Efficacy of BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19 (BREATH Study): A Randomized Pilot Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Trial Population
2.2.1. Inclusion Criteria
- Positive COVID-19 test by PCR test based on nasopharyngeal swab specimen or antigen swab test performed at the local laboratory;
- Patients above 18 years old;
- A diagnosis of mild COVID-19 within 24 h of enrolment with at least one infection symptom: (i) fever for more than 24 h, (ii) headache, (iii) sore throat, (iv) nasal obstruction, (v) cough, (vi) fatigue, (vii) chest pain, (viii) dyspnea, (ix) myalgia, (x) anosmia, (xi) ageusia, or (xii) gastrointestinal changes.
2.2.2. Exclusion Criteria
- Pulse oximetry arterial saturation (SpO2) < 92%;
- Tachypnoea (respiratory rate ≥ 30 breaths/min);
- Current treatment in-hospital;
- Immediate consideration for hospital admission;
- Positive pregnancy test in women of childbearing age;
- Lactating women;
- Patients with unstable chronic disease and organ failure, judged by physician discretion;
- Patients with asthma, chronic obstructive lung disease, or other chronic respiratory diseases;
- Use of inhaled, oral, or intravenous corticosteroid in the ten days prior to randomization;
- Previous treatment with hydroxychloroquine and ivermectin in the last 10 days;
- Use of any investigational or unregistered product within the previous 3-month or the 5-half-life period before baseline, whichever is longer;
- Baseline biochemistry showing hemoglobin < 9.0 g/dL, absolute neutrophil count ≤ 1000/mm3, platelets ≥ 100,000/mm3, or creatinine clearance ≤ 30 mL/min (using the Cockcroft–Gault formula).
2.3. Outcomes
2.4. Safety
2.5. Trial Procedures
2.6. Clinical and Laboratory Data
2.7. Intervention
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO COVID-19 Dashboard; World Health Organization: Geneva, Switzerland, 2020; Available online: https://covid19.who.int/ (accessed on 24 April 2023).
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Geng, Y.; Xu, X.; Chen, X.; Gao, J.; Li, J.; Zhang, X. The features comparison between patients in the ICU and general wards and between patients with different outcomes: A 2020 COVID-19 study. Ann. Palliat. Med. 2021, 10, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, P497–P506. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 22 April 2023).
- Brito, S.B.P.; Braga, I.O.; Cunha, C.C.; Takenami, I. Mecanismos imunopatológicos envolvidos na infecção por SARS-CoV-2. J. Bras. Patol. Med. Lab. 2020, 56, e3352020. [Google Scholar]
- Jin, Y.H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.Q. From SARS to COVID-19: Pathogens, receptor, pathogenesis and principles of the treatment. Zhonghua Bing Li Xue Za Zhi 2020, 49, 647–652. [Google Scholar] [PubMed]
- Machado, R.R.G.; Glaser, T.; Araujo, D.B.; Petiz, L.L.; Oliveira, D.B.L.; Durigon, G.S.; Leal, A.L.; Pinho, J.R.R.; Ferreira, L.C.S.; Ulrich, H.; et al. Inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 Replication by Hypertonic Saline Solution in Lung and Kidney Epithelial Cells. ACS Pharmacol. Transl. Sci. 2021, 4, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Silva De Souza, A.; Rivera, J.D.; Almeida, V.M.; Ge, P.; de Souza, R.F.; Farah, C.S.; Ulrich, H.; Marana, S.R.; Salinas, R.K.; Guzzo, C.R. Molecular Dynamics Reveals Complex Compensatory Effects of Ionic Strength on the Severe Acute Respiratory Syndrome Coronavirus 2 Spike/Human Angiotensin-Converting Enzyme 2 Interaction. J. Phys. Chem. Lett. 2020, 11, 10446–10453. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Schoeffei, R.E.; Anderson, S.D.; Altounyan, R.E.C. Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. Br. Med. J. 1981, 283, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.; Mcdonald, V.M. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 2018, CD001506. [Google Scholar] [CrossRef] [PubMed]
- Taube, C.; Holz, O.; Mücke, M.; Jorres, R.A.; Magnussen, H. Airway response to inhaled hypertonic saline in patients with moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Rytilä, P.H.; Lindqvist, A.E.; Laitinen, L.A. Safety of sputum induction in chronic obstructive pulmonary disease. Eur. Respir. J. 2000, 15, 1116–1119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- COVID-19 Rapid Guideline: Managing COVID-19—The National Institute for Health and Care Excellence (NICE). 28.0 published on 29.03.2023. Available online: https://www.nice.org.uk/guidance/ng191 (accessed on 22 April 2023).

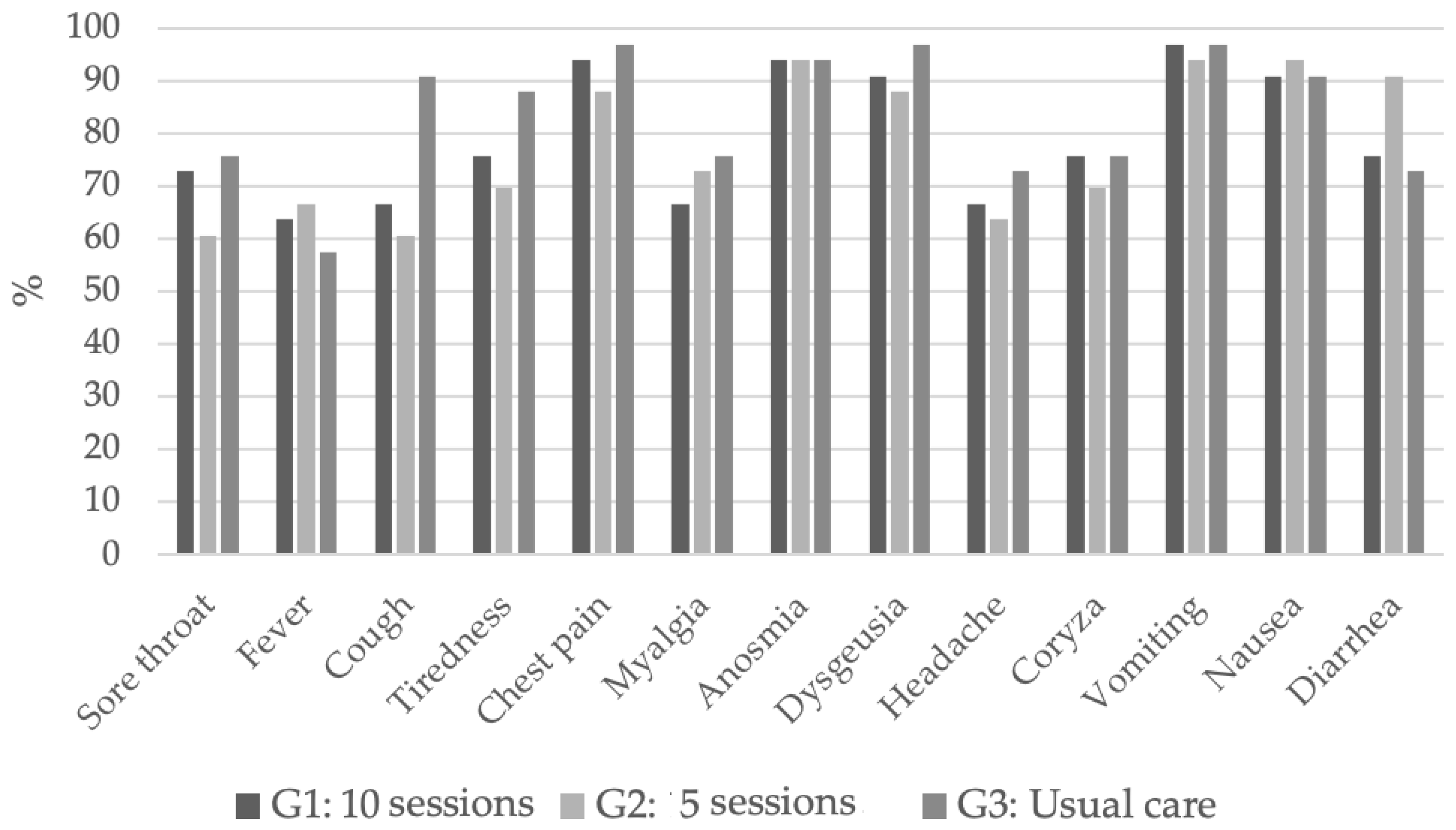
| G1 | G2 | G3 | p | |
|---|---|---|---|---|
| Age, years (mean, IIQ) | 41 (33–47) | 44.5 (31–51.3) | 40 (28–48) | 0.672 |
| Sex (female, %) | 63.6 | 60.6 | 60.6 | 0.958 |
| Skin color (white, %) | 78.8 | 63.6 | 69.7 | 0.409 |
| Comorbidities (%) | 24.2 | 27.3 | 24.2 | 0.948 |
| Type 1 diabetes (%) | 0 | 0 | 3.03 | 0.364 |
| Diabetes mellitus (%) | 3.03 | 6.06 | 9.09 | 0.587 |
| Obesity (%) | 15.2 | 15.2 | 18.2 | 0.928 |
| Arterial hypertension (%) | 15.2 | 21.1 | 15.2 | 0.753 |
| Coronary artery disease (%) | 0 | 3.03 | 6.1 | 0.357 |
| HIV (%) | 0 | 0 | 3.03 | 0.364 |
| Auto-immune disease (%) | 12.1 | 6.06 | 6.06 | 0.580 |
| Allergy (%) | 12.1 | 15.2 | 15.2 | 0.920 |
| Day 10 | Day 28 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes Symptoms | G1 | G2 | G1 | G2 | ||||||
| HR | 95% CI | HR | 95% CI | p-Value | HR | 95% CI | HR | 95% CI | p-Value | |
| Sore throat | 1.39 | 0.80–2.42 | 1.63 | 0.92–2.91 | 0.234 | 1.04 | 0.61–1.78 | 1.45 | 0.84–2.51 | 0.365 |
| Fever | 0.96 | 0.57–1.62 | 0.87 | 0.51–1.47 | 0.895 | 0.83 | 0.49–1.40 | 0.82 | 0.48–1.38 | 0.700 |
| Cough | 2.17 | 1.17–4.04 | 2.01 | 1.06–3.81 | 0.034 | 1.64 | 0.96–2.81 | 1.46 | 0.86–2.50 | 0.163 |
| Tiredness | 0.99 | 0.55–1.76 | 1.16 | 0.65–2.06 | 0.831 | 0.87 | 0.52–1.47 | 0.99 | 0.58–1.67 | 0.862 |
| Chest pain | 0.98 | 0.59–1.62 | 0.93 | 0.55–1.57 | 0.963 | 0.86 | 0.52–1.42 | 0.87 | 0.52–1.46 | 0.805 |
| Myalgia | 1.24 | 0.72–2.14 | 1.06 | 0.60–1.89 | 0.729 | 0.96 | 0.56–1.65 | 0.90 | 0.53–1.56 | 0.936 |
| Anosmia | 0.97 | 0.57–1.65 | 0.88 | 0.51–1.51 | 0.897 | 0.85 | 0.51–1.43 | 0.84 | 0.50–1.41 | 0.759 |
| Dysgeusia | 1.05 | 0.62–1.77 | 1.00 | 0.59–1.69 | 0.980 | 0.89 | 0.53–1.49 | 0.89 | 0.53–1.48 | 0.871 |
| Headache | 1.33 | 0.77–2.29 | 1.01 | 0.58–1.76 | 0.506 | 1.17 | 0.67–2.03 | 1.07 | 0.63–1.82 | 0.859 |
| Coryza | 0.95 | 0.54–1.67 | 0.65 | 0.36–1.19 | 0.340 | 0.90 | 0.52–1.55 | 0.72 | 0.42–1.24 | 0.487 |
| Vomiting | 1.00 | 0.60–1.66 | 0.96 | 0.58–1.61 | 0.988 | 0.87 | 0.52–1.45 | 0.90 | 0.54–1.50 | 0.857 |
| Nausea | 1.05 | 0.63–1.76 | 1.02 | 0.61–1.72 | 0.980 | 0.92 | 0.55–1.54 | 0.96 | 0.58–1.59 | 0.948 |
| Diarrhea | 1.03 | 0.61–1.73 | 0.99 | 0.59–1.68 | 0.990 | 0.89 | 0.53–1.50 | 0.93 | 0.55–1.55 | 0.911 |
| Day 10 | Day 28 | |||||
|---|---|---|---|---|---|---|
| Symptom | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Sore throat | 1.49 | 0.91–2.44 | 0.111 | 1.20 | 0.76–1.91 | 0.433 |
| Fever | 0.91 | 0.58–1.44 | 0.700 | 0.82 | 0.53–1.29 | 0.399 |
| Cough | 2.10 | 1.20–3.67 | 0.010 | 1.54 | 0.98–2.45 | 0.063 |
| Tiredness | 1.07 | 0.65–1.76 | 0.790 | 0.93 | 0.59–1.44 | 0.732 |
| Chest pain | 0.96 | 0.62–1.48 | 0.839 | 0.86 | 0.56–1.34 | 0.513 |
| Myalgia | 1.15 | 0.71–1.87 | 0.565 | 0.93 | 0.59–1.48 | 0.763 |
| Anosmia | 0.92 | 0.58–1.47 | 0.736 | 0.85 | 0.55–1.31 | 0.458 |
| Dysgeusia | 1.02 | 0.65–1.61 | 0.930 | 0.89 | 0.57–1.38 | 0.599 |
| Headache | 1.16 | 0.72–1.86 | 0.545 | 1.11 | 0.70–1.77 | 0.649 |
| Coryza | 0.79 | 0.48–1.30 | 0.360 | 0.80 | 0.50–1.26 | 0.333 |
| Vomiting | 0.98 | 0.6–1.53 | 0.931 | 0.89 | 0.57–1.37 | 0.590 |
| Nausea | 1.04 | 0.66–1.62 | 0.867 | 0.94 | 0.60–1.46 | 0.775 |
| Earache | 1.00 | 0.65–1.54 | 0.989 | 0.90 | 0.59–1.39 | 0.647 |
| Skin rash | 1.00 | 0.65–1.54 | 0.989 | 0.90 | 0.59–1.39 | 0.647 |
| Diarrhea | 1.01 | 0.64–1.59 | 0.960 | 0.91 | 0.58–1.42 | 0.682 |
| G1 | G2 | G3 | |
|---|---|---|---|
| Blood pressure increase | 0 | 1 | 0 |
| Neck pain | 1 | 0 | 0 |
| Cystitis | 0 | 1 | 0 |
| Dyslipidemia | 0 | 0 | 1 |
| Dyspnea | 1 | 0 | 0 |
| Ankle pain | 0 | 1 | 0 |
| Sneezing | 0 | 1 | 0 |
| Pharyngitis | 0 | 0 | 2 |
| Nausea | 1 | 0 | 0 |
| Bacterial rhinosinusitis | 0 | 1 | 0 |
| Dry mouth | 0 | 1 | 0 |
| Nasal burning | 6 | 9 | 0 |
| Chest pain | 0 | 1 | 1 |
| Sore or itchy nose | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanni, S.; Wehrmeister, F.; Prudente, R.; Damatto, F.; Breda Neto, C.; Oliveira, L.; Pagan, L.; Gatto, M.; Vieira, L.; Coelho, L.; et al. Efficacy of BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19 (BREATH Study): A Randomized Pilot Clinical Trial. J. Clin. Med. 2023, 12, 6075. https://doi.org/10.3390/jcm12186075
Tanni S, Wehrmeister F, Prudente R, Damatto F, Breda Neto C, Oliveira L, Pagan L, Gatto M, Vieira L, Coelho L, et al. Efficacy of BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19 (BREATH Study): A Randomized Pilot Clinical Trial. Journal of Clinical Medicine. 2023; 12(18):6075. https://doi.org/10.3390/jcm12186075
Chicago/Turabian StyleTanni, Suzana, Fernando Wehrmeister, Robson Prudente, Felipe Damatto, Carlos Breda Neto, Leiliane Oliveira, Luana Pagan, Mariana Gatto, Letícia Vieira, Liana Coelho, and et al. 2023. "Efficacy of BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19 (BREATH Study): A Randomized Pilot Clinical Trial" Journal of Clinical Medicine 12, no. 18: 6075. https://doi.org/10.3390/jcm12186075
APA StyleTanni, S., Wehrmeister, F., Prudente, R., Damatto, F., Breda Neto, C., Oliveira, L., Pagan, L., Gatto, M., Vieira, L., Coelho, L., Rezende, D., Machado, L., Mota, G., Gaiato, M., Santaella, F., Campos, E., Franco, E., Callegari, M., Okoshi, M. P., & Weinreich, U. (2023). Efficacy of BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19 (BREATH Study): A Randomized Pilot Clinical Trial. Journal of Clinical Medicine, 12(18), 6075. https://doi.org/10.3390/jcm12186075







