Effectiveness of Dry Needling and Ischaemic Trigger Point Compression of the Levator Scapulae in Patients with Chronic Neck Pain: A Short-Term Randomized Clinical Trial
Abstract
1. Introduction
1.1. Myofascial Trigger Points (MTrPs) and Myofascial Pain Syndrome (MPS)
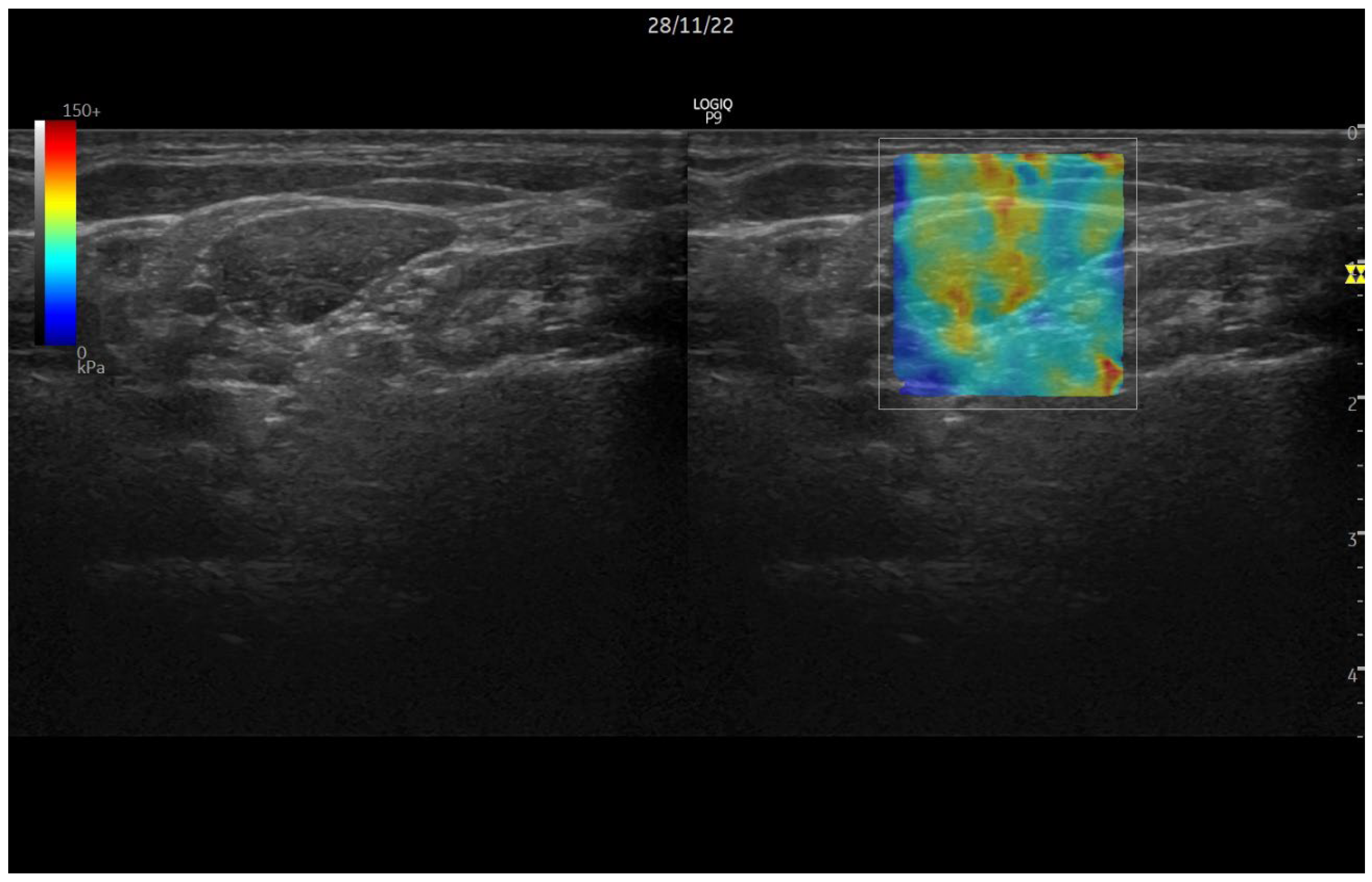
1.2. Ultrasound Elastography
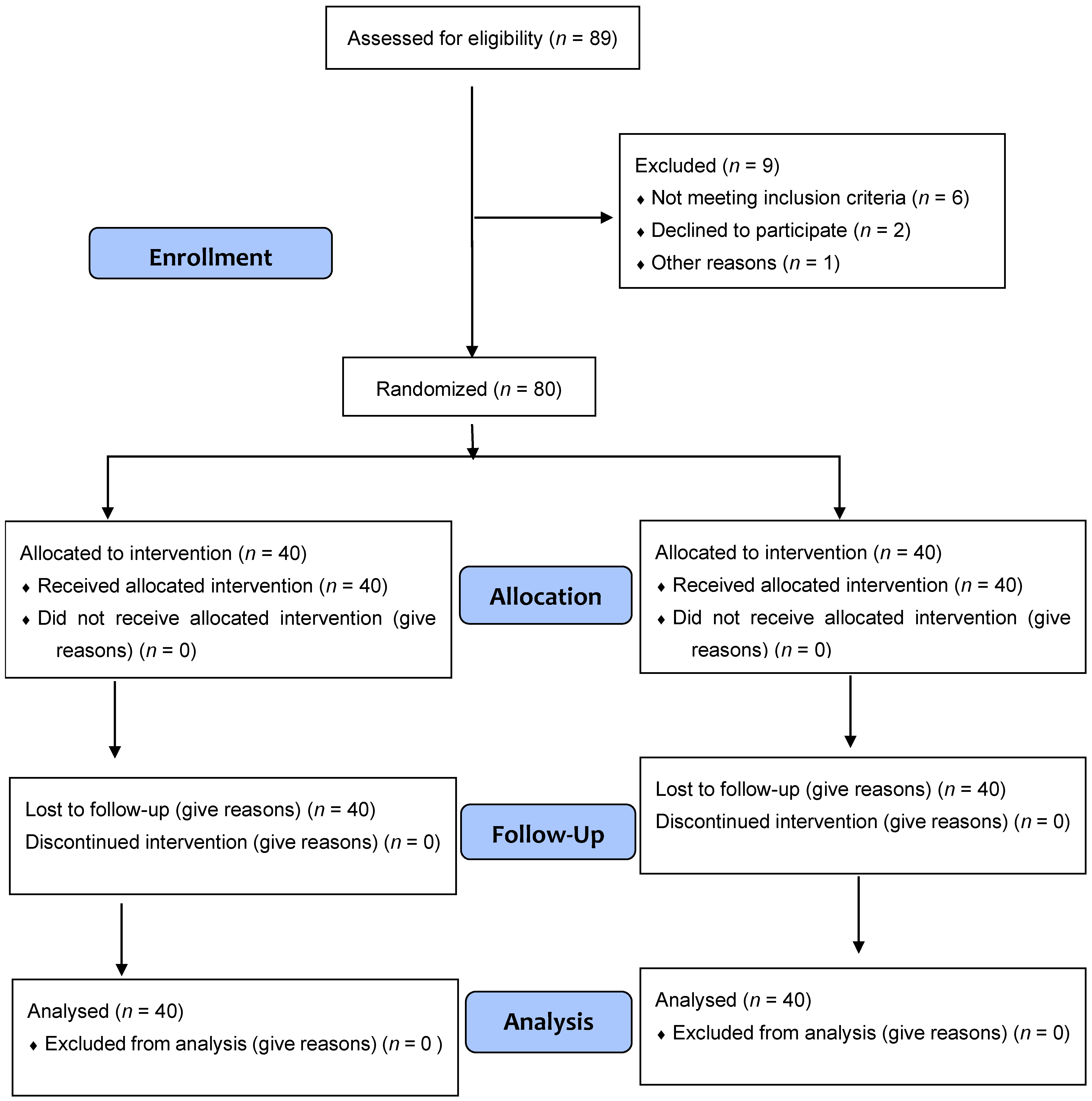
2. Materials and Methods
2.1. Common Treatment Parts
2.2. Identification of Latent MTrPs
2.3. Invasive Technique: DN Group (n = 40)
2.4. IC Group with Conservative Technique (n = 40)
2.5. Outcome Measures
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Chronic neck pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of chronic neck pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Griswold, D.; Gargano, F.; Learman, K.E. A randomized clinical trial comparing non-thrust manipulation with segmental and distal dry needling on pain, disability, and rate of recovery for patients with chronic neck pain. J. Man. Manip. Ther. 2019, 27, 141. [Google Scholar] [CrossRef] [PubMed]
- Ramsook, R.R.; Malanga, G.A. Myofascial chronic neck pain. Curr. Pain. Headache Rep. 2012, 16, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Bron, C.; Dommerholt, J.D. Etiology of Myofascial Trigger Points. Curr. Pain. Headache Rep. 2012, 16, 439. [Google Scholar] [CrossRef]
- Hong, C.Z. Treatment of myofascial pain syndrome. Curr. Pain. Headache Rep. 2006, 10, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H.; Schneider, M. Chiropractic Management of Myofascial Trigger Points and Myofascial Pain Syndrome: A Systematic Review of the Literature. J. Manip. Physiol. Ther. 2009, 32, 14–24. [Google Scholar] [CrossRef]
- Tekin, L.; Akarsu, S.; Durmuş, O.; Çakar, E.; Dinçer, Ü.; Kiralp, M.Z. The effect of dry needling in the treatment of myofascial pain syndrome: A randomized double-blinded placebo-controlled trial. Clin. Rheumatol. 2013, 32, 309–315. [Google Scholar] [CrossRef]
- Lizier, D.T.; Perez, M.V.; Sakata, R.K. Exercises for treatment of nonspecific chronic neck pain. Rev. Bras. Anestesiol. 2012, 62, 838–846. [Google Scholar] [CrossRef]
- Hemmer, C.R. Evaluation and Treatment of Chronic neck pain in Adult Patients. Orthop. Nurs. 2021, 40, 336–342. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What chronic neck pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Chenot, J.F.; Greitemann, B.; Kladny, B.; Petzke, F.; Pfingsten, M.; Schorr, S.G. Chronic neck pain. Dtsch. Arztebl. Int. 2017, 114, 883. [Google Scholar] [CrossRef] [PubMed]
- Dolor de Origen Muscular: Dolor Miofascial y Fibromialgia. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1134-80462007000100006 (accessed on 29 March 2022).
- Trigger Points: Diagnosis and Management—American Family Physician. Available online: https://www.aafp.org/afp/2002/0215/p653.html (accessed on 29 March 2022).
- Borg-Stein, J.; Simons, D.G. Focused review: Myofascial pain. Arch. Phys. Med. Rehabil. 2002, 83 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Gallego, J.; Solana Galdámez, R. Liberación Miofascial: Síndrome de Dolor Miofascial y Puntos Gatillo. 1998. Available online: https://www.casadellibro.com/libro-sindrome-de-dolor-miofascial-y-puntos-gatillo-liberacion-miofasc-ial/9788495052148/628807 (accessed on 29 March 2022).
- Shah, J.P.; Thaker, N.; Heimur, J.; Aredo, J.V.; Sikdar, S.; Gerber, L. Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective. PMR 2015, 7, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Dolor y Disfunción Miofascial: El Manual de Puntos Gatillo—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2484855/ (accessed on 7 June 2022).
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Declaración de Helsinki de la AMM—Principios Éticos para las Investigaciones Médicas en Seres Humanos—WMA—The World Medical Association. Available online: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ (accessed on 9 April 2022).
- Simons, D.G. New views of myofascial trigger points: Etiology and diagnosis. Arch. Phys. Med. Rehabil. 2008, 89, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Cummings, T.M.; White, A.R. Needling therapies in the management of myofascial trigger point pain: A systematic review. Arch. Phys. Med. Rehabil. 2001, 82, 986–992. [Google Scholar] [CrossRef]
- Travell, J.G.; Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1992. [Google Scholar]
- Cagnie, B.; Castelein, B.; Pollie, F.; Steelant, L.; Verhoeyen, H.; Cools, A. Evidence for the use of ischemic compression and dry needling in the management of trigger points of the upper trapezius in Patients with Neck Pain: A Systematic Review. Am. J. Phys. Med. Rehabil. 2015, 94, 573–583. [Google Scholar] [CrossRef]
- Bernardelli, R.S.; Santos, B.C.; Scharan, K.O.; Corrêa, K.P.; Silveira, M.I.B.; de Moser, A.D.L. Application of the refinements of ICF linking rules to the Visual Analogue Scale, Roland Morris questionnaire and SF-36. Cien Saude Colet. 2021, 26, 1137–1152. [Google Scholar] [CrossRef]
- Badia, X.; Alonso, J. La Medida de la Salud, GuÍa de Escalas de Medición en Español; Tecnología y Ediciones del Conocimiento, S.L.: Barcelona, Spain, 2007; pp. 95–98. ISBN 978-84-611-8288-6. [Google Scholar]
- Wolan-Nieroda, A.; Guzik, A.; Mocur, P.; Drużbicki, M.; Maciejczak, A. Assessment of Interrater and Intrarater Reliability of Cervical Range of Motion (CROM) Goniometer. Biomed. Res. Int. 2020, 2020, 8908035. [Google Scholar] [CrossRef]
- Vanderweeën, L.; Oostendorp, R.A.B.; Vaes, P.; Duquet, W. Pressure algometry in manual therapy. Man. Ther. 1996, 1, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and Usefulness of the Pressure Pain Threshold Measurement in Patients with Myofascial Pain. Ann. Rehabil. Med. 2011, 35, 412. [Google Scholar] [CrossRef] [PubMed]
- Unalan, H.; Majlesi, J.; Aydin, F.Y.; Palamar, D. Comparison of high-power pain threshold ultrasound therapy with local injection in the treatment of active myofascial trigger points of the upper trapezius muscle. Arch. Phys. Med. Rehabil. 2011, 92, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Swanenburg, J.; Humphreys, K.; Langenfeld, A.; Brunner, F.; Wirth, B. Validity and reliability of a German version of the Neck Disability Index (NDI-G). Manual Ther. 2014, 19, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Rah, U.W.; Sheen, S.S.; Cho, K.H. Comparison of 3 needle sizes for trigger point injection in myofascial pain syndrome of upper- and middle-trapezius muscle: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2009, 90, 1332–1339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, C.T.; Hsieh, L.F.; Kuan, T.S.; Kao, M.J.; Chou, L.W.; Hong, C.Z. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am. J. Phys. Med. Rehabil. 2010, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ziaeifar, M.; Arab, A.M.; Nourbakhsh, M.R. Clinical Effectiveness of Dry Needling Immediately After Application on Myofascial Trigger Point in Upper Trapezius Muscle. J. Chiropr. Med. 2016, 15, 252. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Pacheco-Da-Costa, S.; Hita-Herranz, E. Efficacy of Deep Dry Needling on Latent Myofascial Trigger Points in Older Adults with Nonspecific Shoulder Pain: A Randomized, Controlled Clinical Trial Pilot Study. J. Geriatr. Phys. Ther. 2017, 40, 63. [Google Scholar] [CrossRef]
- Mejuto-Vázquez, M.J.; Salom-Moreno, J.; Ortega-Santiago, R.; Truyols-Domínguez, S.; Fernández-de-Las-Peñas, C. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Therapy 2014, 44, 252–260. [Google Scholar] [CrossRef]
- Velázquez-Saornil, J.; Ruíz-Ruíz, B.; Rodríguez-Sanz, D.; Romero-Morales, C.; López-López, D.; Calvo-Lobo, C. Efficacy of quadriceps vastus medialis dry needling in a rehabilitation protocol after surgical reconstruction of complete anterior cruciate ligament rupture. Medicine 2017, 96, e6726. [Google Scholar] [CrossRef]
- Espejo-Antúnez, L.; Tejeda, J.F.; Albornoz-Cabello, M.; Rodríguez-Mansilla, J.; de la Cruz-Torres, B.; Ribeiro, F.; Silva, A.G. Dry needling in the management of myofascial trigger points: A systematic review of randomized controlled trials. Compl. Ther. Med. 2017, 33, 46–57. [Google Scholar] [CrossRef]
- Biella, G.; Sotgiu, M.L.; Pellegata, G.; Paulesu, E.; Castiglioni, I.; Fazio, F. Acupuncture produces central activations in pain regions. Neuroimage 2001, 14 Pt 1, 60–66. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Simons, D.G. Myofascial Trigger Points: Translating Molecular Theory into Manual Therapy. J. Man. Manip. Ther. 2006, 14, 232–239. [Google Scholar] [CrossRef]
- Harden, R.N.; Bruehl, S.P.; Gass, S.; Niemiec, C.; Barbick, B. Signs and symptoms of the myofascial pain syndrome: A national survey of pain management providers. Clin. J. Pain. 2000, 16, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Loizidis, T.; Nikodelis, T.; Bakas, E.; Kollias, I. The effects of dry needling on pain relief and functional balance in patients with sub-chronic chronic neck pain. J. Back Musculoskelet. Rehabil. 2020, 33, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Z.; Simons, D.G. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch. Phys. Med. Rehabil. 1998, 79, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.D.; Velázquez Saornil, J.; Sánchez Milá, Z.; Jaén Crespo, G.; Campón Chekroun, A.; Barragán Casas, J.M.; Frutos Llanes, R.; Rodríguez Sanz, D. Effectiveness of Dry Needling and Ischemic Trigger Point Compression in the Gluteus Medius in Patients with Non-Specific Low Back Pain: A Randomized Short-Term Clinical Trial. Int. J. Environ. Res. Public. Health 2022, 19, 12468. [Google Scholar] [CrossRef]
- Irnich, D.; Behrens, N.; Gleditsch, J.M.; Stör, W.; Schreiber, M.A.; Schöps, P.; Vickers, A.J.; Beyer, A. Immediate effects of dry needling and acupuncture at distant points in chronic neck pain: Results of a randomized, double-blind, sham-controlled crossover trial. Pain 2002, 99, 83–89. [Google Scholar] [CrossRef]
- Roland, M.; Fairbank, J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef]
- Simons, D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef]
- Hou, C.R.; Tsai, L.C.; Cheng, K.F.; Chung, K.C.; Hong, C.Z. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch. Phys. Med. Rehabil. 2002, 83, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Kietrys, D.M.; Palombaro, K.M.; Azzaretto, E.; Hubler, R.; Schaller, B.; Schlussel, J.M.; Tucker, M. Effectiveness of dry needling for upper-quarter myofascial pain: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Gunn, C.C.; Milbrandt, W.E.; Little, A.S.; Mason, K.E. Dry needling of muscle motor points for chronic low-back pain: A randomized clinical trial with long-term follow-up. Spine 1980, 5, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Ramos, R.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Llamas-Ramos, I.; Plaza-Manzano, G.; Ortega-Santiago, R.; Cleland, J.; Fernandez-De-Las-Penas, C. Comparison of the short-term outcomes between trigger point dry needling and trigger point manual therapy for the management of chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014, 44, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, F.J.M.; Martín, D.P.; Masanet, R.A.; Botella, A.C.; Soler, L.B.; Morell, F.B. Immediate effect of ultrasound and ischemic compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: A randomized controlled study. J. Manip. Physiol. Ther. 2009, 32, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Benito-de-Pedro, M.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; López-López, D.; Cosín-Matamoros, J.; Martínez-Jiménez, E.M.; Calvo-Lobo, C. Effectiveness between Dry Needling and Ischemic Compression in the Triceps Surae Latent Myofascial Trigger Points of Triathletes on Pressure Pain Threshold and Thermography: A Single Blinded Randomized Clinical Trial. J. Clin. Med. 2019, 8, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.S.; Singh, H.; Mushtaq, S.; Mohanty, P.; Pattnaik, M. Effect of cervical mobilization and ischemic compression therapy on contralateral cervical side flexion and pressure pain threshold in latent upper trapezius trigger points. J. Bodyw. Mov. Ther. 2016, 20, 477–483. [Google Scholar] [CrossRef]


| Control Variables | ||||
|---|---|---|---|---|
| DN Group n = 40 | IC Group n = 40 | |||
| M | SD | M | SD | |
| Age | 52.03 | 16.92 | 55.76 | 13.84 |
| Weight (kg) | 76.27 | 12.78 | 81.22 | 9.09 |
| Height (cm) | 179.03 | 4.01 | 180.88 | 3.79 |
| Body mass index | 24.37 | 3.52 | 23.96 | 3.28 |
| Student’s t | p < 0.05 | |
|---|---|---|
| Age | 0.412 | 0.355 |
| Weight (kg) | −0.796 | 0.196 |
| Height (cm) | −0.985 | 0.137 |
| Body mass index | −0.529 | 0.335 |
| DN Group n = 40 | IC Group n = 40 | |||
|---|---|---|---|---|
| M | DT | M | SD | |
| VAS | ||||
| Pre | 6.96 | 0.91 | 7.72 | 0.87 |
| Post | 6.68 | 1.79 | 3.56 | 2.21 |
| 48 h | 4.49 | 0.96 | 4.32 | 1.19 |
| 1 week | 4.31 | 1.85 | 4.21 | 4.58 |
| ALGOMETRY | ||||
| Pre | 5.26 | 0.68 | 5.35 | 1.32 |
| Post | 4.66 | 0.60 | 5.88 | 1.02 |
| 48 h | 5.58 | 0.48 | 5.40 | 1.17 |
| 1 week | 5.56 | 0.68 | 5.34 | 1.30 |
| ROM | ||||
| Pre | 0.33 | 0.51 | 0.43 | 0.53 |
| 1 week | 0.07 | 0.37 | 0.14 | 0.37 |
| Neck Disability Index | ||||
| Pre | 22.17 | 6.49 | 23.86 | 5.84 |
| Post 1 week | 15.50 | 6.09 | 20.86 | 4.33 |
| Group | Pre | Post | 48 h | 1 Week | |
|---|---|---|---|---|---|
| M | DN | 7.41 | 7.09 | 4.60 | 4.20 |
| IC | 8.07 | 3.87 | 4.80 | 5.00 | |
| SD | DN | 0.82 | 2.37 | 0.98 | 1.26 |
| IC | 1.03 | 2.06 | 1.20 | 1.81 | |
| p value (p > 0.05) | DN | 0.292 | 0.987 | 0.001 | 0.001 |
| IC | 0.271 | 0.001 | 0.001 | 0.002 |
| Group | Pre | Post | 48 h | 1 Week | |
|---|---|---|---|---|---|
| M | DN | 3.97 | 3.96 | 4.61 | 4.61 |
| IC | 4.53 | 4.91 | 5.02 | 5.09 | |
| SD | DN | 0.89 | 0.81 | 0.89 | 0.89 |
| IC | 0.96 | 0.89 | 0.99 | 1.23 | |
| p value (p > 0.05) | DN | 0.405 | 0.036 | 0.031 | 0.051 |
| IC | 0.725 | 0.022 | 0.501 | 0.806 |
| Group | Pre | 1 Week | |
|---|---|---|---|
| M | DN | 0.20 | 0.10 |
| IC | 0.27 | 0.10 | |
| SD | DN | 0.41 | 0.25 |
| IC | 0.45 | 0.25 | |
| p value | DN | 0.082 | 0.182 |
| IC | 0.086 | 0.182 |
| Group | Pre | 1 Week | |
|---|---|---|---|
| M | DN | 21.09 | 14.80 |
| IC | 23.08 | 21.25 | |
| SD | DN | 6.07 | 3.05 |
| IC | 5.26 | 5.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez Saornil, J.; Sánchez Milá, Z.; Campón Chekroun, A.; Barragán Casas, J.M.; Frutos Llanes, R.; Rodríguez Sanz, D. Effectiveness of Dry Needling and Ischaemic Trigger Point Compression of the Levator Scapulae in Patients with Chronic Neck Pain: A Short-Term Randomized Clinical Trial. J. Clin. Med. 2023, 12, 6136. https://doi.org/10.3390/jcm12196136
Velázquez Saornil J, Sánchez Milá Z, Campón Chekroun A, Barragán Casas JM, Frutos Llanes R, Rodríguez Sanz D. Effectiveness of Dry Needling and Ischaemic Trigger Point Compression of the Levator Scapulae in Patients with Chronic Neck Pain: A Short-Term Randomized Clinical Trial. Journal of Clinical Medicine. 2023; 12(19):6136. https://doi.org/10.3390/jcm12196136
Chicago/Turabian StyleVelázquez Saornil, Jorge, Zacarías Sánchez Milá, Angélica Campón Chekroun, José Manuel Barragán Casas, Raúl Frutos Llanes, and David Rodríguez Sanz. 2023. "Effectiveness of Dry Needling and Ischaemic Trigger Point Compression of the Levator Scapulae in Patients with Chronic Neck Pain: A Short-Term Randomized Clinical Trial" Journal of Clinical Medicine 12, no. 19: 6136. https://doi.org/10.3390/jcm12196136
APA StyleVelázquez Saornil, J., Sánchez Milá, Z., Campón Chekroun, A., Barragán Casas, J. M., Frutos Llanes, R., & Rodríguez Sanz, D. (2023). Effectiveness of Dry Needling and Ischaemic Trigger Point Compression of the Levator Scapulae in Patients with Chronic Neck Pain: A Short-Term Randomized Clinical Trial. Journal of Clinical Medicine, 12(19), 6136. https://doi.org/10.3390/jcm12196136







