Pulp Revascularization in an Autotransplanted Mature Tooth: Visualization with Magnetic Resonance Imaging and Histopathologic Correlation
Abstract
:1. Introduction
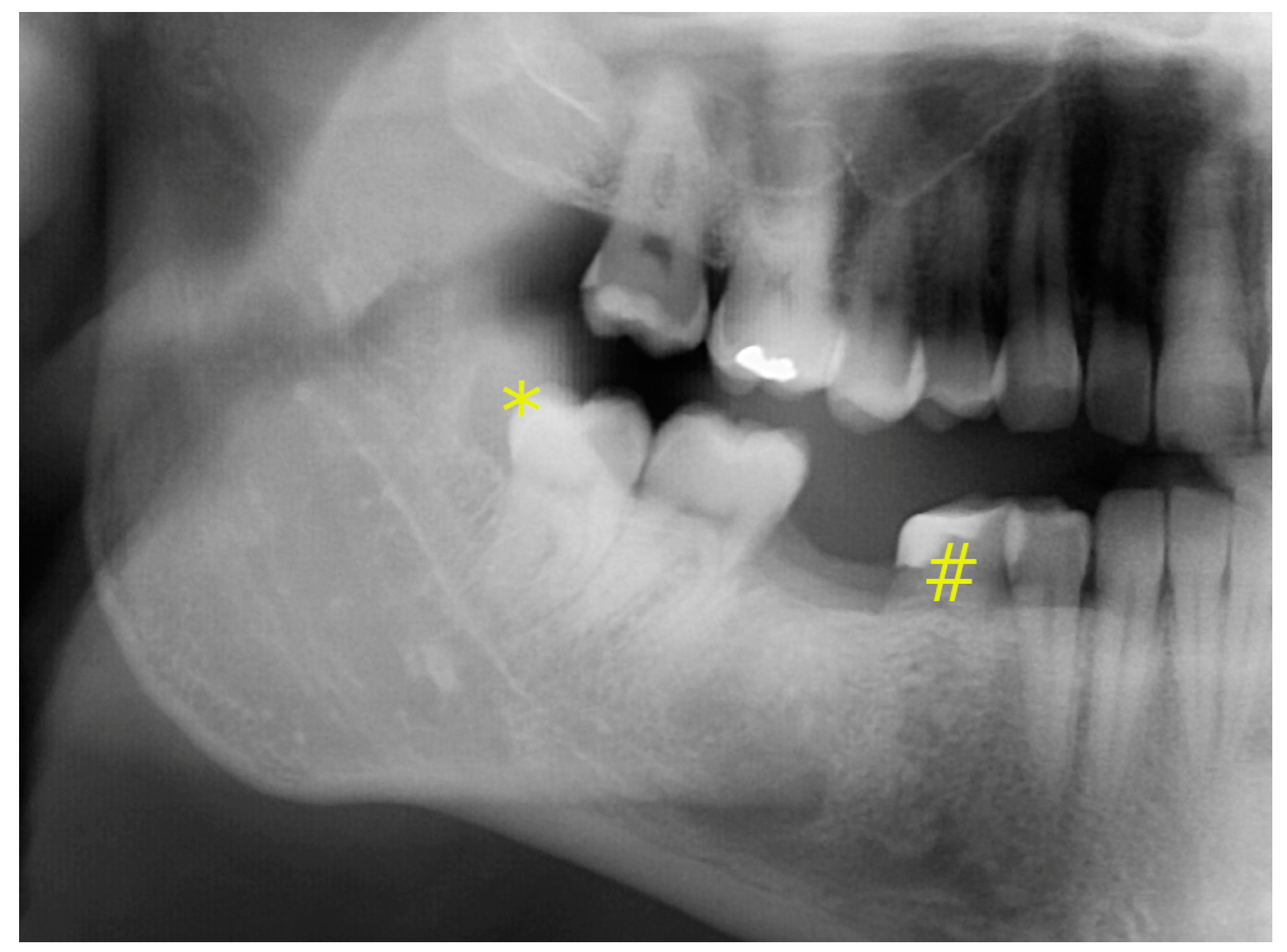
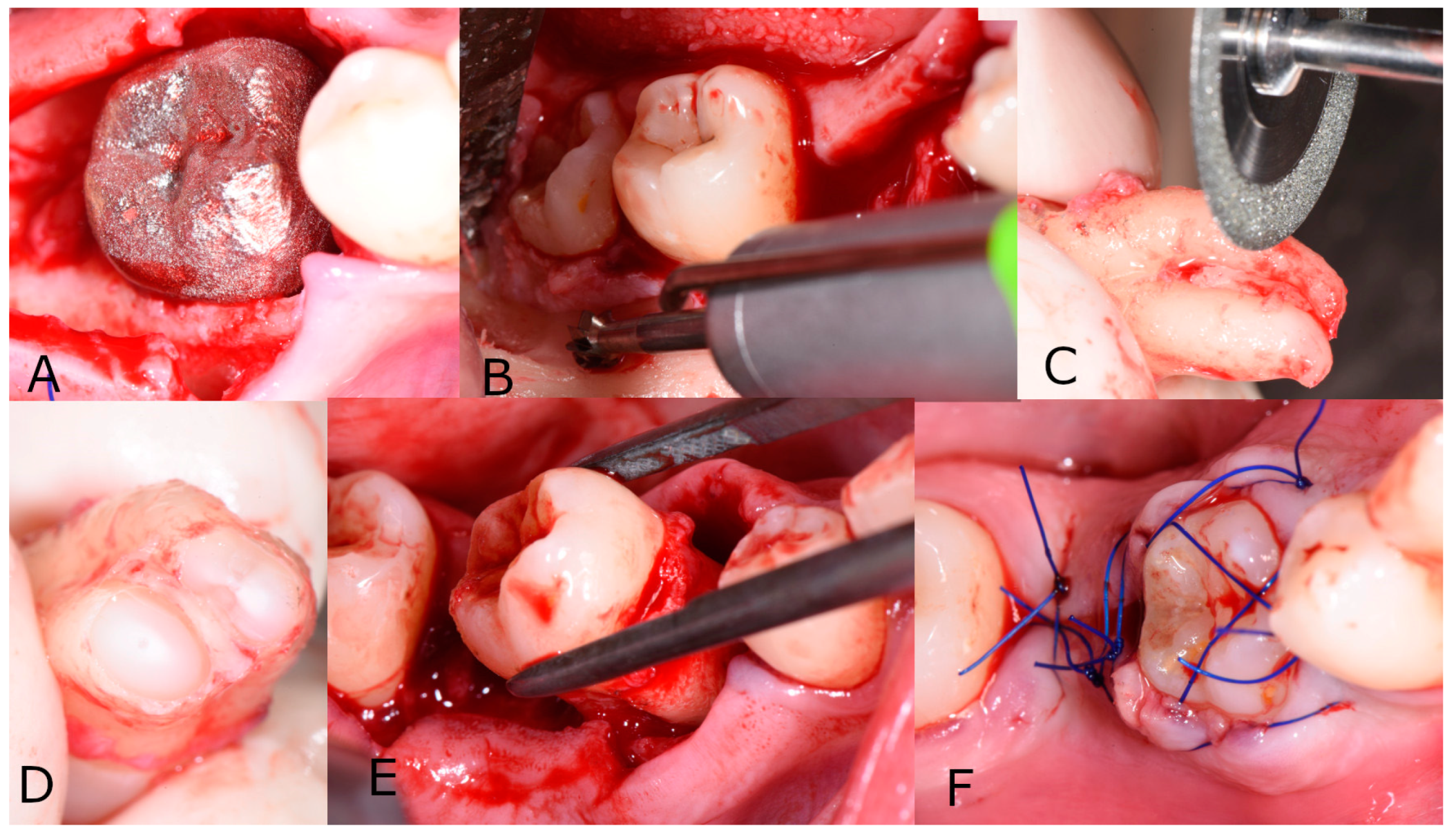
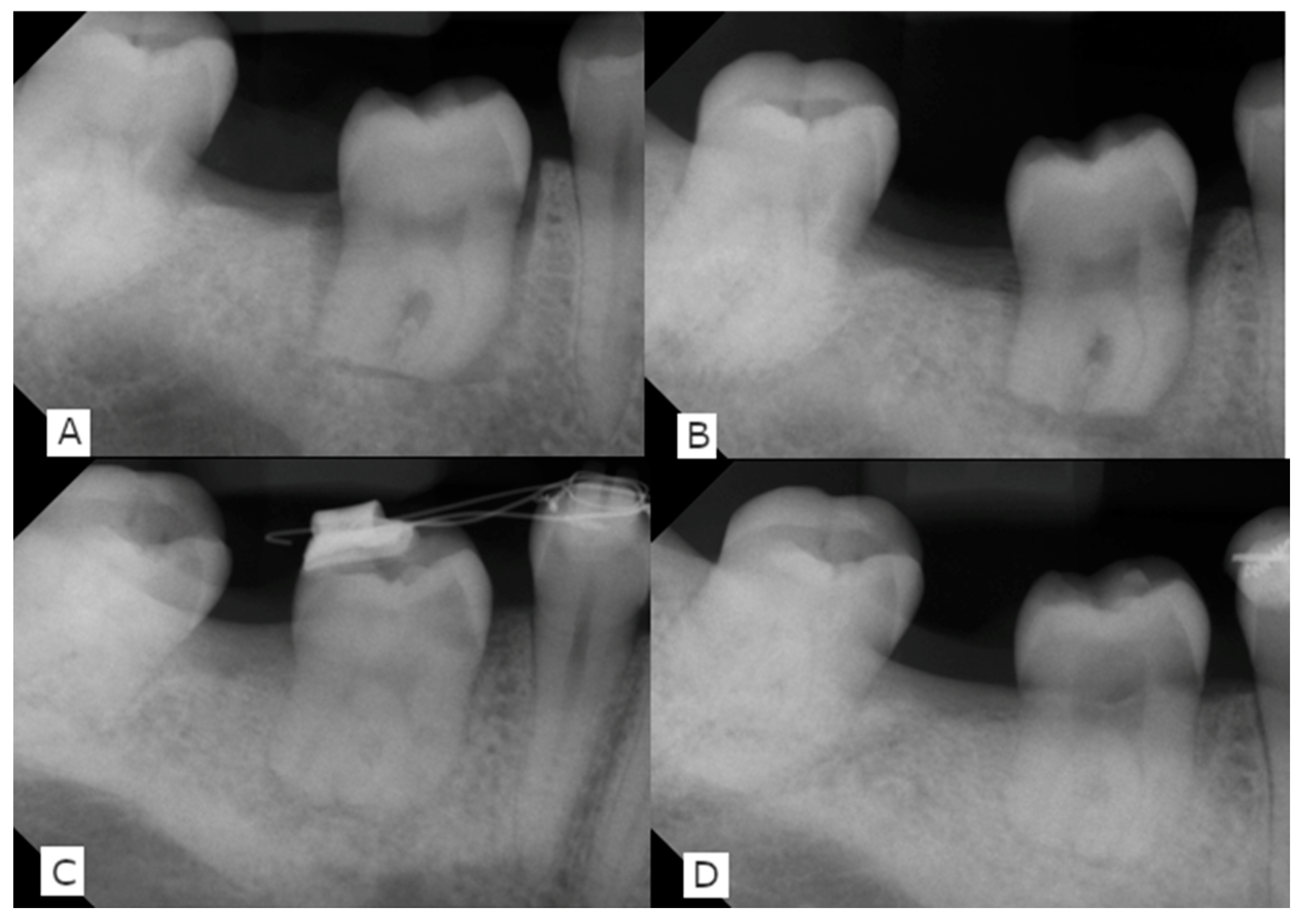
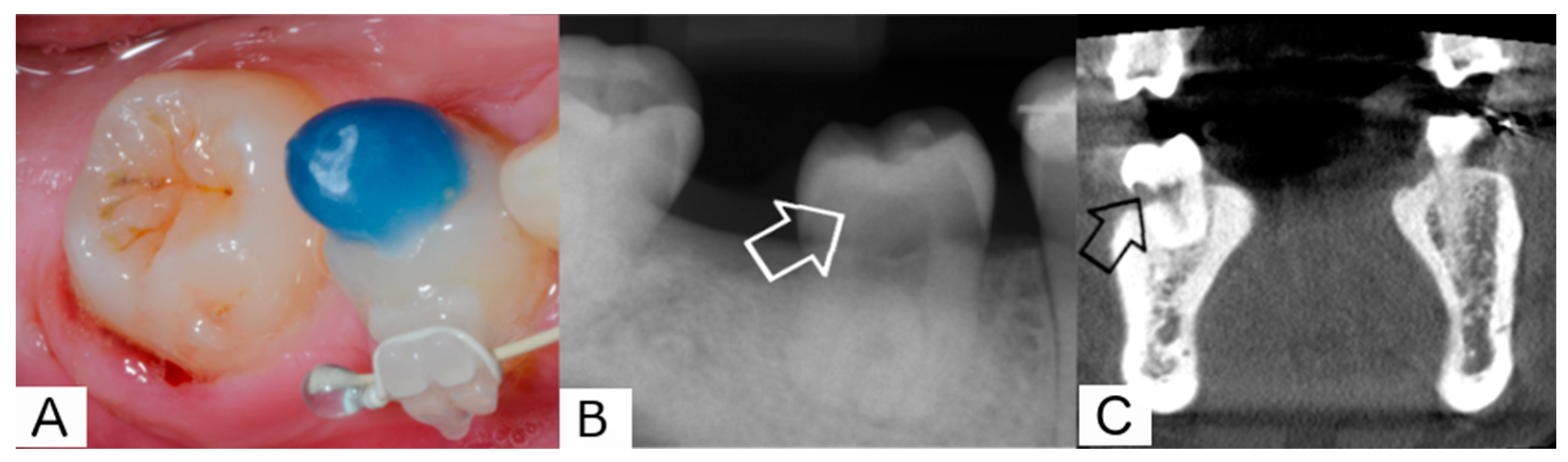
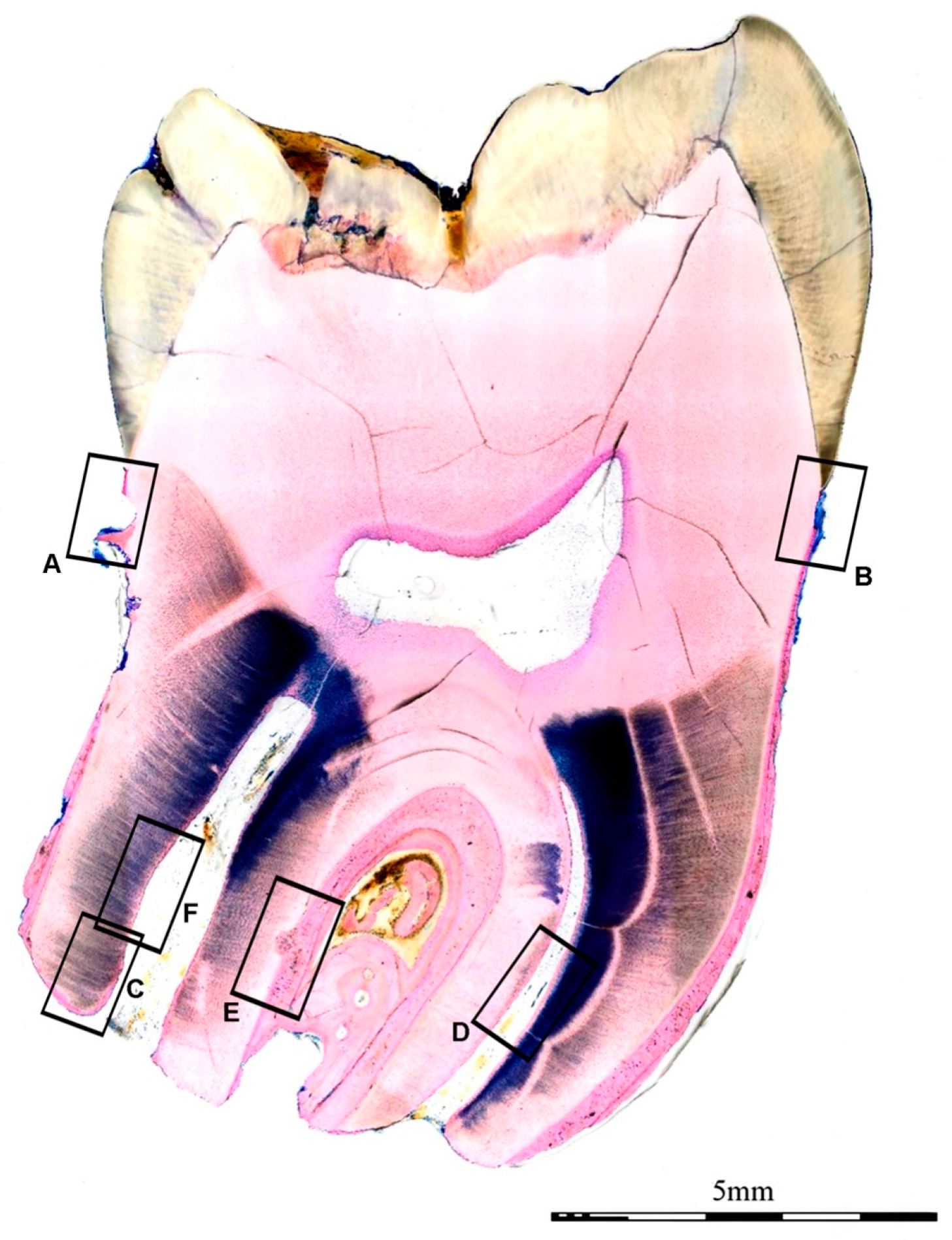
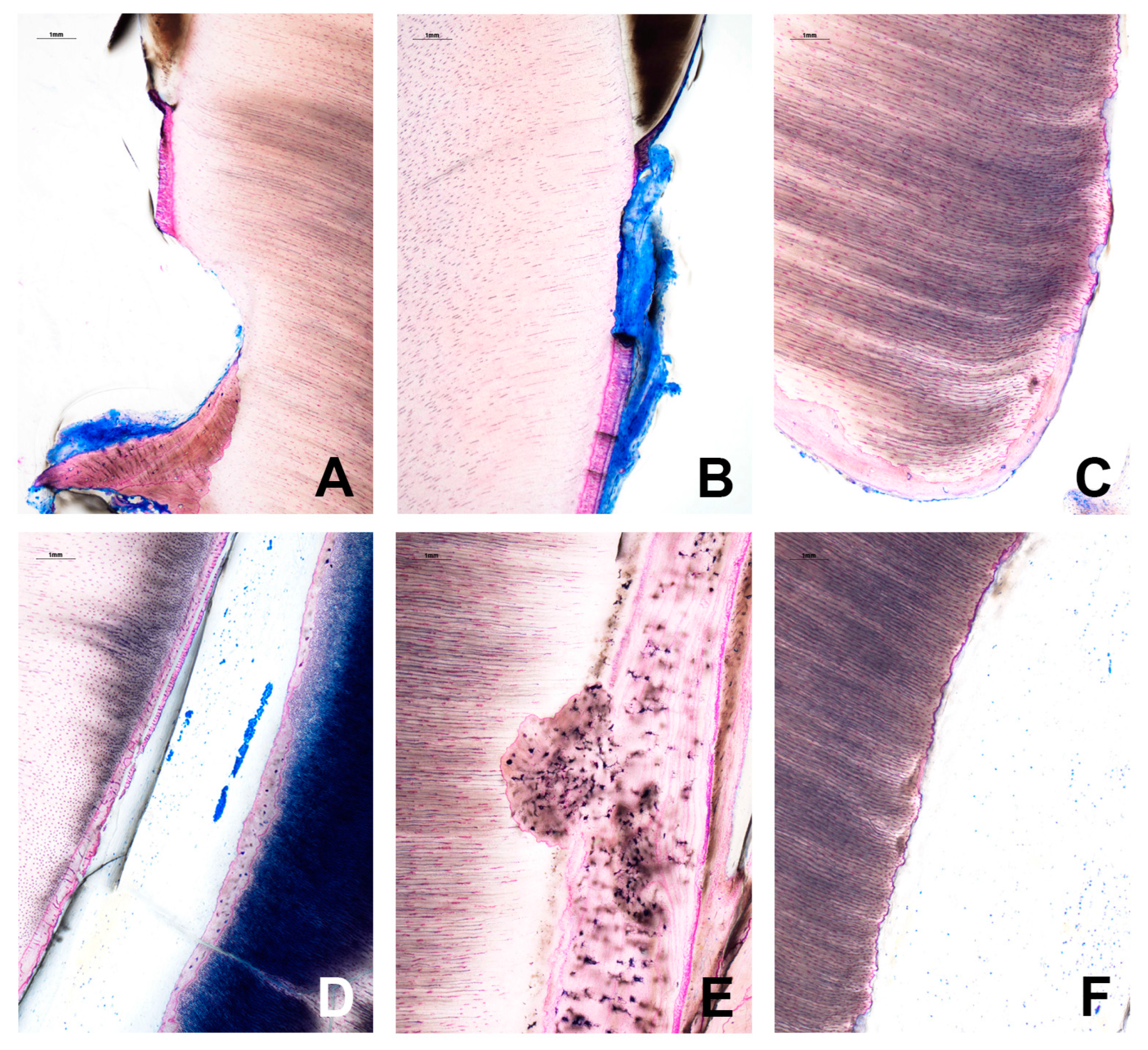
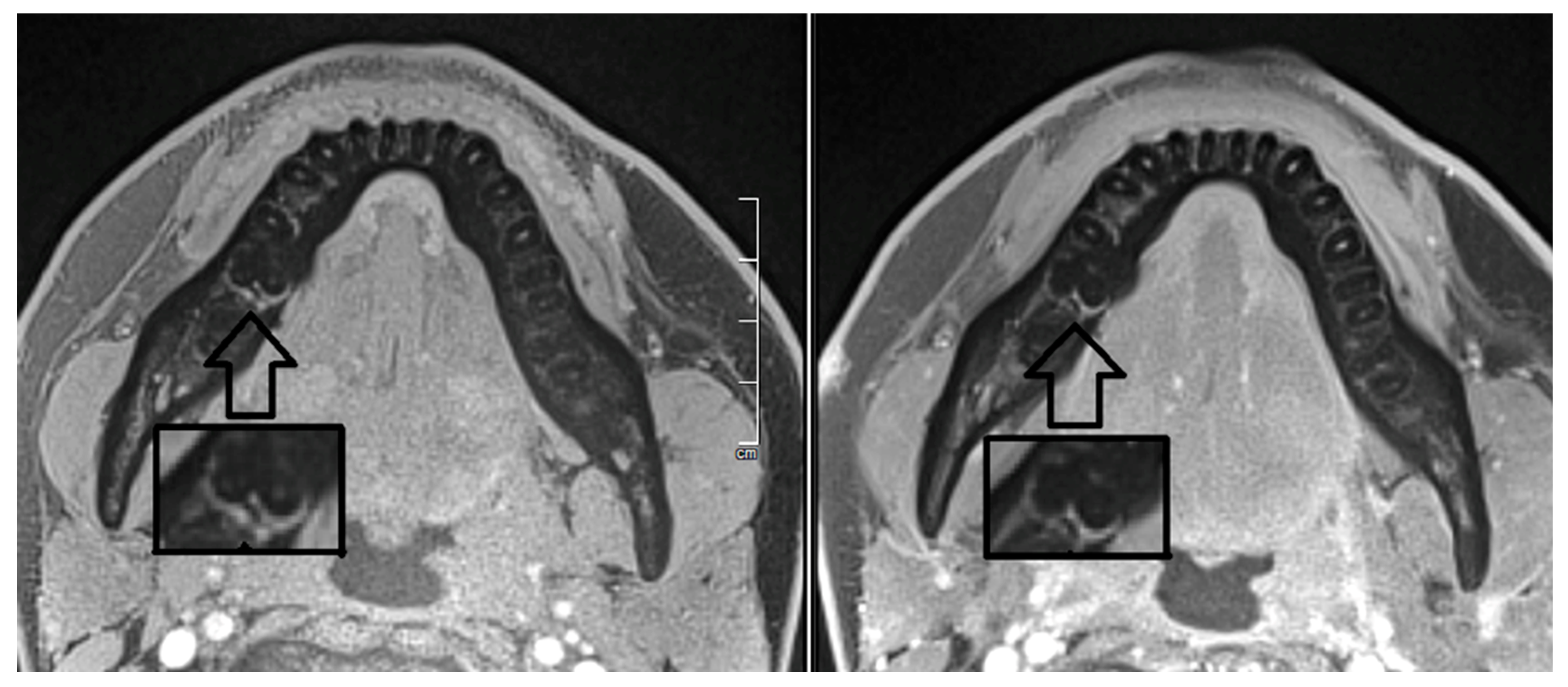
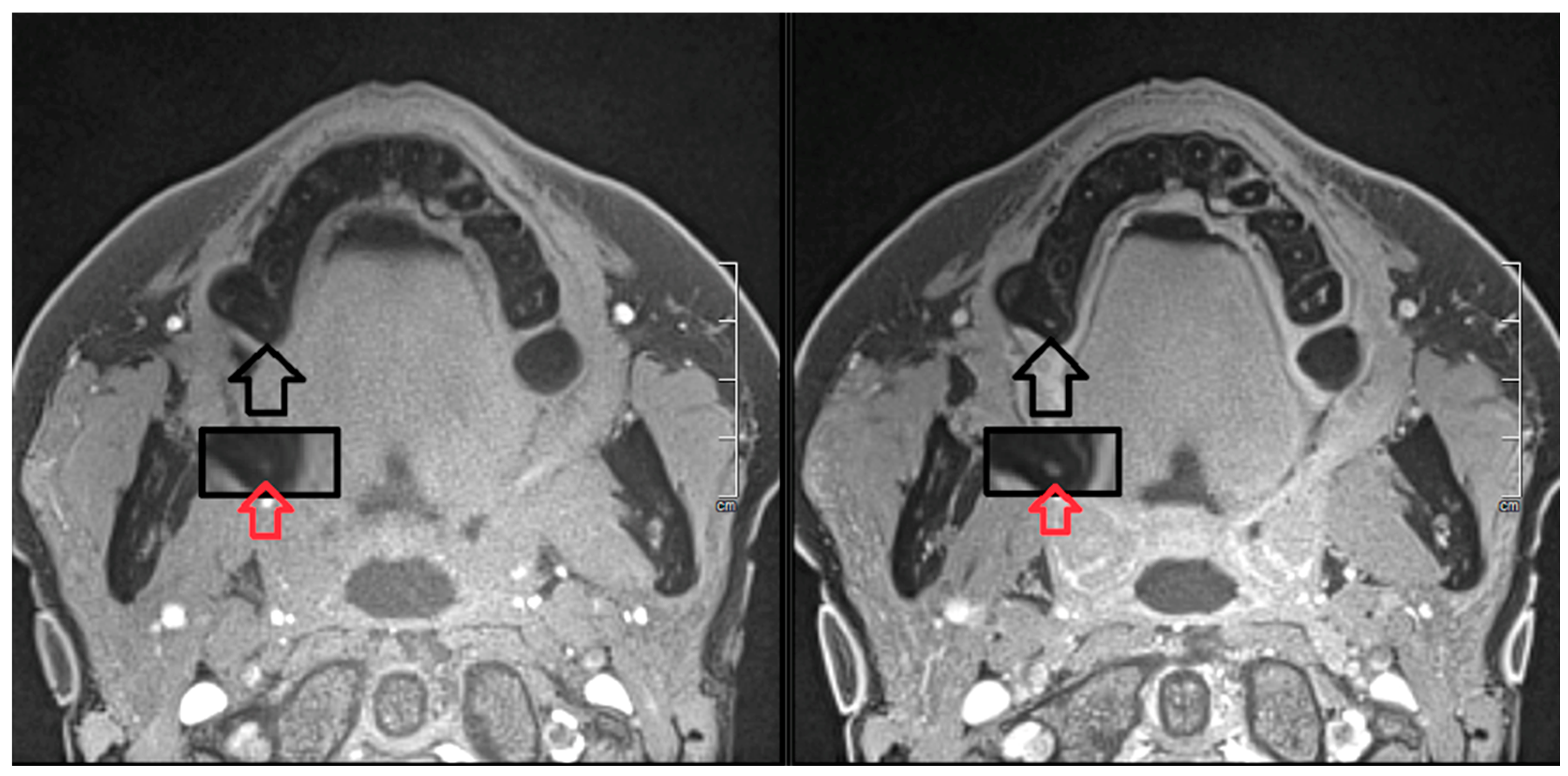
2. Report
- Presence/absence of local swelling or sinus tract
- Sensitivity to percussion
- Pulpal sensitivity to an electric pulp tester (Digitest®, Parkell, NY, USA).
3. Discussion
- (a)
- A larger diameter of the apical foramen facilitates cell migration and ingrowth of new vessels into the ischemic pulp space.
- (b)
- Immature teeth display a shorter root; therefore, the migration distance for the ingrown tissue is short.
- (c)
- Immature teeth are endowed with more and a larger spectrum of stem cells.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verweij, J.P.; van Westerveld, K.J.H.; Anssari Moin, D.; Mensink, G.; van Merkesteyn, J.P.R. Autotransplantation With a 3-Dimensionally Printed Replica of the Donor Tooth Minimizes Extra-Alveolar Time and Intraoperative Fitting Attempts: A Multicenter Prospective Study of 100 Transplanted Teeth. J. Oral. Maxillofac. Surg. 2020, 78, 35–43. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, X.; Zhu, J.; Su, C.; Yang, Y.; Meng, L. Influence of apical diameter on the outcome of regenerative endodontic treatment in teeth with pulp necrosis: A review. J. Endod. 2018, 44, 414–431. [Google Scholar] [CrossRef]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y. Regeneration of dental-pulp-like tissue by chemotaxis—Induced cell homing. Tissue Eng. A 2010, 16, 3023–3031. [Google Scholar] [CrossRef]
- Wolf, T.G.; Kim, P.; Campus, G.; Stiebritz, M.; Siegrist, M.; Briseño-Marroquín, B. 3-Dimensional analysis and systematic review of root canal morphology and physiological foramen geometry of 109 mandibular first premolars by micro-computed tomography in a mixed Swiss-German population. J. Endod. 2020, 46, 801–809. [Google Scholar] [CrossRef]
- Kokai, S.; Kanno, Z.; Koike, S.; Uesugi, S.; Takahashi, Y.; Ono, T.; Soma, K. Retrospective study of 100 autotransplanted teeth with complete root formation and subsequent orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Tsukiboshi, M. Autotransplantation of teeth: Requirements for predictable success. Dent. Traumatol. 2002, 18, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Ploder, O.; Partik, B.; Rand, T.; Fock, N.; Voracek, M.; Undt, G.; Baumann, A. Reperfusion of autotransplanted teeth—Comparison of clinical measurements by means of dental magnetic resonance imaging. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2001, 92, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akamine, A. The Application of Tissue Engineering to Regeneration of Pulp and Dentin in Endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Bellavia, D.; Ottolenghi, L.; Cicconetti, A.; Cristalli, M.P.; Quaranta, R.; Pilloni, A. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: Preliminary data. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 102, 815–825. [Google Scholar] [CrossRef]
- Assaf, A.T.; Zrnc, T.A.; Remus, C.C.; Khokale, A.; Habermann, C.R.; Schulze, D.; Fiehler, J.; Heiland, M.; Sedlacik, J.; Friedrich, R.E. Early detection of pulp necrosis and dental vitality after traumatic dental injuries in children and adolescents by 3-Tesla magnetic resonance imaging. J. Cranio Maxillofac. Surg. 2015, 43, 1088–1093. [Google Scholar] [CrossRef]
- Jakse, N.; Ruckenstuhl, M.; Rugani, P.; Kirnbauer, B.; Sokolowski, A.; Ebeleseder, K. Influence of extraoral apicoectomy on revascularization of an autotransplanted tooth: A case report. J. Endod. 2018, 44, 1298–1302. [Google Scholar] [CrossRef]
- Gaviño Orduña, J.F.; García García, M.; Dominguez, P.; Caviedes Bucheli, J.; Martin Biedma, B.; Abella Sans, F.; Manzanares Céspedes, M.C. Successful pulp revascularization of an autotransplantated mature premolar with fragile fracture apicoectomy and plasma rich in growth factors: A 3-year follow-up. Int. Endod. J. 2020, 53, 421–433. [Google Scholar] [CrossRef]
- Raabe, C.; Bornstein, M.M.; Ducommun, J.; Sendi, P.; Von Arx, T.; Janner, S.F.M. A retrospective analysis of autotransplanted teeth including an evaluation of a novel surgical technique. Clin. Oral. Investig. 2021, 25, 3513–3525. [Google Scholar] [CrossRef]
- Rugani, P.; Kirnbauer, B.; Mischak, I.; Ebeleseder, K.; Jakse, N. Extraoral Root-End Resection May Promote Pulpal Revascularization in Autotransplanted Mature Teeth—A Retrospective Study. J. Clin. Med. 2022, 11, 7199. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Chong, B.S.; McCabe, P.; Shah, P.K.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Dummer, P.M.H. PRICE 2020 guidelines for reporting case reports in Endodontics: Explanation and elaboration. Int. Endod. J. 2020, 53, 922–947. [Google Scholar] [CrossRef]
- Donath, K. [The separating thin section technique for the production of histological preparations from tissues and materials that cannot be sectioned: Description of apparatus and methods] German. Die Trenn-Dünnschliff-Technik zur Herstellung histologischer Präparate von nicht schneidbaren Geweben und Materialien. Präparator 1998, 34, 197–206. [Google Scholar]
- Laczko, J.; Levai, G. A simple differential staining method for semi-thin sections of ossyfying cartilage and bone tissue embedded in epoxy resin. Mikroskopie 1975, 31, 1–4. [Google Scholar]
- Kress, B.; Buhl, Y.; Anders, L.; Stippich, C.; Palm, F.; Bähren, W.; Sartor, K. Quantitative analysis of MRI signal intensity as a tool for evaluating tooth pulp vitality. Dentomaxillofac. Radiol. 2004, 33, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.; Zanza, A.; Mazzoni, A.; Cicconetti, A.; Testarelli, L.; Di Nardo, D. An Update of the Possible Applications of Magnetic Resonance Imaging (MRI) in Dentistry: A Literature Review. J. Imaging 2021, 7, 75. [Google Scholar] [CrossRef]
- Shah, R.; D’Arco, F.; Soares, B.; Cooper, J.; Brierley, J. Use of gadolinium contrast agents in paediatric population: Donald Rumsfeld meets Hippocrates! Br. J. Radiol. 2019, 92, 20180746. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.R.; Lee, H.G.; Lee, C.H.; Youn, S.W.; Lee, I.H.; Yoon, W.; Yang, W.; Wang, H.; Wang, J.; Shih, T.T.; et al. Safety of gadobutrol in over 23,000 patients: The GARDIAN study, a global multicentre, prospective, non-interventional study. Eur. Radiol. 2017, 27, 286–295. [Google Scholar] [CrossRef]
- Heshmatzadeh Behzadi, A.; McDonald, J. Gadolinium-based contrast agents for imaging of the central nervous system: A multicenter European prospective study. Medicine 2022, 101, e30163. [Google Scholar] [CrossRef]
- Skoglund, A. Pulpal changes in replanted and autotransplanted apicoectomized mature teeth of dogs. Int. J. Oral. Surg. 1981, 10, 111–121. [Google Scholar]
- Skoglund, A. Vascular changes in replanted and autotransplanted apicoectomized mature teeth of dogs. Int. J. Oral. Surg. 1981, 10, 100–110. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Hosseini, F.; Mehrizi, E.A.; Verma, P.; Torabinejad, M. Histologic outcomes of uninfected human immature teeth treated with regenerative endodontics: 2 case reports. J. Endod. 2015, 41, 1725–1729. [Google Scholar] [CrossRef]
- Martin, G.; Ricucci, D.; Gibbs, J.L.; Lin, L.M. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J. Endod. 2013, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Paulsen, H.U.; Yu, Z.; Schwartz, O. A long-term study of 370 autotransplanted premolars. Part III. Periodontal healing subsequent to transplantation. Eur. J. Orthod. 1990, 12, 25–37. [Google Scholar] [CrossRef]
- Filippi, A. [Tooth transplantation] German. Quintessenz 2008, 59, 497–504. [Google Scholar]
- Paulsen, H.U.; Andreasen, J.O.; Schwartz, O. Pulp and periodontal healing, root development and root resorption subsequent to transplantation and orthodontic rotation: A long-term study of autotransplanted premolars. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 630–640. [Google Scholar] [CrossRef]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J. Dent. Res. 2015, 12, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, Y.; Zhou, R.; Huang, X.; Cai, Z. Histologic and Immunohistochemical Findings of a Human Immature Permanent Tooth with Apical Periodontitis after Regenerative Endodontic Treatment. J. Endod. 2015, 41, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pei, X.; Zhao, Y.; Tulu, U.S.; Liu, B.; Helms, J.A. A Wnt-Responsive PDL Population Effectuates Extraction Socket Healing. J. Dent. Res. 2018, 7, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, J.; Wang, K.; Li, C.; Zhang, S.; Jing, D.; Xu, C.; Wang, X.; Zhao, H.; Feng, J.Q. Axin2+-Mesenchymal PDL Cells, Instead of K14+ Epithelial Cells, Play a Key Role in Rapid Cementum Growth. J. Dent. Res. 2019, 11, 1262–1270. [Google Scholar] [CrossRef]
- Eramo, S.; Natali, A.; Pinna, R.; Milia, E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018, 51, 405–419. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Fouad, A.M.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Cell-Based Transplantation versus Cell Homing Approaches for Pulp-Dentin Complex Regeneration. Stem Cells Int. 2021, 8483668. [Google Scholar] [CrossRef] [PubMed]
- Yanpiset, K.; Trope, M. Pulp revascularization of replanted immature dog teeth after different treatment methods. Endod. Dent. Traumatol. 2000, 16, 211–217. [Google Scholar] [CrossRef]
- Skoglund, A.; Tronstad, L. Pulpal changes in replanted and autotransplanted immature teeth of dogs. J. Endod. 1981, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, N.; Nagaoka, H.; Yamauchi, S.; Teixeira, F.B.; Miguez, P.; Yamauchi, M. Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J. Endod. 2011, 37, 1636–1641. [Google Scholar] [CrossRef]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugani, P.; Brcic, I.; Magyar, M.; Schwarze, U.Y.; Jakse, N.; Ebeleseder, K. Pulp Revascularization in an Autotransplanted Mature Tooth: Visualization with Magnetic Resonance Imaging and Histopathologic Correlation. J. Clin. Med. 2023, 12, 6008. https://doi.org/10.3390/jcm12186008
Rugani P, Brcic I, Magyar M, Schwarze UY, Jakse N, Ebeleseder K. Pulp Revascularization in an Autotransplanted Mature Tooth: Visualization with Magnetic Resonance Imaging and Histopathologic Correlation. Journal of Clinical Medicine. 2023; 12(18):6008. https://doi.org/10.3390/jcm12186008
Chicago/Turabian StyleRugani, Petra, Iva Brcic, Marton Magyar, Uwe Yacine Schwarze, Norbert Jakse, and Kurt Ebeleseder. 2023. "Pulp Revascularization in an Autotransplanted Mature Tooth: Visualization with Magnetic Resonance Imaging and Histopathologic Correlation" Journal of Clinical Medicine 12, no. 18: 6008. https://doi.org/10.3390/jcm12186008
APA StyleRugani, P., Brcic, I., Magyar, M., Schwarze, U. Y., Jakse, N., & Ebeleseder, K. (2023). Pulp Revascularization in an Autotransplanted Mature Tooth: Visualization with Magnetic Resonance Imaging and Histopathologic Correlation. Journal of Clinical Medicine, 12(18), 6008. https://doi.org/10.3390/jcm12186008






