Efficacy of Transcutaneous 4.4 MHz Radiofrequency Diathermy versus Therapeutic Ultrasound for Pain Relief and Functional Recovery in Patients with Knee Osteoarthritis: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
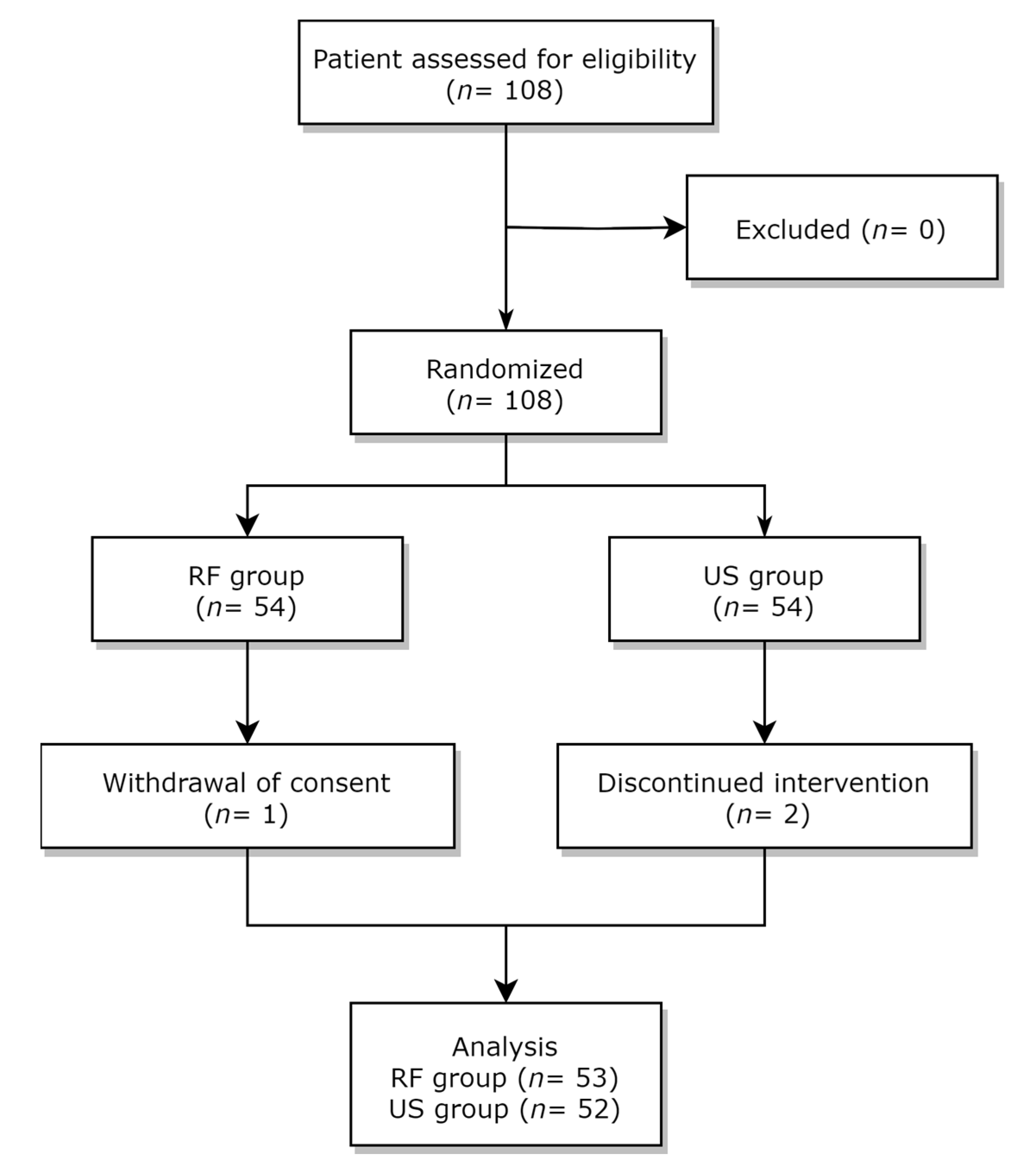
2.1. Study Design and Participants
2.2. Random Allocation and Blinding Method
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Mok, A.; Khaw, K.T.; Luben, R.; Wareham, N.; Brage, S. Physical activity trajectories and mortality: Population based cohort study. BMJ 2019, 365, l2323. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L.; Yonge, K.A.; Robinson, V.; Marchand, S.; Judd, M.; Wells, G.; Tugwell, P. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst. Rev. 2003, 2003, CD004522. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.S.; Mani, R. The Role of Ultrasound Therapy in the Management of Musculoskeletal Soft Tissue Pain. Int. J. Low Extrem. Wounds 2020, 19, 350–358. [Google Scholar] [CrossRef]
- van der Windt, D.; van der Heijden, G.; van den Berg, S.G.M.; Ter Riet, G.; de Winter, A.F.; Bouter, L.M. Ultrasound therapy for musculoskeletal disorders: A systematic review. Pain 1999, 81, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- Dantas, L.O.; Osani, M.C.; Bannuru, R.R. Therapeutic ultrasound for knee osteoarthritis: A systematic review and meta-analysis with grade quality assessment. Braz. J. Phys. Ther. 2021, 25, 688–697. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperth. 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Lee, J.H.; Do, J.G.; Park, H.J.; Lee, Y.T.; Kim, S.J. A Comparison of the Effect of a 4.4-MHz Radiofrequency Deep Heating Therapy and Ultrasound on Low Back Pain: A Randomized, Double-Blind, Multicenter Trial. J. Clin. Med. 2022, 11, 5011. [Google Scholar] [CrossRef]
- Kim, G.W.; Won, Y.H.; Park, S.H.; Seo, J.H.; Kim, D.H.; Lee, H.N.; Ko, M.H. Effects of a Newly Developed Therapeutic Deep Heating Device Using High Frequency in Patients with Shoulder Pain and Disability: A Pilot Study. Pain Res. Manag. 2019, 2019, 8215371. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marin, M.; Rodriguez-Almagro, D.; Castellote-Caballero, Y.; Achalandabaso-Ochoa, A.; Lomas-Vega, R.; Ibanez-Vera, A.J. Efficacy of Non-Invasive Radiofrequency-Based Diathermy in the Postoperative Phase of Knee Arthroplasty: A Double-Blind Randomized Clinical Trial. J. Clin. Med. 2021, 10, 1611. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, B.; Watson, T. Treatment using 448kHz capacitive resistive monopolar radiofrequency improves pain and function in patients with osteoarthritis of the knee joint: A randomised controlled trial. Physiotherapy 2019, 105, 98–107. [Google Scholar] [CrossRef]
- Kumaran, B.; Watson, T. Thermophysiological responses to capacitive resistive monopolar radiofrequency electromagnetic radiation in patients with osteoarthritis of the knee joint: A randomised controlled experimental study. Electromagn. Biol. Med. 2021, 40, 210–221. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Ibanez-Vera, A.J.; Barrios-Quinta, C.J.; Lara-Palomo, I.C.; Cardero-Duran, M.L.A.; Espejo-Antunez, L. Effects of Radiofrequency Diathermy Plus Therapeutic Exercises on Pain and Functionality of Patients with Patellofemoral Pain Syndrome: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 2348. [Google Scholar] [CrossRef]
- Tae Sung, K.; Seong Yeol, K.; Jong Soo, L. Reliability and Validity of the Korean Western Ontario and McMaster Universities(WOMAC) Osteoarthritis Index in Patients with Osteoarthritis of the Knee. J. Orient. Rehabil. Med. 2009, 19, 251–260. [Google Scholar]
- Kwon, O. Health-related quality of Life Instrument in Neuromuscular Disorders. J. Korean Neurol. Assoc. 2021, 39, 93–109. [Google Scholar] [CrossRef]
- Lequesne, M. Indices of severity and disease activity for osteoarthritis. Semin Arthritis Rheum. 1991, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.A.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2015, 127, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.F.; Weingand, K.; Kruse, R.J. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 2004, 7, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanz, J.; Perez-Bellmunt, A.; Lopez-de-Celis, C.; Lucha-Lopez, O.M.; Gonzalez-Rueda, V.; Tricas-Moreno, J.M.; Simon, M.; Hidalgo-Garcia, C. Thermal and non-thermal effects of capacitive-resistive electric transfer application on different structures of the knee: A cadaveric study. Sci. Rep. 2020, 10, 22290. [Google Scholar] [CrossRef] [PubMed]
- Albornoz-Cabello, M.; Ibanez-Vera, A.J.; Aguilar-Ferrandiz, M.E.; Espejo-Antunez, L. Monopolar dielectric diathermy by emission of radiofrequency in Patellofemoral pain. A single-blind-randomized clinical trial. Electromagn. Biol. Med. 2020, 39, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Park, E.H.; Park, J.S.; Lee, S.W.; Suh, H.R.; Park, S.H.; Yoon, S.Z.; Park, K.W.; Han, H.C. Pain-Relieving Effect of 4.4 MHz of Pulsed Radiofrequency on Acute Knee Arthritis in Rats. Pain Med. 2020, 21, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; Felson, D.T. Mechanisms of Osteoarthritis (OA) Pain. Curr. Osteoporos. Rep. 2018, 16, 611–616. [Google Scholar] [CrossRef]
- Kose, S.G.; Kose, H.C.; Celikel, F.; Akkaya, O.T. Predictive factors associated with successful response to utrasound guided genicular radiofrequency ablation. Korean J. Pain 2022, 35, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Fonkoue, L.; Behets, C.; Kouassi, J.K.; Coyette, M.; Detrembleur, C.; Thienpont, E.; Cornu, O. Distribution of sensory nerves supplying the knee joint capsule and implications for genicular blockade and radiofrequency ablation: An anatomical study. Surg. Radiol. Anat. 2019, 41, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Toktas, H.; Dundar, U.; Adar, S.; Solak, O.; Ulasli, A.M. Ultrasonographic assessment of pes anserinus tendon and pes anserinus tendinitis bursitis syndrome in patients with knee osteoarthritis. Mod Rheumatol. 2015, 25, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Osteoarthritis year in review 2021: Biology. Osteoarthr. Cartil. 2022, 30, 207–215. [Google Scholar] [CrossRef]

| RF Group (n = 53) | US Group (n = 52) | p-Value | ||
|---|---|---|---|---|
| Age, yr | 61.45 ± 5.05 | 60.85 ± 5.11 | 0.5420 (1) | |
| Sex | 0.1625 (2) | |||
| Male | 1 (1.89%) | 4 (7.69%) | ||
| Female | 52 (98.11%) | 48 (92.31%) | ||
| Height, cm | 154.17 ± 5.42 | 154.96 ± 4.82 | 0.4324 (1) | |
| Weight, kg | 56.82 ± 6.70 | 58.27 ± 7.06 | 0.2847 (1) | |
| Pulse rate, bpm | 75.57 ± 7.61 | 77.50 ± 7.48 | 0.1921 (1) | |
| SBP, mmHg | 127.51 ± 11.51 | 130.56 ± 11.85 | 0.1842 (1) | |
| DBP, mmHg | 78.06 ± 8.59 | 78.56 ± 8.45 | 0.7638 (1) | |
| RF Group (n = 53) | US Group (n = 52) | Within-Group Mean Difference | Between-Group Mean Difference | p-Value (1) | ||
|---|---|---|---|---|---|---|
| RF Group | US Group | |||||
| Visit 1 | 4.42 ± 1.18 | 4.56 ± 1.06 | −0.16 [−0.60/0.27] | 0.5165 | ||
| Visit 2 | 2.91 ± 1.39 | 3.08 ± 1.12 | −1.51 [−1.79/−1.23] ** | −1.51 [−1.78/−1.24] ** | −0.19 [−0.68/0.30] | 0.4887 |
| Visit 3 | 2.23 ± 1.12 | 2.41 ± 1.31 | −2.19 [−2.48/−1.90] ** | −2.12 [−2.47/−1.76] ** | −0.24 [−0.72/0.25] | 0.4401 |
| Visit 4 | 1.98 ± 1.25 | 2.25 ± 1.48 | −2.43 [−2.80/−2.07] ** | −2.31 [−2.73/−1.90] ** | −0.27 [−0.81/0.26] | 0.3097 |
| RF Group (n = 53) | US Group (n = 52) | Within-Group Mean Difference | Between-Group Mean Difference | p-Value (1) | |||
|---|---|---|---|---|---|---|---|
| RF Group | US Group | ||||||
| Pain | Visit 1 | 5.53 ± 2.97 | 4.87 ± 3.41 | 0.59 [−0.66/1.83] | 0.2902 | ||
| Visit 2 | 4.34 ± 3.06 | 3.50 ± 2.80 | −1.19 [−2.11/−0.26] * | −1.37 [−2.4/−0.35] * | 0.76 [−0.38/1.91] | 0.1462 | |
| Visit 3 | 3.26 ± 2.70 | 2.73 ± 2.75 | −2.26 [−3.08/−1.45] ** | −2.13 [−2.97/−1.30] ** | 0.46 [−0.61/1.52] | 0.3157 | |
| Visit 4 | 3.87 ± 3.15 | 3.29 ± 3.12 | −1.66 [−2.76/−0.56] * | −1.59 [−2.56/−0.62] * | 0.57 [−0.65/1.79] | 0.3530 | |
| Stiffness | Visit 1 | 2.09 ± 1.60 | 1.54 ± 1.51 | 0.56 [−0.05/1.16] | 0.0701 | ||
| Visit 2 | 1.60 ± 1.45 | 1.10 ± 1.45 | −0.49 [−0.99/0.01] | −0.47 [−1.02/0.08] | 0.55 [−0.01/1.11] | 0.0749 | |
| Visit 3 | 1.23 ± 1.32 | 0.88 ± 1.13 | −0.87 [−1.35/−0.38] ** | −0.60 [−1.09/−0.10] * | 0.28 [−0.21/0.77] | 0.1573 | |
| Visit 4 | 1.30 ± 1.55 | 1.06 ± 1.41 | −0.79 [−1.26/−0.32] ** | −0.49 [−0.94/−0.04] * | 0.24 [−0.33/0.82] | 0.4050 | |
| Activity | Visit 1 | 17.47 ± 11.51 | 15.69 ± 11.82 | 1.38 [−3.17/5.92] | 0.4362 | ||
| Visit 2 | 13.06 ± 9.85 | 10.13 ± 8.16 | −4.42 [−7.91/−0.92] * | −6.02 [−8.82/−3.22] ** | 2.79 [−0.78/6.35] | 0.1012 | |
| Visit 3 | 10.53 ± 9.49 | 9.67 ± 9.01 | −6.94 [−10.10/−3.79] ** | −5.90 [−8.82/−2.99] ** | 0.34 [−3.38/4.05] | 0.6362 | |
| Visit 4 | 10.11 ± 10.08 | 9.61 ± 9.67 | −7.36 [−10.70/−4.01] ** | −6.20 [−9.36/−3.03] ** | 0.51 [−3.34/4.35] | 0.7949 | |
| Total | Visit 1 | 25.09 ± 14.66 | 22.10 ± 15.22 | 2.52 [−3.3/8.33] | 0.3062 | ||
| Visit 2 | 19.00 ± 13.53 | 14.73 ± 10.94 | −6.09 [−10.45/−1.74] * | −7.86 [−11.67/−4.06] ** | 4.10 [−0.73/8.93] | 0.0787 | |
| Visit 3 | 15.02 ± 12.54 | 13.27 ± 12.23 | −10.08 [−14.03/−6.12] ** | −8.63 [−12.33/−4.94] ** | 1.08 [−3.87/6.03] | 0.4746 | |
| Visit 4 | 15.28 ± 13.64 | 13.96 ± 13.31 | −9.81 [−14.23/−5.39] ** | −8.27 [−12.36/−4.18] ** | 1.32 [−3.92/6.57] | 0.6182 | |
| RF Group (n = 53) | US Group (n = 52) | Within-Group Mean Difference | Between-Group Mean Difference | p-Value (1) | ||
|---|---|---|---|---|---|---|
| RF Group | US Group | |||||
| Visit 1 | 7.25 (3.20) | 6.38 (3.41) | 0.87 [−0.41/2.15] | 0.1847 | ||
| Visit 2 | 6.16 (3.55) | 5.43 (3.10) | −1.08 [−2.01/−0.16] * | −0.95 [−1.75/−0.16] * | 0.66 [−0.64/1.96] | 0.2661 |
| Visit 3 | 4.98 (3.38) | 4.56 (3.21) | −2.26 [−3.21/−1.32] ** | −1.75 [−2.43/−1.07] ** | 0.36 [−0.92/1.63] | 0.5152 |
| Visit 4 | 5.06 (3.31) | 4.75 (3.77) | −2.19 [−3.2/−1.17] ** | −1.64 [−2.48/−0.8] ** | 0.30 [−1.08/1.68] | 0.6652 |
| RF Group (n = 53) | US Group (n = 52) | Within-Group Mean Difference | Between-Group Mean Difference | p-Value (1) | |||
|---|---|---|---|---|---|---|---|
| RF Group | US Group | ||||||
| Physical functioning | Visit 1 | 62.08 ± 21.27 | 66.15 (21.39) | −4.18 [−12.42/4.07] | 0.3295 | ||
| Visit 4 | 69.53 ± 21.29 | 72.16 (18.36) | 7.45 [0.51/14.39] * | 5.69 [−0.23/11.6] | −2.63 [−10.37/5.12] | 0.5024 | |
| Role limitation-physical | Visit 1 | 61.79 ± 29.66 | 64.42 ± 31.45 | −2.15 [−13.99/9.69] | 0.6601 | ||
| Visit 4 | 67.45 ± 32.74 | 70.59 ± 32.67 | 5.66 [−3.57/14.89] | 6.37 [−1.31/14.05] | −3.14 [−15.86/9.59] | 0.6261 | |
| Bodily pain | Visit 1 | 64.43 ± 17.35 | 69.76 ± 13.69 | −5.52 [−11.58/0.54] | 0.0841 | ||
| Visit 4 | 74.95 ± 16.67 | 76.52 ± 17.59 | 10.52 [4.49/16.54] * | 6.72 [1.77/11.66] * | −1.57 [−8.23/5.1] | 0.6419 | |
| General health | Visit 1 | 53.87 ± 15.37 | 53.46 ± 16.79 | 0.5 [−5.74/6.74] | 0.8973 | ||
| Visit 4 | 56.70 ± 15.87 | 57.65 ± 18.61 | 2.83 [−1.18/6.84] | 4.02 [0.72/7.32] * | −0.95 [−7.67/5.77] | 0.7799 | |
| Vitality | Visit 1 | 54.91 ± 17.31 | 57.50 ± 14.87 | −2.4 [−8.66/3.85] | 0.4123 | ||
| Visit 4 | 57.36 ± 14.86 | 60.20 ± 18.22 | 2.45 [−1.98/6.88] | 2.75 [−1.78/7.27] | −2.84 [−9.29/3.62] | 0.3853 | |
| Social functioning | Visit 1 | 67.45 ± 18.24 | 68.51 ± 17.06 | −0.82 [−7.65/6.02] | 0.7598 | ||
| Visit 4 | 68.87 ± 19.71 | 72.06 ± 17.96 | 1.42 [−3.98/6.81] | 3.68 [−1.78/9.14] * | −3.19 [−10.53/4.15] | 0.3906 | |
| Role limitation-emotion | Visit 1 | 69.18 ± 38.59 | 78.22 ± 32.93 | −9.03 [−22.93/4.87] | 0.2004 | ||
| Visit 4 | 79.25 ± 35.34 | 86.28 ± 27.63 | 10.06 [−0.98/21.11] | 8.49 [−2.89/19.87] | −7.03 [−19.4/5.34] | 0.2623 | |
| Mental health | Visit 1 | 63.47 ± 16.19 | 66.92 ± 14.33 | −3.53 [−9.45/2.4] | 0.2504 | ||
| Visit 4 | 68.60 ± 15.20 | 71.14 ± 15.95 | 5.13 [1.16/9.1] * | 4.24 [0.88/7.59] * | −2.53 [−8.59/3.52] | 0.4087 | |
| Total | Visit 1 | 497.18 ± 125.01 | 524.95 ± 106.71 | −27.12 [−72.14/17.91] | 0.2242 | ||
| Visit 4 | 542.71 ± 114.34 | 566.58 ± 114.93 | 45.53 [13.89/77.16] | 41.94 [14.04/69.84] * | −23.87 [−68.47/20.73] | 0.2909 | |
|
RF Group (n = 53) |
US Group (n = 52) | Within-Group Mean Difference | Between-Group Mean Difference | p-Value (1) | |||
|---|---|---|---|---|---|---|---|
| RF Group | US Group | ||||||
| Stride length (cm) | Visit 1 | 1.16 ± 0.19 | 1.18 ± 0.16 | −0.02 [−0.08/0.05] | 0.7047 | ||
| Visit 2 | 1.19 ± 0.17 | 1.18 ± 0.17 | 0.02 [−0.02/0.07] | 0.02 [−0.01/0.05] | −0.02 [−0.08/0.04] | 0.9740 | |
| Visit 3 | 1.22 ± 0.11 | 1.21 ± 0.12 | 0.06 [0.01/0.11] * | 0.03 [−0.01/0.07] | 0.02 [−0.03/0.06] | 0.4361 | |
| Visit 4 | 1.23 ± 0.10 | 1.24 ± 0.13 | 0.06 [0.02/0.11] * | 0.06 [0.02/0.1] * | −0.02 [−0.06/0.03] | 0.4321 | |
| %Stride length (%height) | Visit 1 | 75.45 ± 11.96 | 76.19 ± 9.80 | −0.73 [−4.97/3.5] | 0.8014 | ||
| Visit 2 | 76.78 ± 10.49 | 77.81 ± 8.84 | 1.33 [−1.56/4.21] | 1.23 [−0.92/3.38] | −1.03 [−4.79/2.73] | 0.9057 | |
| Visit 3 | 79.41 ± 7.63 | 78.07 ± 7.85 | 3.99 [0.84/7.14] * | 1.88 [−0.56/4.32] | 1.34 [−1.67/4.35] | 0.3901 | |
| Visit 4 | 79.49 ± 6.67 | 79.59 ± 7.81 | 4.04 [1.05/7.03] * | 3.29 [0.76/5.82] * | −0.10 [−2.92/2.72] | 0.9442 | |
| Stride time (sec) | Visit 1 | 1.03 ± 0.07 | 1.03 ± 0.08 | 0.01 [−0.02/0.04] | 0.5335 | ||
| Visit 2 | 1.03 ± 0.07 | 1.04 ± 0.08 | −0.01 [−0.02/0.01] | 0.01 [−0.01/0.03] | −0.01 [−0.04/0.02] | 0.4924 | |
| Visit 3 | 1.03 ± 0.06 | 1.03 ± 0.07 | 0.00 [−0.02/0.02] | 0.00 [−0.02/0.02] | 0.01 [−0.02/0.03] | 0.6223 | |
| Visit 4 | 1.02 ± 0.07 | 1.02 ± 0.07 | −0.02 [−0.03/0] | 0.00 [−0.02/0.02] | 0.00 [−0.03/0.02] | 0.7332 | |
| Stride velocity (cm/s) | Visit 1 | 1.14 ± 0.20 | 1.16 ± 0.18 | −0.03 [−0.1/0.05] | 0.6472 | ||
| Visit 2 | 1.18 ± 0.19 | 1.17 ± 0.21 | 0.03 [−0.01/0.08] | 0.02 [−0.02/0.06] | −0.01 [−0.08/0.06] | 0.8277 | |
| Visit 3 | 1.21 ± 0.14 | 1.21 ± 0.14 | 0.07 [0.02/0.12] * | 0.04 [−0.01/0.08] | 0.01 [−0.05/0.06] | 0.8687 | |
| Visit 4 | 1.23 ± 0.13 | 1.23 ± 0.15 | 0.08 [0.03/0.13] * | 0.07 [0.02/0.11] * | −0.01 [−0.06/0.05] | 0.8151 | |
| %Stride velocity (%) | Visit 1 | 74.19 ± 13.38 | 75.79 ± 11.73 | −1.60 [−6.47/3.28] | 0.6774 | ||
| Visit 2 | 76.27 ± 12.16 | 76.88 ± 11.38 | 2.08 [−0.95/5.11] | 1.07 [−1.28/3.43] | −0.61 [−5.17/3.95] | 0.7810 | |
| Visit 3 | 78.66 ± 9.56 | 77.89 ± 9.32 | 4.47 [1.19/7.75] * | 2.1 [−0.92/5.12] | 0.77 [−2.89/4.42] | 0.7430 | |
| Visit 4 | 79.55 ± 8.86 | 79.6 ± 9.45 | 5.36 [2.07/8.64] * | 4.06 [1.15/6.96] * | −0.05 [−3.61/3.51] | 0.9783 | |
| Cadence (steps/min) | Visit 1 | 115.98 ± 7.11 | 115.99 ± 7.52 | −0.12 [−2.93/2.7] | 0.9991 | ||
| Visit 2 | 116.94 ± 7.65 | 115.90 ± 7.78 | 0.95 [−0.39/2.29] | −0.14 [−1.85/1.58] | 0.95 [−2.03/3.93] | 0.4913 | |
| Visit 3 | 116.69 ± 7.20 | 117.63 ± 7.42 | 0.7 [−0.74/2.14] | 1.31 [−0.21/2.84] | −0.73 [−3.57/2.12] | 0.5141 | |
| Visit 4 | 118.34 ± 7.80 | 117.67 ± 7.37 | 2.35 [0.53/4.17] * | 1.53 [−0.13/3.19] | 0.67 [−2.29/3.62] | 0.6553 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.; Je, L.G.; Lee, S.; Na, D.; Shin, H.; Choi, J.B.; Koh, J.C. Efficacy of Transcutaneous 4.4 MHz Radiofrequency Diathermy versus Therapeutic Ultrasound for Pain Relief and Functional Recovery in Patients with Knee Osteoarthritis: A Randomized Controlled Study. J. Clin. Med. 2023, 12, 6040. https://doi.org/10.3390/jcm12186040
Jang Y, Je LG, Lee S, Na D, Shin H, Choi JB, Koh JC. Efficacy of Transcutaneous 4.4 MHz Radiofrequency Diathermy versus Therapeutic Ultrasound for Pain Relief and Functional Recovery in Patients with Knee Osteoarthritis: A Randomized Controlled Study. Journal of Clinical Medicine. 2023; 12(18):6040. https://doi.org/10.3390/jcm12186040
Chicago/Turabian StyleJang, Yookyung, Lee Gyeong Je, Sunhee Lee, Donghyun Na, Hyekyung Shin, Jong Bum Choi, and Jae Chul Koh. 2023. "Efficacy of Transcutaneous 4.4 MHz Radiofrequency Diathermy versus Therapeutic Ultrasound for Pain Relief and Functional Recovery in Patients with Knee Osteoarthritis: A Randomized Controlled Study" Journal of Clinical Medicine 12, no. 18: 6040. https://doi.org/10.3390/jcm12186040
APA StyleJang, Y., Je, L. G., Lee, S., Na, D., Shin, H., Choi, J. B., & Koh, J. C. (2023). Efficacy of Transcutaneous 4.4 MHz Radiofrequency Diathermy versus Therapeutic Ultrasound for Pain Relief and Functional Recovery in Patients with Knee Osteoarthritis: A Randomized Controlled Study. Journal of Clinical Medicine, 12(18), 6040. https://doi.org/10.3390/jcm12186040






