree, M.; Janca, A.; Waters, F. Sleep in Schizophrenia: Exploring Subjective Experiences of Sleep Problems, and Implications for Treatment. Psychiatr. Q. 2016, 87, 633–648. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Sun, L.; Wang, J.; Xia, L.; Yang, Y.; Sun, F.; Li, W.; Yao, X.; Yang, R.; et al. Physical activity levels associated with insomnia and depressive symptoms in middle-aged and elderly patients with chronic schizophrenia. Front. Psychiatry 2023, 13, 1045398. [Google Scholar] [CrossRef]
- Minato, M.; Zemke, R. Time use of people with schizophrenia living in the community. Occup. Ther. Int. 2004, 11, 177–191. [Google Scholar] [CrossRef]
- Dunn, E.C.; Wewiorski, N.J.; Rogers, E.S. The meaning and importance of employment to people in recovery from serious mental illness: Results of a qualitative study. Psychiatr. Rehabil. J. 2008, 32, 59–62. [Google Scholar] [CrossRef]
- Messari, S.; Hallam, R. CBT for psychosis: A qualitative analysis of clients’ experiences. Br. J. Clin. Psychol. 2003, 42, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 62627. [Google Scholar] [CrossRef] [PubMed]
- López Martín, E.; Ardura Martínez, D. Effect size in scientific publication. Educ. XX1 2023, 26, 9–17. [Google Scholar] [CrossRef]
- Geiger-Brown, J.M.; Rogers, V.E.; Liu, W.; Ludeman, E.M.; Downton, K.D.; Diaz-Abad, M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med. Rev. 2015, 23, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Tsiachristas, A.; Waite, F.; Freeman, D.; Luengo-Fernandez, R. Cost-effectiveness of cognitive-behavioural therapy for sleep disorder added to usual care in patients with schizophrenia: The BEST study. BJPsych Open 2018, 4, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; MacMahon, K.M.A.; Kelly, H.L.; Broomfield, N.M.; Douglas, N.J.; Engleman, H.M.; McKinstry, B.; Morin, C.M.; Walker, A.; Wilson, P. Randomized Clinical Effectiveness Trial of Nurse-Administered Small-Group Cognitive Behavior Therapy for Persistent Insomnia in General Practice. Sleep 2007, 30, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Keliat, B.A.; Riasmini, N.M.; Daulima, N.H.C.; Erawati, E. Applying the community mental health nursing model among people with schizophrenia. Enferm. Clin. 2022, 32, 131–138. [Google Scholar] [CrossRef]
- Nagai, M.; Oe, Y.; Horikoshi, M.; Nakajima, S.; Oi, H.; Kita, Y. Evaluation of a Japanese brief CBT-I administered by a nurse: A pilot study. Prim. Health Care Res. Dev. 2022, 23, e42. [Google Scholar] [CrossRef]
- Waters, F.; Chiu, V.W.; Janca, A.; Atkinson, A.; Ree, M. Preferences for different insomnia treatment options in people with schizophrenia and related psychoses: A qualitative study. Front. Psychol. 2015, 6, 990. [Google Scholar] [CrossRef]
- Birling, Y.; Wang, J.; Li, G.; Wu, E.; Yu, Z.; Feng, Y.; Wu, Y. Culturally Adapted CBTI for Chinese Insomnia Patients: A One-Arm Pilot Trial. Int. J. Behav. Med. 2018, 25, 331–340. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef]
- Chiu, V.W.; Ree, M.; Janca, A.; Iyyalol, R.; Dragovic, M.; Waters, F. Sleep profiles and CBT-I response in schizophrenia and related psychoses. Psychiatry Res. 2018, 268, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Olsen, M.K.; Stechuchak, K.M.; Means, M.K.; Lineberger, M.D.; Kirby, A.; Carney, C.E. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: A randomized clinical trial. Sleep 2009, 32, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Tegeler, C.L.; Munger Clary, H.; Shaltout, H.A.; Simpson, S.L.; Gerdes, L.; Tegeler, C.H. Cereset Research Standard Operating Procedures for Insomnia: A Randomized, Controlled Clinical Trial. Glob. Adv. Integr. Med. Health 2023, 12, 275361302211474. [Google Scholar] [CrossRef]
- Savard, M.-H.; Savard, J.; Simard, S.; Ivers, H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology 2005, 14, 429–441. [Google Scholar] [CrossRef]
- Morin, C.M. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med. Rev. 2003, 7, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601. [Google Scholar] [CrossRef]
- Yang, M.; Morin, C.M.; Schaefer, K.; Wallenstein, G.V. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Curr. Med. Res. Opin. 2009, 25, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Sidey-Gibbons, C. Use of the Pittsburgh Sleep quality index in people with schizophrenia spectrum disorders: A mixed methods study. Front. Psychiatry 2019, 10, 284. [Google Scholar] [CrossRef]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H. Insomnia as a Health Risk Factor. Behav. Sleep Med. 2003, 1, 227–247. [Google Scholar] [CrossRef]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of insomnia, depression, and anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef]
- Chalet, F.-X.; Saskin, P.; Ahuja, A.; Thompson, J.; Olopoenia, A.; Modi, K.; Morin, C.M.; Wickwire, E.M. The Associations between Insomnia Severity and Health Outcomes in the United States. J. Clin. Med. 2023, 12, 2438. [Google Scholar] [CrossRef] [PubMed]
- Zeitlhofer, J.; Schmeiser-Rieder, A.; Tribl, G.; Rosenberger, A.; Bolitschek, J.; Kapfhammer, G.; Saletu, B.; Katschnig, H.; Holzinger, B.; Popovic, R.; et al. Sleep and quality of life in the Austrian population. Acta Neurol. Scand. 2000, 102, 249–257. [Google Scholar] [CrossRef]
- Chalet, F.X.; Bujaroska, T.; Germeni, E.; Ghandri, N.; Maddalena, E.T.; Modi, K.; Olopoenia, A.; Thompson, J.; Togninalli, M.; Briggs, A.H. Mapping the Insomnia Severity Index Instrument to EQ-5D Health State Utilities: A United Kingdom Perspective. PharmacoEconomics-Open 2023, 7, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Schotanus, A.Y.; Dozeman, E.; Ikelaar, S.L.C.; van Straten, A.; Beekman, A.T.F.; van Nassau, F.; Bosmans, J.E.; van Schaik, A. Internet-delivered cognitive behavioural therapy for insomnia disorder in depressed patients treated at an outpatient clinic for mood disorders: Protocol of a randomised controlled trial. BMC Psychiatry 2023, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Raya-Tena, A.; Fernández-San-martin, M.I.; Martin-Royo, J.; Casañas, R.; Sauch-Valmaña, G.; Cols-Sagarra, C.; Navas-Mendez, E.; Masa-Font, R.; Casajuana-Closas, M.; Foguet-Boreu, Q.; et al. Effectiveness of a Psychoeducational Group Intervention Carried Out by Nurses for Patients with Depression and Physical Comorbidity in Primary Care: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 2948. [Google Scholar] [CrossRef]
- Enomoto, K.; Adachi, T.; Fujino, H.; Kugo, M.; Tatsumi, S.; Sasaki, J. Comparison of the effectiveness of cognitive behavioral therapy for insomnia, cognitive behavioral therapy for pain, and hybrid cognitive behavioral therapy for insomnia and pain in individuals with comorbid insomnia and chronic pain: A systematic review and network meta-analysis. Sleep Med. Rev. 2022, 66, 101693. [Google Scholar] [CrossRef]
- Van Der Zweerde, T.; Lancee, J.; Slottje, P.; Bosmans, J.E.; Van Someren, E.J.W.; Van Straten, A. Nurse-Guided Internet-Delivered Cognitive Behavioral Therapy for Insomnia in General Practice: Results from a Pragmatic Randomized Clinical Trial. Psychother. Psychosom. 2020, 89, 174–184. [Google Scholar] [CrossRef]
- Belleville, G.; Cousineau, H.; Levrier, K.; St-Pierre-Delorme, M.E. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin. Psychol. Rev. 2011, 31, 638–652. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Jafari, E.; Broström, A.; Ohayon, M.M.; Lin, C.Y.; Griffiths, M.D.; Blom, K.; Jernelöv, S.; Kaldo, V.; Pakpour, A.H. Effects of cognitive behavioral therapy for insomnia (CBT-I) on quality of life: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 64, 101646. [Google Scholar] [CrossRef]

| Session | Objective | Measure of Adaptation |
|---|---|---|
| 1 | First group meeting Sleep and insomnia’s basic concepts | Promoting CBT intervention potential Increasing motivation for change Patient narratives |
| 2 | Sleep hygiene | Increased physical activity Patient narratives |
| 3 | Stimulus control Reducing time in bed | Patient narratives |
| 4 | Relaxation techniques | Making mandalas Patient narratives |
| 5 | Cognitive therapy | Intervention for rumination control Patient narratives |
| 6 | Summary of previous sessions Group farewell | Encourage the linking of community and rehabilitative resources to reduce social isolation. Patient narratives |
| Control Group | Intervention Group | χ2/t | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | 0.000 | 1 | ||||
| Male | 12 | 60.0 | 12 | 60.0 | ||
| Female | 8 | 40.0 | 8 | 40.0 | ||
| Age (mean ± SD) | 50.35 (±12.75) | 50.25 (±11.22) | 0.026 | 0.979 | ||
| Marital status | 1.233 | 0.745 | ||||
| Single | 13 | 65.0 | 11 | 55.0 | ||
| Married/In relationship | 4 | 20.0 | 6 | 30.0 | ||
| Separated/Divorced | 1 | 5.0 | 2 | 10.0 | ||
| Widowed | 2 | 10.0 | 1 | 5.0 | ||
| Educational attainment | 1.118 | 0.773 | ||||
| No primary school | 1 | 5.0 | 1 | 5.0 | ||
| Primary school | 9 | 45.0 | 8 | 40.0 | ||
| Secondary school | 9 | 45.0 | 8 | 40.0 | ||
| University | 1 | 5.0 | 3 | 15.0 | ||
| Employment status | 1.467 | 0.690 | ||||
| Special work centre | 0 | 0.0 | 0 | 0.0 | ||
| Freelancer | 0 | 0.0 | 0 | 0.0 | ||
| Salaried employee | 2 | 10.0 | 1 | 5.0 | ||
| Unemployed | 1 | 5.0 | 0 | 0.00 | ||
| Disability | 14 | 70.0 | 16 | 80.0 | ||
| Retired | 3 | 15.0 | 3 | 15.0 | ||
| Degree of disability (mean ± SD) | 65.74 (±4.86) | 64.70 (±6.49) | 0.562 | 0.577 | ||
| Link to resources | 1.382 | 0.710 | ||||
| No link | 12 | 60.0 | 10 | 50.0 | ||
| SRC | 2 | 10.0 | 2 | 10.0 | ||
| Pre-employment | 3 | 15.0 | 2 | 10.0 | ||
| Social club | 3 | 15.0 | 6 | 30.0 | ||
| Income level | 1.048 | 0.592 | ||||
| No income | 0 | 0.0 | 0 | 0.0 | ||
| Less than minimum wage | 11 | 55.0 | 10 | 50.0 | ||
| Minimum wage | 0 | 0.0 | 1 | 5.0 | ||
| More than minimum wage | 9 | 45.0 | 9 | 45.0 | ||
| BMI (mean ± SD) | 31.27 (±7.34) | 32.06 (±6.38) | −0.365 | 0.717 | ||
| Antipsychotics | ||||||
| Yes | 20 | 100.0 | 20 | 100. | ||
| No | 0 | 0.0 | 0 | 0.0 | ||
| Antidepressant | 0.902 | 0.342 | ||||
| Yes | 11 | 55.0 | 8 | 40.0 | ||
| No | 9 | 45.0 | 12 | 60.0 | ||
| Mood stabiliser | 1.558 | 0.212 | ||||
| Yes | 5 | 25.0 | 2 | 10.0 | ||
| No | 15 | 75.0 | 18 | 80.0 | ||
| Anxiolytics | 0.102 | 0.749 | ||||
| Yes | 11 | 55.0 | 12 | 60.0 | ||
| No | 9 | 45.0 | 8 | 40.0 | ||
| Control Group | Intervention Group | χ2/t | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Insomnia Severity Index Ordinal | 3.604 | 0.165 | ||||
| No insomnia | 0 | 0.0 | 0 | 0.0 | ||
| Mild insomnia | 6 | 30.0 | 11 | 55.0 | ||
| Moderate insomnia | 13 | 65.0 | 7 | 35.0 | ||
| Severe insomnia | 1 | 5.0 | 2 | 10.0 | ||
| Index Severity Insomnia (mean ± SD) | 15.65 (±4.45) | 15.00 (±4.18) | 0.476 | 0.637 | ||
| Pittsburgh (mean ± SD) | 13.80 (±4.31) | 13.70 (± 3.29) | 0.082 | 0.935 | ||
| EQ-5D—Mobility | 0.960 | 0.327 | ||||
| No problems | 11 | 55.0 | 14 | 70.0 | ||
| Moderate problems | 9 | 45.0 | 6 | 30.0 | ||
| Severe problems | 0 | 0.0 | 0 | 0.0 | ||
| EQ-5D Self-Care | 2.850 | 0.091 | ||||
| No problems | 11 | 55.0 | 16 | 80.0 | ||
| Moderate problems | 9 | 45.0 | 4 | 20.0 | ||
| Severe problems | 0 | 0.0 | 0 | 0.0 | ||
| Usual activities | 4.570 | 0.083 | ||||
| No problems | 5 | 25.0 | 11 | 55.0 | ||
| Moderate problems | 13 | 65.0 | 9 | 45.0 | ||
| Severe problems | 2 | 10.0 | 0 | 0.0 | ||
| Pain/Discomfort | 4.570 | 0.102 | ||||
| No problems | 6 | 30.0 | 4 | 20.0 | ||
| Moderate problems | 9 | 45.0 | 15 | 75.0 | ||
| Severe problems | 5 | 25.0 | 1 | 5.0 | ||
| Anxiety/Depression | 1.360 | 0.507 | ||||
| No problems | 3 | 15.0 | 3 | 15.0 | ||
| Moderate problems | 14 | 70.0 | 11 | 55.0 | ||
| Severe problems | 3 | 15.0 | 6 | 30.0 | ||
| EQ-VAS (mean ± SD) | 56.85 (±13.30) | 54.75 (±19.63) | 0.396 | 0.694 | ||
| ISI | ||
|---|---|---|
| Control | Intervention | |
| M (SD) | M (SD) | |
| Initial time point | 15.65 (4.45) | 15.00 (4.18) |
| 6 months | 15.30 (4.86) | 8.10 (4.00) |
| 9 months | 15.75 (4.34) | 6.70 (3.50) |
| F | ηp2 | |
| Intra-subject | 29.29 ** (<0.000) | 0.435 (Large) |
| Intersection | 28.36 ** (<0.000) | 0.427 (Large) |
| Inter-group | 23.67 ** (<0.000) | 0.384 (Large) |
| MD | IC 95% | |
| 0 vs. 6 months | 3.63 ** (<0.000) | 2.02–5.23 |
| 0 vs. 9 months | 4.10 ** (<0.000) | 2.45–5.75 |
| 6 vs. 9 months | 0.48 NS (0.841) | −0.61–1.56 |
| Groups | 5.63 ** (<0.000) | 3.29–7.98 |
| Initial Time Point | 6 Months | 9 Months | ||||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No Insomnia | 0.0% (0) | 0.0% (0) | 15.0% (3) | 50.0% (10) | 0.0% (0) | 55.0% (11) |
| Mild Insomnia | 30.0% (6) | 50.0% (10) | 25.0% (5) | 40.0% (8) | 40.0% (8) | 45.0% (9) |
| Moderate Insomnia | 65.0% (13) | 40.0% (8) | 55.0% (11) | 10.0% (2) | 50.0% (10) | 0.0% (0) |
| Severe Insomnia | 5.0% (1) | 10.0% (2) | 5.0% (1) | 0.0% (0) | 10.0% (2) | 0.0% (0) |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 3.60 (0.165 NS) | 0.300 | 11.69 (0.009 **) | 0.541 | 23.06 (0.000 **) | 0.759 | |
| PSQI | ||
|---|---|---|
| Control | Intervention | |
| M (SD) | M (SD) | |
| Initial time point | 13.80 (3.31) | 13.70 (3.29) |
| 6 months | 13.50 (4.20) | 8.00 (3.58) |
| 9 months | 14.30 (3.59) | 8.60 (3.36) |
| F | ηp2 | |
| Intra-subject | 17.87 ** (<0.000) | 0.320 (Large) |
| Intersection | 18.31 ** (<0.000) | 0.325 (Large) |
| Inter-group | 13.73 ** (0.001) | 0.265 (Large) |
| MD | IC 95% | |
| 0 vs. 6 months | 3.00 ** (<0.000) | 1.53–4.47 |
| 0 vs. 9 months | 2.30 ** (<0.000) | 0.85–3.75 |
| 6 vs. 9 months | −0.70 NS (0.240) | −1.67–0.27 |
| Groups | 3.77 ** (<0.000) | 1.71–5.83 |
| EQ-VAS | ||
|---|---|---|
| Control | Intervention | |
| M (SD) | M (SD) | |
| Initial time point | 56.85 (13.30) | 54.75 (19.63) |
| 6 Months | 49.60 (16.50) | 64.05 (20.78) |
| 9 Months | 47.83 (13.60) | 66.90 (14.01) |
| F | ηp2 | |
| Intra-subject | 0.24 NS (0.783) | 0.006 (Small) |
| Intersection | 12.05 ** (<0.000) | 0.241 (Large) |
| Inter-group | 5.33 * (0.027) | 0.123 (Medium) |
| MD | IC 95% | |
| 0 vs. 6 months | −1.03 NS (0.999) | −7.04–4.99 |
| 0 vs. 9 months | −1.56 NS (0.999) | −7.55–4.42 |
| 6 vs. 9 months | −0.54 NS (0.999) | −5.52–4.44 |
| Groups | −10.48 * (0.027) | −19.66–−1.29 |
| EQ-5D Mobility | Initial Time point | 6 Months | 9 Months | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No problems | 55.0% (11) | 70.0% (14) | 60.0% (12) | 70.0% (14) | 60.0% (12) | 80.0% (16) |
| Some problems | 45.0% (9) | 30.0% (6) | 40.0% (8) | 30.0% (6) | 40.0% (8) | 20.0% (4) |
| Major problems | - | - | - | - | - | - |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 0.96 (0.327 NS) | 0.155 | 0.44 (0.507 NS) | 0.105 | 1.91 (0.168 NS) | 0.218 | |
| EQ-5D Self-Care | Initial time point | 6 months | 9 months | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No problems | 55.0% (11) | 80.0% (16) | 40.0% (8) | 85.0% (17) | 35.0% (7) | 75.0% (15) |
| Some problems | 45.0% (9) | 20.0% (4) | 60.0% (12) | 15.0% (3) | 65.0% (13) | 25.0% (5) |
| Major problems | - | - | - | - | - | - |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 2.85 (0.091 NS) | 0.267 | 8.64 (0.003 **) | 0.465 | 6.47 (0.011 *) | 0.402 | |
| EQ-5D Usual activities | Initial time point | 6 months | 9 months | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No problems | 25.0% (5) | 55.0% (11) | 40.0% (8) | 60.0% (12) | 35.0% (7) | 55.0% (11) |
| Some problems | 65.0% (13) | 45.0% (9) | 60.0% (12) | 40.0% (8) | 50.0% (10) | 45.0% (9) |
| Major problems | 10.0% (2) | - | - | - | 15.0% (3) | - |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 4.98 (0.083 NS) | 0.353 | 1.60 (0.206 NS) | 0.200 | 3.94 (0.139 NS) | 0.314 | |
| EQ-5D Pain/discomfort | Initial time point | 6 months | 9 months | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No problems | 30.0% (6) | 20.0% (4) | 25.0% (5) | 70.0% (14) | 25.0% (5) | 55.0% (11) |
| Some problems | 45.0% (9) | 75.0% (15) | 55.0% (11) | 25.0% (5) | 45.0% (9) | 40.0% (8) |
| Major problems | 25.0% (5) | 5.0% (1) | 20.0% (4) | 5.0% (1) | 30.0% (6) | 5.0% (1) |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 4.57 (0.102 NS) | 0.338 | 8.31 (0.016 *) | 0.456 | 5.88 (0.053 NS) | 0.383 | |
| EQ-5D Anxiety/depression | Initial time point | 6 months | 9 months | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| No problems | 15.0% (3) | 15.0% (3) | 5.0% (1) | 25.0% (5) | 5.0% (1) | 25.0% (5) |
| Some problems | 70.0% (14) | 55.0% (11) | 70.0% (14) | 60.0% (12) | 80.0% (16) | 60.0% (12) |
| Major problems | 15.0% (3) | 30.0% (6) | 25.0% (5) | 15.0% (3) | 15.0% (3) | 15.0% (3) |
| χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | χ2 (p) | Cramer’s V | |
| 1.36 (0.507NS) | 0.184 | 3.32 (0.190 NS) | 0.288 | 3.24 (0.198 NS) | 0.285 | |
| EQ-5D Mobility | Control Group | Intervention Group | ||||
|---|---|---|---|---|---|---|
| Initial Time Point | 6 Months | 9 Months | Initial Time Point | 6 Months | 9 Months | |
| No problems | 55.0% (11) | 60.0% (12) | 60.0% (12) | 70.0% (14) | 70.0% (14) | 80.0% (16) |
| Some problems | 45.0% (9) | 40.0% (8) | 40.0% (8) | 30.0% (6) | 30.0% (6) | 20.0% (4) |
| Major problems | - | - | - | - | - | - |
| Initial time point–6 months | Initial time point–9 months | 6 months–9 months | Initial time point–6 months | Initial time point–9 months | 6 months–9 months | |
| Mc Nemar (p) | - (0.999 NS) | - (0.999 NS) | - (0.999 NS) | - (0.999 NS) | - (0.687 NS) | - (0.500 NS) |
| EQ-5D Self-Care | Control Group | Intervention Group | ||||
| Initial time point | 6 Months | 9 Months | Initial time point | 6 Months | 9 Months | |
| No problems | 55.0% (11) | 40.0% (8) | 35.0% (7) | 80.0% (16) | 85.0% (17) | 75.0% (15) |
| Some problems | 45.0% (9) | 60.0% (12) | 65.0% (13) | 20.0% (4) | 15.0% (3) | 25.0% (5) |
| Major problems | - | - | - | - | - | - |
| Initial time point–6 months | Initial time point–9 months | 6 months–9 months | Initial time point–6 months | Initial time point–9 months | 6 months–9 months | |
| Mc Nemar (p) | - (0.453 NS) | - (0.289 NS) | - (0.999 NS) | - (0.999 NS) | - (0.999 NS) | - (0.500 NS) |
| EQ-5D Usual activities | Control Group | Intervention Group | ||||
| Initial time point | 6 Months | 9 Months | Initial time point | 6 Months | 9 Months | |
| No problems | 25.0% (5) | 40.0% (8) | 35.0% (7) | 55.0% (11) | 60.0% (12) | 55.0% (11) |
| Some problems | 65.0% (13) | 60.0% (12) | 50.0% (10) | 45.0% (9) | 40.0% (8) | 45.0% (9) |
| Major problems | 10.0% (2) | - | 15.0% (3) | - | - | - |
| Initial time point–6 months | Initial time point–9 months | 6 months–9 months | Initial time point–6 months | Initial time point–9 months | 6 months–9 months | |
| Mc Nemar (p) | - (0.999 NS) | 1.00 (0.607 NS) | - (0.999 NS) | - (0.999 NS) | - (0.999 NS) | - (0.999 NS) |
| EQ-5D Pain/discomfort | Control Group | Intervention Group | ||||
| Initial time point | 6 Months | 9 Months | Initial time point | 6 Months | 9 Months | |
| No problems | 30.0% (6) | 25.0% (5) | 25.0% (5) | 20.0% (4) | 70.0% (14) | 55.0% (11) |
| Some problems | 45.0% (9) | 55.0% (11) | 45.0% (9) | 75.0% (15) | 25.0% (5) | 40.0% (8) |
| Major problems | 25.0% (5) | 20.0% (4) | 30.0% (6) | 5.0% (1) | 5.0% (1) | 5.0% (1) |
| Initial time point–6 months | Initial time point–9 months | 6 months–9 months | Initial time point–6 months | Initial time point–9 months | 6 months–9 months | |
| Mc Nemar (p) | 0.48 (0.788 NS) | 1.00 (0.801 NS) | 1.00 (0.607 NS) | 10.00 (0.002 **) | 7.0 (0.030 *) | 1.80 (0.407 NS) |
| EQ-5D Anxiety/depression | Control Group | Intervention Group | ||||
| Initial time point | 6 Months | 9 Months | Initial time point | 6 Months | 9 Meses | |
| No problems | 15.0% (3) | 5.0% (1) | 5.0% (1) | 15.0% (3) | 25.0% (5) | 25.0% (5) |
| Some problems | 70.0% (14) | 70.0% (14) | 80.0% (16) | 55.0% (11) | 60.0% (12) | 60.0% (12) |
| Major problems | 15.0% (3) | 25.0% (5) | 15.0% (3) | 30.0% (6) | 15.0% (3) | 15.0% (3) |
| Initial time point–6 months | Initial time point–9 months | 6 months–9 months | Initial time point–6 months | Initial time point–9 months | 6 months–9 months | |
| Mc Nemar (p) | 1.67 (0.435 NS) | 1.00 (0.607 NS) | 3.80 (0.284 NS) | 5.00 (0.082 NS) | 5.00 (0.082 NS) | - (0.999 NS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batalla-Martin, D.; Martorell-Poveda, M.-A.; Belzunegui-Eraso, A.; Marieges Gordo, A.; Batlle Lleal, H.; Pasqual Melendez, R.; Querol Girona, R.; López-Ruiz, M. A Pilot Nurse-Administered CBT Intervention for Insomnia in Patients with Schizophrenic Disorder: A Randomized Clinical Effectiveness Trial. J. Clin. Med. 2023, 12, 6147. https://doi.org/10.3390/jcm12196147
Batalla-Martin D, Martorell-Poveda M-A, Belzunegui-Eraso A, Marieges Gordo A, Batlle Lleal H, Pasqual Melendez R, Querol Girona R, López-Ruiz M. A Pilot Nurse-Administered CBT Intervention for Insomnia in Patients with Schizophrenic Disorder: A Randomized Clinical Effectiveness Trial. Journal of Clinical Medicine. 2023; 12(19):6147. https://doi.org/10.3390/jcm12196147
Chicago/Turabian StyleBatalla-Martin, David, Maria-Antonia Martorell-Poveda, Angel Belzunegui-Eraso, Alejandro Marieges Gordo, Helena Batlle Lleal, Raquel Pasqual Melendez, Raquel Querol Girona, and Marina López-Ruiz. 2023. "A Pilot Nurse-Administered CBT Intervention for Insomnia in Patients with Schizophrenic Disorder: A Randomized Clinical Effectiveness Trial" Journal of Clinical Medicine 12, no. 19: 6147. https://doi.org/10.3390/jcm12196147
APA StyleBatalla-Martin, D., Martorell-Poveda, M.-A., Belzunegui-Eraso, A., Marieges Gordo, A., Batlle Lleal, H., Pasqual Melendez, R., Querol Girona, R., & López-Ruiz, M. (2023). A Pilot Nurse-Administered CBT Intervention for Insomnia in Patients with Schizophrenic Disorder: A Randomized Clinical Effectiveness Trial. Journal of Clinical Medicine, 12(19), 6147. https://doi.org/10.3390/jcm12196147








