The Role of Spatial Aortic Arch Architecture in Type B Aortic Dissection
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. CAT Scan Analysis
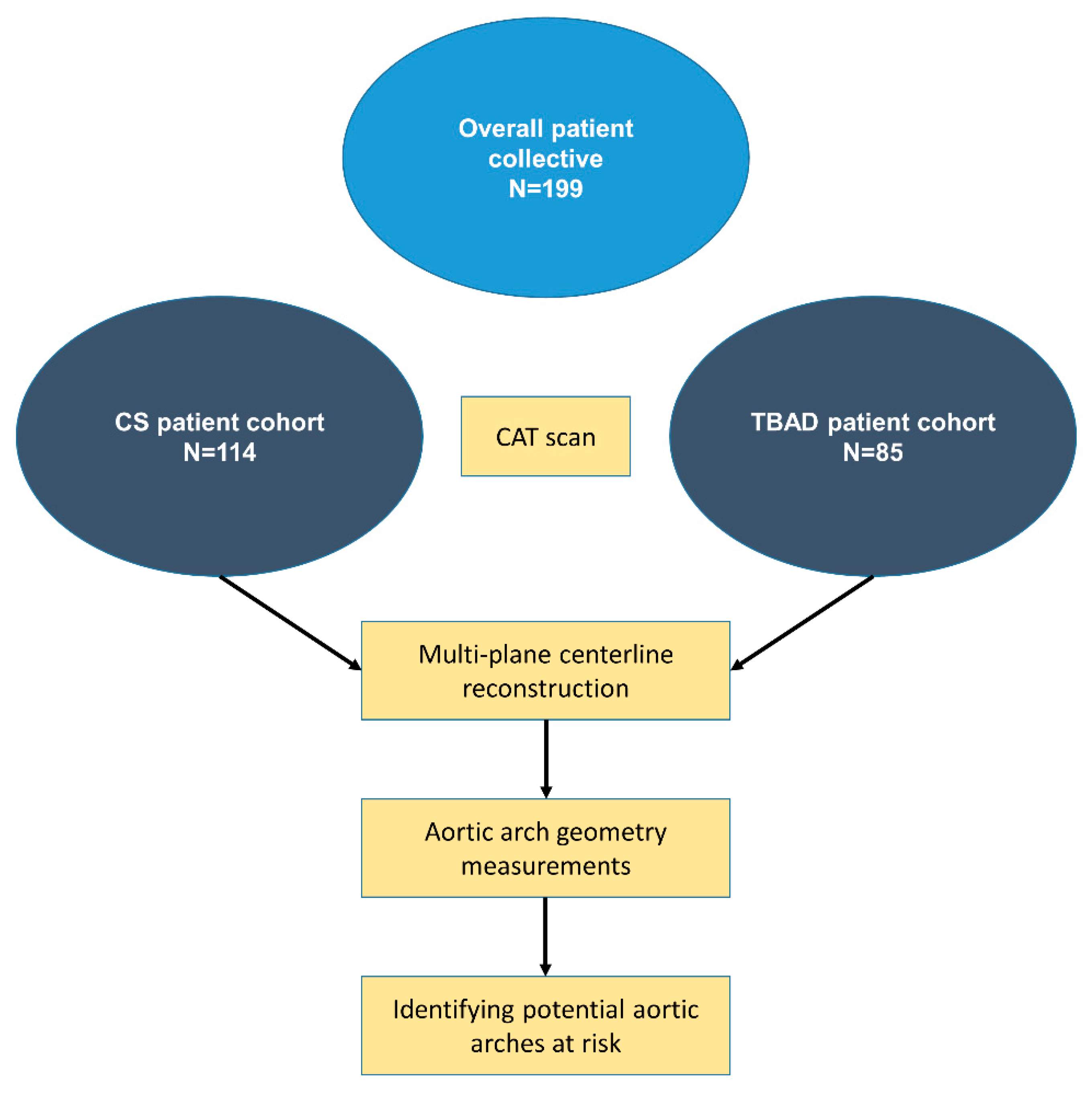

2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
3.1. Patient Characteristics
3.2. Anatomical Parameters of the Aortic Arch and Supra-Aortic Branches
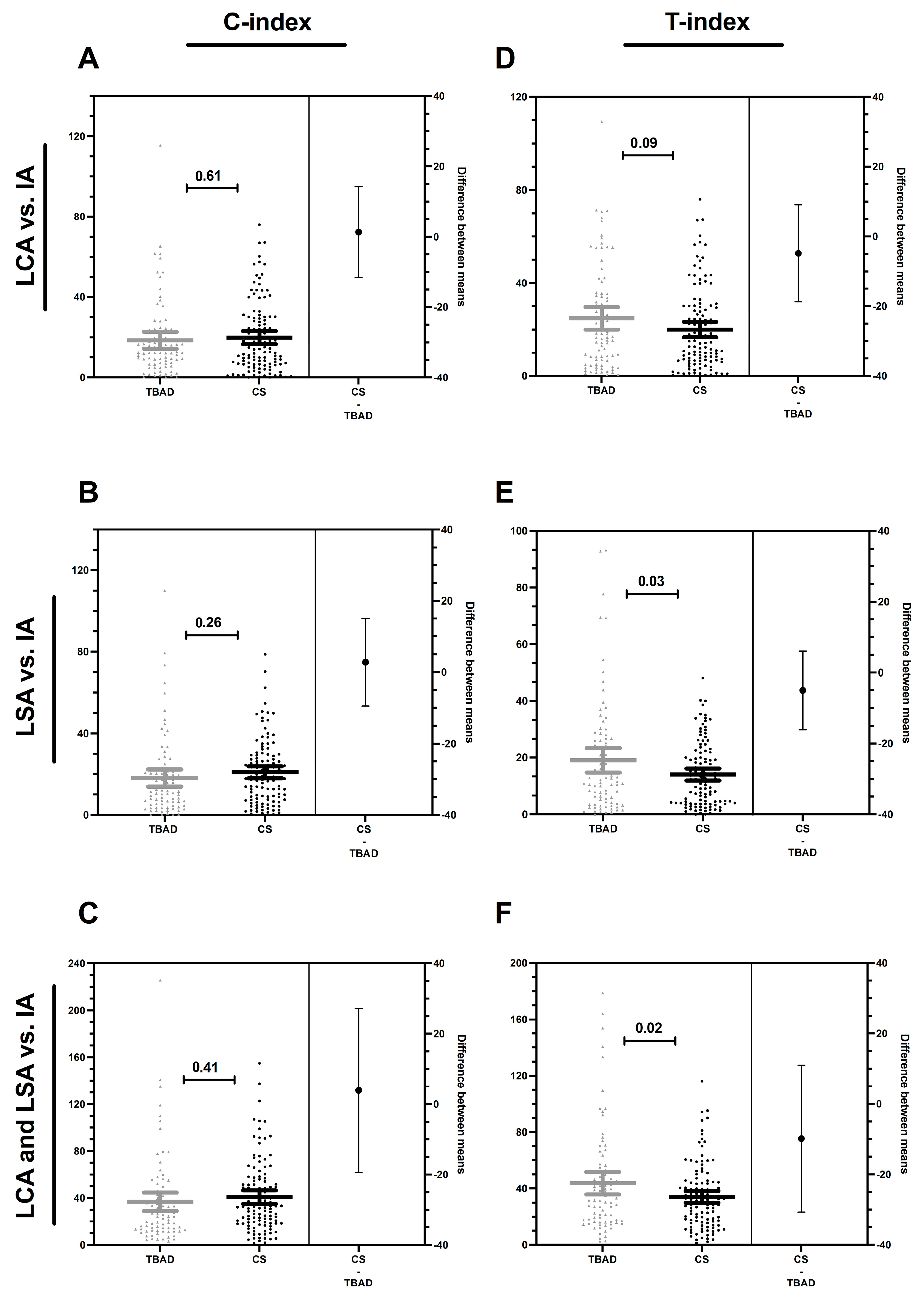
3.3. Compression and Torsion Index
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamaguchi, T.; Nakai, M.; Yano, T.; Matsuyama, M.; Yoshino, H.; Miyamoto, Y.; Sumita, Y.; Matsuda, H.; Inoue, Y.; Okita, Y.; et al. Population-Based Incidence and Outcomes of Acute Aortic Dissection in Japan. Eur. Hear. J. Acute Cardiovasc. Care 2021, 10, 701–709. [Google Scholar] [CrossRef]
- Hallberg, S.; Gandra, S.R.; Fox, K.M.; Mesterton, J.; Banefelt, J.; Johansson, G.; Levin, L.; Sobocki, P. Healthcare Costs Associated with Cardiovascular Events in Patients with Hyperlipidemia or Prior Cardiovascular Events: Estimates from Swedish Population-Based Register Data. Eur. J. Health Econ. 2016, 17, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Criado, F.J. Aortic Dissection: A 250-Year Perspective. Tex. Hear. Inst. J. 2011, 38, 694–700. [Google Scholar]
- Zhou, Z.; Cecchi, A.C.; Prakash, S.K.; Milewicz, D.M. Risk Factors for Thoracic Aortic Dissection. Genes 2022, 13, 1814. [Google Scholar] [CrossRef]
- Oberhuber, A.; Raddatz, A.; Betge, S.; Ploenes, C.; Ito, W.; Janosi, R.A.; Ott, C.; Langheim, E.; Czerny, M.; Puls, R.; et al. Interdisciplinary German Clinical Practice Guidelines on the Management of Type B Aortic Dissection. Gefasschirurgie 2023, 28, 1. [Google Scholar] [CrossRef]
- Shetty, P. Grey Matter: Ageing in Developing Countries. Lancet 2012, 379, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Shalhub, S.; Schäfer, M.; Hatsukami, T.S.; Sweet, M.P.; Reynolds, J.J.; Bolster, F.A.; Shin, S.H.; Reece, T.B.; Singh, N.; Starnes, B.W.; et al. Association of Variant Arch Anatomy with Type B Aortic Dissection and Hemodynamic Mechanisms. J. Vasc. Surg. 2018, 68, 1640–1648. [Google Scholar] [CrossRef]
- Marrocco-Trischitta, M.M.; Rylski, B.; Schofer, F.; Secchi, F.; Piffaretti, G.; De Beaufort, H.; Belvroy, V.; Bismuth, J.; Czerny, M.; Trimarchi, S. Prevalence of Type III Arch Configuration in Patients with Type B Aortic Dissection. Eur. J. Cardiothorac. Surg. 2019, 56, 1075–1080. [Google Scholar] [CrossRef]
- Madhwal, S.; Rajagopal, V.; Bhatt, D.L.; Bajzer, C.T.; Whitlow, P.; Kapadia, S.R. Predictors of Difficult Carotid Stenting as Determined By Aortic Arch Angiography. J. Invasive Cardiol. 2008, 20, 200–204. [Google Scholar]
- Demertzis, S.; Hurni, S.; Stalder, M.; Gahl, B.; Herrmann, G.; Van Den Berg, J. Aortic Arch Morphometry in Living Humans. J. Anat. 2010, 217, 588–596. [Google Scholar] [CrossRef]
- Salmasi, M.Y.; Pirola, S.; Mahuttanatan, S.; Fisichella, S.M.; Sengupta, S.; Jarral, O.A.; Oo, A.; O’Regan, D.; Xu, X.Y.; Athanasiou, T. Geometry and Flow in Ascending Aortic Aneurysms Are Influenced by Left Ventricular Outflow Tract Orientation: Detecting Increased Wall Shear Stress on the Outer Curve of Proximal Aortic Aneurysms. J. Thorac. Cardiovasc. Surg. 2023, 166, 11–21.e1. [Google Scholar] [CrossRef] [PubMed]
- Shum, J.; Martufi, G.; Di Martino, E.; Washington, C.B.; Grisafi, J.; Muluk, S.C.; Finol, E.A. Quantitative Assessment of Abdominal Aortic Aneurysm Geometry. Ann. Biomed. Eng. 2011, 39, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Van Bogerijen, G.H.W.; Tolenaar, J.L.; Rampoldi, V.; Moll, F.L.; Van Herwaarden, J.A.; Jonker, F.H.W.; Eagle, K.A.; Trimarchi, S. Predictors of Aortic Growth in Uncomplicated Type B Aortic Dissection. J. Vasc. Surg. 2014, 59, 1134–1143. [Google Scholar] [CrossRef]
- Malone, C.D.; Urbania, T.H.; Crook, S.E.S.; Hope, M.D. Bovine Aortic Arch: A Novel Association with Thoracic Aortic Dilation. Clin. Radiol. 2012, 67, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.; Velásquez, F.; Mertens, N.; Vargas, F.; Torrealba, I.; Mariné, L.; Bergoeing, M.; Valdés, F. Higher Prevalence of Bovine Aortic Arch Configuration in Patients Undergoing Blunt Isthmic Aortic Trauma Repair. Ann. Vasc. Surg. 2020, 67, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Wang, L.; Li, Q.; He, H.; Li, X.; Li, M.; Wang, T.; Zhao, C.; Zhang, X.; et al. Aortic Geometric Alteration Associated With Acute Type B Aortic Dissection: Angulation, Tortuosity, and Arch Type. Front. Physiol. 2021, 12, 708651. [Google Scholar] [CrossRef]
- Alhafez, B.A.; Truong, V.T.T.; Ocazionez, D.; Sohrabi, S.; Sandhu, H.; Estrera, A.; Safi, H.J.; Evangelista, A.; Hurtado, L.D.S.; Guala, A.; et al. Aortic Arch Tortuosity, a Novel Biomarker for Thoracic Aortic Disease, Is Increased in Adults with Bicuspid Aortic Valve. Int. J. Cardiol. 2019, 284, 84–89. [Google Scholar] [CrossRef]
- Geeraert, P.; Jamalidinan, F.; Burns, F.; Jarvis, K.; Bristow, M.S.; Lydell, C.; Hidalgo Tobon, S.S.; de Celis Alonso, B.; Fedak, P.W.M.; White, J.A.; et al. Hemodynamic Assessment in Bicuspid Aortic Valve Disease and Aortic Dilation: New Insights From Voxel-By-Voxel Analysis of Reverse Flow, Stasis, and Energetics. Front. Bioeng. Biotechnol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A Syndrome of Altered Cardiovascular, Craniofacial, Neurocognitive and Skeletal Development Caused by Mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef]
- Veger, H.T.C.; Pasveer, E.H.; Westenberg, J.J.M.; Wever, J.J.; van Eps, R.G.S. Wall Shear Stress Assessment of the False Lumen in Acute Type B Aortic Dissection Visualized by 4-Dimensional Flow Magnetic Resonance Imaging: An Ex-Vivo Study. Vasc. Endovasc. Surg. 2021, 55, 696–701. [Google Scholar] [CrossRef]
- Akutsu, K. Etiology of Aortic Dissection. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, E.; Nishigami, K.; Miyamoto, S.; Sakamoto, T.; Nakao, K. Impact of Shear Stress and Atherosclerosis on Entrance-Tear Formation in Patients with Acute Aortic Syndromes. Heart Vessel. 2014, 29, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, F.M.; Grieve, S.M. Normal Patterns of Thoracic Aortic Wall Shear Stress Measured Using Four-Dimensional Flow MRI in a Large Population. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1174–H1181. [Google Scholar] [CrossRef]
- Wen, J.; Yan, T.; Su, Z.; Huang, H.; Gao, Q.; Chen, X.; Wong, K.K.L.; Peng, L. Risk Evaluation of Type B Aortic Dissection Based on WSS-Based Indicators Distribution in Different Types of Aortic Arch. Comput. Methods Programs Biomed. 2022, 221, 106872. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Celermajer, D.S.; Raisky, O.; Jolivet, O.; Buyens, F.; Herment, A.; Sidi, D.; Bonnet, D.; Mousseaux, E. Angular (Gothic) Aortic Arch Leads to Enhanced Systolic Wave Reflection, Central Aortic Stiffness, and Increased Left Ventricular Mass Late after Aortic Coarctation Repair: Evaluation with Magnetic Resonance Flow Mapping. J. Thorac. Cardiovasc. Surg. 2008, 135, 62–68. [Google Scholar] [CrossRef]
- Fortunato, R.N.; Huckaby, L.V.; Emerel, L.V.; Schlosser, V.; Yang, F.; Phillippi, J.A.; Vorp, D.A.; Maiti, S.; Gleason, T.G. The Predictive Capability of Aortic Stiffness Index for Aortic Dissection among Dilated Ascending Aortas. J. Thorac. Cardiovasc. Surg. 2022, in press. [Google Scholar] [CrossRef]
- Mulorz, J.; Ibing, W.; Cappallo, M.; Braß, S.M.; Takeuchi, K.; Raaz, U.; Schellinger, I.N.; Krott, K.J.; Schelzig, H.; Aubin, H.; et al. Ethanol Enhances Endothelial Rigidity by Targeting VE-Cadherin—Implications for Acute Aortic Dissection. J. Clin. Med. 2023, 12, 4967. [Google Scholar] [CrossRef] [PubMed]
- Luebke, T.; Brunkwall, J. Type B Aortic Dissection. Ann. Cardiothorac. Surg. 2014, 3, 265–278. [Google Scholar] [CrossRef]
- Hughes, G.C. Commentary: Lack of Screening Makes Primary Prevention Most Effective to Reduce the Mortality of Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 1202–1203. [Google Scholar] [CrossRef]

| TBAD (N = 85) | CS (N = 114) | TBAD vs. CS p-Value | |
|---|---|---|---|
| Age (years) | 61.5 ± 13.1 | 71 ± 9.3 | <0.000 |
| Age groups (years) | |||
| <34 | 3 (3.5) | - | 0.04 |
| 35–49 | 9 (10.6) | - | 0.000 |
| 50–64 | 40 (47.1) | 27 (23.7) | 0.001 |
| 65–79 | 26 (30.6) | 64 (56.1) | 0.000 |
| 80–95 | 7 (8.2) | 23 (20.2) | 0.02 |
| Sex | |||
| Male | 63 (74.1) | 82 (71.9) | 0.73 |
| Female | 22 (25.9) | 32 (28.1) | 0.73 |
| Medication | |||
| Beta-Blockers | 61 (71.8) | 68 (59.6) | 0.07 |
| ACE-inhibitors | 36 (42.4) | 60 (52.6) | 0.15 |
| AT1-blockers | 11 (12.9) | 30 (26.3) | 0.02 |
| Calcium-antagonists | 48 (56.5) | 63 (55.3) | 0.86 |
| Diuretics | 35 (41.2) | 44 (38.6) | 0.71 |
| Comorbidities | |||
| aHT | 66 (77.6) | 107 (93.9) | 0.001 |
| T1DM/T2DM | 5 (5.9) | 25 (21.9) | 0.002 |
| CHD | 24 (28.2) | 38 (33.3) | 0.44 |
| History of tobacco use (prior and current) | 12 (14.1) | 44 (38.6) | 0.000 |
| Dimensions | N = 66 | N = 111 | N = 177 |
| Height (cm) | 177.7 ± 9.5 | 172.8 ± 7.9 | 0.000 |
| Weight (kg) | 85.7 ± 21.7 | 80.9 ± 12.7 | 0.28 |
| BMI (classes) | 26.9 ± 5.6 | 27 ± 3.7 | 0.35 |
| Underweight | 3 (4.5) | 2 (1.8) | 0.43 |
| Normal weight | 19 (28.8) | 34 (30.6) | 0.24 |
| Overweight | 29 (43.9) | 51 (45.9) | 0.16 |
| Obesity I° | 10 (15.2) | 23 (20.7) | 0.11 |
| Obesity II° | 3 (4.5) | 1 (0.9) | 0.19 |
| Obesity III° | 2 (3) | - | 0.10 |
| TBAD | CS | TBAD vs. CS p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aortic Arch Classification | N | Absolute Frequency (%) | N | Absolute Frequency (%) | |||||||
| Type I | 85 | 15 (17.6) | 114 | 29 (25.4) | 0.18 | ||||||
| Type II | 85 | 13 (15.3) | 114 | 41 (36.0) | 0.001 | ||||||
| Type III | 85 | 57 (67.1) | 114 | 44 (38.6) | 0.000 | ||||||
| Angles (°) | N | Mean | SD | Upper limit | Lower limit | N | Mean | SD | Upper limit | Lower limit | |
| IA sag. | 85 | 80.6 | 17.14 | 84.3 | 76.9 | 114 | 83.8 | 16.24 | 86.8 | 80.7 | 0.12 |
| IA cor. | 85 | 96 | 20.6 | 100.4 | 91.6 | 114 | 95 | 20.4 | 98.8 | 91.2 | 0.97 |
| IA p-sag. | 85 | 85 | 19 | 89.1 | 80 | 114 | 86.9 | 16.5 | 90 | 83.9 | 0.44 |
| IA p-cor. | 85 | 102.8 | 22.8 | 107.7 | 102.3 | 114 | 97.4 | 16.6 | 100.5 | 94.3 | 0.05 |
| LCA sag. | 85 | 70.6 | 14.1 | 73.7 | 67.6 | 114 | 76 | 17.1 | 79.2 | 72.8 | 0.13 |
| LCA cor. | 85 | 71.3 | 17.8 | 75.2 | 67.5 | 114 | 64.34 | 15.7 | 67.2 | 61.4 | 0.001 |
| LCA p-sag. | 85 | 71.7 | 14.8 | 74.9 | 68.5 | 114 | 68.7 | 13.5 | 71.2 | 66.2 | 0.12 |
| LCA p-cor. | 85 | 83.6 | 20 | 88 | 79.3 | 114 | 73 | 15.1 | 75.8 | 70.2 | 0.000 |
| LSA sag. | 85 | 74.6 | 15.7 | 78 | 71.2 | 114 | 71.1 | 14.3 | 73.8 | 68.5 | 0.11 |
| LSA cor. | 85 | 84.2 | 14.3 | 87.3 | 81.1 | 114 | 79.1 | 12.1 | 81.3 | 76.8 | 0.007 |
| LSA p-sag. | 85 | 74.1 | 13.6 | 77 | 71.2 | 114 | 69 | 13.6 | 71.5 | 66.4 | 0.009 |
| LSA p-cor. | 85 | 97.7 | 17.9 | 101.6 | 93.8 | 114 | 91.3 | 12.3 | 93.6 | 89 | 0.003 |
| Arch angulation | 85 | 64.2 | 9.7 | 66.3 | 62.1 | 114 | 74.7 | 9.4 | 76.4 | 72.9 | 0.000 |
| Distances (cm) | |||||||||||
| ∆ IA–LCA | 85 | 1.6 | 0.5 | 1.7 | 1.5 | 114 | 1.2 | 0.3 | 1.2 | 1.1 | 0.000 |
| ∆ LCA–LSA | 85 | 1.5 | 0.5 | 1.6 | 1.4 | 114 | 1.3 | 0.4 | 1.4 | 1.2 | 0.04 |
| ∆ IA–LSA | 85 | 3.1 | 0.8 | 3.3 | 2.9 | 114 | 2.6 | 0.5 | 2.7 | 2.5 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulorz, J.; Garcon, F.; Arnautovic, A.; De Somer, C.; Knapsis, A.; Aubin, H.; Fleissner, F.; Rembe, J.-D.; Vockel, M.; Oberhuber, A.; et al. The Role of Spatial Aortic Arch Architecture in Type B Aortic Dissection. J. Clin. Med. 2023, 12, 5963. https://doi.org/10.3390/jcm12185963
Mulorz J, Garcon F, Arnautovic A, De Somer C, Knapsis A, Aubin H, Fleissner F, Rembe J-D, Vockel M, Oberhuber A, et al. The Role of Spatial Aortic Arch Architecture in Type B Aortic Dissection. Journal of Clinical Medicine. 2023; 12(18):5963. https://doi.org/10.3390/jcm12185963
Chicago/Turabian StyleMulorz, Joscha, Franziska Garcon, Amir Arnautovic, Casper De Somer, Artis Knapsis, Hug Aubin, Felix Fleissner, Julian-Dario Rembe, Malwina Vockel, Alexander Oberhuber, and et al. 2023. "The Role of Spatial Aortic Arch Architecture in Type B Aortic Dissection" Journal of Clinical Medicine 12, no. 18: 5963. https://doi.org/10.3390/jcm12185963
APA StyleMulorz, J., Garcon, F., Arnautovic, A., De Somer, C., Knapsis, A., Aubin, H., Fleissner, F., Rembe, J.-D., Vockel, M., Oberhuber, A., Lichtenberg, A., Schelzig, H., & Wagenhäuser, M. U. (2023). The Role of Spatial Aortic Arch Architecture in Type B Aortic Dissection. Journal of Clinical Medicine, 12(18), 5963. https://doi.org/10.3390/jcm12185963








