Abstract
Background: ANRIL rs4977574 gene polymorphism has been associated with arterial thrombosis and cardiovascular disease development. ANRIL rs4977574 gene polymorphism could also be associated with recurrent pregnancy loss (RPL) since there is increasing evidence in favor of a potential shared pathophysiological mechanism with cardiovascular disease, potentially through arterial thrombosis. This study’s goal is to investigate the differences in ANRIL rs4977574 gene polymorphism between women with and without RPL, if any, as well as a potential association with the number of pregnancy losses. Methods: DNA was isolated from peripheral blood samples, and the sequence containing the polymorphism of interest was amplified with PCR. Results were visualized under UV light following electrophoresis in 3% agarose gel with ethidium bromide. ANRIL rs4977574 (A>G) prevalence was compared between 56 women with and 69 without RPL. Results were adjusted for women’s age and BMI, while a stratified analysis was performed according to number of pregnancy losses. Results: Allele A was significantly more prevalent in the control group compared to RPL women [31 (44.9%) vs. 14 (25%), p = 0.021]. Although not reaching statistical significance, a gradually decreasing prevalence of allele A with an increasing number of pregnancy losses was observed [31 (44.9%) in control, eight (30.7%) with two, six (23.1%) with three, and 0 (0.0%) with four pregnancy losses, p = 0.078]. Results were also similar following adjustment. Conclusions: This is the first study that demonstrates an association between RPL presence and ANRIL rs4977574 gene polymorphism (lower prevalence of allele A), while a difference according to the number of pregnancy losses cannot be excluded.
1. Introduction
Recurrent pregnancy loss (RPL) affects approximately 1–4% of women attempting pregnancy in Europe and the United States [1,2]. Despite its wide prevalence, shedding light on RPL pathophysiology and risk factors remains a challenge.
Many factors could interfere with the complex process of embryo–endometrium interaction and lead to pregnancy loss [3,4]. Amongst them, the role of vascular thrombosis in RPL pathophysiology has been recognized for more than 25 years [5]. Pregnancy, being a prothrombotic state, favors blood coagulation, which in turn could lead to pregnancy loss or other placenta-mediated complications [6]. Arterial thrombosis might have a more predominant role in RPL pathophysiology compared to venous thrombosis. Previous data suggest that even in diagnosed cases of venous thromboembolic disease, there is no clear benefit of a diagnostic workup and treatment [7]. On the other hand, many conditions leading to arterial thromboses have been linked to RPL. Lupus anticoagulant or anticardiolipin antibodies have been associated with arterial thromboses and may increase the risk of RPL [8]. The European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) recommend investigating the presence of such antibodies in women with RPL, while they do not recommend investigating potential inherited thrombophilias [3,4]. Finally, a recent multicenter study found a correlation between pregnancy loss and cardiovascular risk [9], implying a shared underlying pathophysiological mechanism, at least partially, between the two disease processes.
A non-coding region in chromosome 9 (9p21) containing the Antisense Non-coding RNA in the INK4 Locus the CDKN2B antisense RNA 1 gene (CDKN2B-AS1 or ANRIL) was discovered following Genome-Wide Association Studies (GWAS) that investigated the genetic basis of atheromatosis [10]. The ANRIL gene can form at least 20 circular or linear transcripts through alternative splicing [11,12,13], and its discovery remains very promising with many disease associations and implications [14,15,16,17,18,19,20,21]. One of its most prominent correlations seems to be with cardiovascular disease [10,14]. Ever since it was first described, there has been a body of steadily increasing evidence suggesting the critical role of ANRIL in the formation and progression of atheromatous plaques through vascular endothelial, smooth muscular, and mononuclear cells [22,23,24,25]. More specifically, rs4977574 polymorphism has been associated with atheromatosis in various arterial locations [26,27,28], leading to blood flow impediment to the heart [29,30] or the brain [31] and resulting in acute myocardial infarction [29,30] or ischemic stroke [31], respectively. Likewise, a similar event might lead to blood flow impediment in the uterine vessels, leading to the clinical manifestations of RPL. Congrains et al. reported an abnormal expression of CDKN2A/B and a decrease in cell growth when ANRIL was knocked down in cells of vascular smooth muscle [32]. According to Jarinova et al. and Congrains et al., the genetic variants of ANRIL influence atherosclerosis mechanisms such as thrombogenesis, vascular repair, and plaque stability by altering ANRIL expression and cell proliferation [32,33]. Xu Bing proposes that ANRIL changes the expression of the corresponding coding-related genes via mechanisms such as RNAi, gene silencing, or DNA methylation [30]. These data suggest that ANRIL rs4977574 gene polymorphism involvement in vascular disease and arterial thrombosis pathophysiology could provide a link to RPL development, further supporting the hypothesis of a shared underlying mechanism with cardiovascular disease.
One hypothesis would be ANRIL rs4977574 gene polymorphism being associated with arterial thrombosis, uterine blood flow restriction, and RPL manifestation. Consequently, the aim of our study is to investigate whether ANRIL rs4977574 gene polymorphism prevalence differs between women with or without RPL, as well as according to the number of pregnancy losses.
2. Materials and Methods
2.1. Study Design
This study included 125 women from the Alexandra University Hospital, First Department of Obstetrics and Gynecology, Medical School of the National and Kapodistrian University of Athens. The study protocol was approved by the scientific and ethics committee of the institution (Protocol Number 91970). Fifty-six women who had at least 2 prior pregnancy losses and who were aged less than 40 years old were included in the recurrent pregnancy loss (RPL) group. Sixty-nine women who had already had at least one livebirth without any prior pregnancy loss were included in the control group. Patient characteristics, such as age and BMI, were registered for the RPL group. The age and the BMI of the control group were matched to those in the RPL group. ANRIL rs4977574 gene polymorphism (A>G) prevalence was compared between the two groups. Data were not available for all partners and, consequently, were not included.
2.2. DNA Isolation and Detection of ANRIL rs4977574 Gene Polymorphism
Peripheral blood samples (2–3 mL) were collected, and DNA extraction was performed using the PureLink® Genomic DNA Mini Kit (Invitrogen by Life Technologies, Waltham, MA, USA). Primers used for ANRIL polymorphism detection (rs4977574) were forward 5′-TTGAGGGTACATCAAAAGCATTCTATATCG-3′ and reverse 5′-TTTATTAGAGTGACTTGAACATCCCGT-3′. The conditions of the PCR were as follows: 95 °C for 1 min, 65 °C for 1 min, and 72 °C for 1 min. PCR products were visualized using agarose gel electrophoresis, and different fragments at 226 for allele G and 166 for allele A were detected.
2.3. Statistical Analysis
T-test, Mann–Whitney U-test, ANOVA, and Kruskal–Wallis H-test were used for numerical variables depending on each variable’s distribution and the number of groups compared. Chi-square test and Fisher exact test were used to compare categorical variables as appropriate. ANRIL rs4977574 gene polymorphism results were compared between controls and RPL, as well as after stratification of the RPL group according to the number of pregnancy losses. Odds ratios (ORs) and their respective 95% confidence intervals (CIs) were calculated using logistic regression to compare the odds of allele A prevalence between women with 2 and 3 pregnancy losses. The group of women with 4 pregnancy losses was not separately included in the regression models since it was comprised of only 4 women. OR results were adjusted for maternal age and BMI. Statistical significance was determined as a p-value < 0.05. Statistical analysis was performed using R (v4.1.0; R foundation for statistical programming).
3. Results
3.1. Baseline Characteristics
Table 1 summarizes baseline characteristics for the RPL group. In total, there were 56 women with RPL included in our analysis. The mean age was 35 for the women and 37.9 for their partners, while the mean women’s BMI was 23. Following stratification according to number of pregnancy losses, mean ages were 33.1, 36.7, and 35.8 years, their partner’s ages were 34.5, 40.7, and 36.5 years, and women’s BMI was 23.1, 22.8, and 24.7, among women with 2, 3, and 4 pregnancy losses, respectively. Both women (33.1 vs. 36.7 years, p = 0.019) and their partners (34.5 vs. 40.7 years, p = 0.027) with 2 pregnancy losses were younger compared to those with 3.

Table 1.
RPL group baseline characteristics.
3.2. ANRIL rs4977574 Gene Polymorphism in RPL and Control Groups
The results presented were classified according to specific zones following electrophoresis (Figure 1). The ANRIL rs4977574 gene polymorphism results between the control and RPL groups are shown in Table 2. Women in the control group had allele A in a significantly higher frequency compared to RPL [31 (44.9%) control vs. 14 (25%) RPL, p = 0.021]. Of them, nine women (13.0%) in the control group and three (5.4%) in the RPL were homozygous for allele A (genotype A/A). When analysis was based on specific genotypes, the results did not reach statistical significance (p = 0.062) (Figure 2).
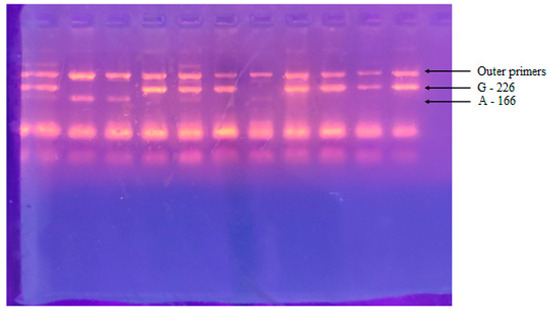
Figure 1.
Agarose electrophoresis after PCR reaction. Lanes 1–11 show amplification of PCR products. A 226bp PCR product corresponds to a G allele. A 166bp PCR product corresponds to A allele. A 330bp PCR product is visualized after amplification with an outer primer pair.

Table 2.
ANRIL rs4977574 polymorphism results between control and RPL.
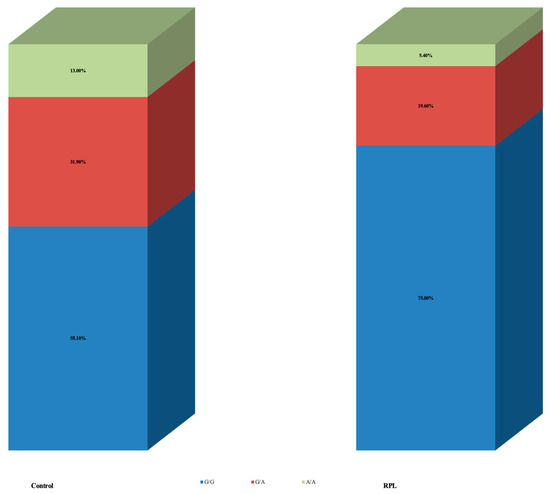
Figure 2.
ANRIL (Antisense non-coding RNA in the INK4 Locus) rs4977574 polymorphism results between control and RPL (recurrent pregnancy loss).
3.3. ANRIL rs4977574 Gene Polymorphism Depending on Number of Pregnancy Losses
Table 3 summarizes the results following stratification according to the number of pregnancy losses. The first group (None) represents the control group, which included women with no pregnancy losses. Even though results did not reach statistical significance (p = 0.078), there was a pattern of gradually decreased prevalence of allele A with an increasing number of pregnancy losses. Thirty-one (44.9%) of women in the control group had at least one allele A, eight (30.7%) with two pregnancy losses, six (23.1%) with three, and none with four pregnancy losses. Of them, nine women (13.0%) in the control group, three (11.5%) with two pregnancy losses, and none with three were homozygous for allele A (genotype A/A) (Figure 3).

Table 3.
ANRIL rs4977574 polymorphism results according to number of pregnancy losses.
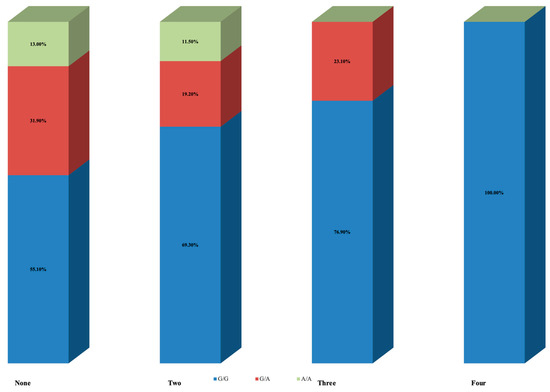
Figure 3.
ANRIL (Antisense Non-coding RNA in the INK4 Locus) rs4977574 polymorphism results according to number of pregnancy losses.
3.4. ANRIL rs4977574 Gene Polymorphism in Women with 2 and 3 Pregnancy Losses
Finally, OR for allele A presence between women with 2 and 3 pregnancy losses was not significant neither before [OR (95%CI): 0.68(0.20–2.32), p = 0.533], nor after adjustment [adjOR(95%CI): 0.54(0.14–2.13), p = 0.375].
4. Discussion
Our study investigated whether ANRIL rs4977574 gene polymorphism prevalence differs between women with or without RPL, as well as according to the number of pregnancy losses. The results suggest that allele A was more prevalent among women with RPL compared to those without, with a high percentage of heterozygosity (G/A) in both groups. After stratification according to the number of pregnancy losses, even though the results did not reach statistical significance, allele A prevalence decreased with an increasing number of pregnancy losses both before and after accounting for women’s age and BMI. Thus, our results suggest that there might be an association of ANRIL rs4977574 gene polymorphism with RPL diagnosis, while an association with the number of pregnancy losses could not be excluded.
Since there are no studies investigating polymorphism rs4977574 in the setting of RPL, we will focus on the literature related to cardiovascular disease and potential molecular mechanisms implicated in the formation, rupture, and thrombosis of the atheromatous plaque. These processes are partially mediated by ANRIL gene expression in vascular endothelial, smooth muscle, and mononuclear cells [22,23,24,25]. ANRIL mediates inflammatory response and enhances vascular endothelial damage through TNF-α-NF-κB-ANRIL/YY1-IL6/8 pathway [34], caspase recruitment domain-containing protein 8 [35], and vascular endothelial growth factor [36] expression increase. Moreover, ANRIL has been associated with abnormal proliferation, migration, aging, and apoptosis of vascular smooth muscle cells [32,37,38,39]. ANRIL has also been found to regulate cyclin-dependent kinase inhibitor 2A and 2B expression, which have a major role in the regulation of the cell cycle, apoptosis, and cell aging [37,40]. Finally, Alu elements, a family of short interspersed repeat elements, are associated with atheromatosis by facilitating mononuclear cell adhesion and proliferation [41] and have been found to be functionally related to ANRIL in knock-out studies [42,43]. Taken together, this evidence highlights the role of ANRIL in many aspects of atheromatosis and cardiovascular disease pathophysiology, which might also be associated with RPL.
To the best of our knowledge, there are no published data about ANRIL gene polymorphism prevalence in women with RPL. Therefore, we provide the first preliminary data on ANRIL rs4977574 gene polymorphism among women with RPL as part of a novel investigation of the common pathophysiology shared between RPL and cardiovascular disease. We found a significant association between ANRIL rs4977574 gene polymorphism and RPL diagnosis. Two recent metanalyses have found an association between ANRIL rs4977574 gene polymorphism and a higher risk for coronary artery disease [30,44]. Both studies concluded that allele G was associated with increased risk [30,44]. Similarly, our results suggest a higher prevalence of allele G in women with RPL. Taking into consideration the higher probability of cardiovascular disease development later in life among women with pregnancy loss [9], these results further support the hypothesis of a shared underlying pathophysiological mechanism that contributes to both conditions.
Formation and progression of the atheromatous plaque might explain, at least partially, the basis of the shared underlying mechanism. ANRIL rs4977574 gene polymorphism has been associated with atheromatosis in coronary [27] and carotid arteries [26,28], which is probably independent of hypertension [45]. Additionally, consumption of vegetables, wine [46], and smoking [47] seem to further modify the risk for cardiovascular disease associated with the polymorphism. The ultimate result is atheromatous plaque rupture and vessel thrombosis [27]. Depending on the location of the affected vessel, there is the respective clinical manifestation of acute myocardial infarction [29,30] or ischemic stroke [31]. Therefore, one potential explanation would be that the presence of allele G in rs4977574 increases the risk for atheromatosis [26,27,28] and, thus, arterial thrombosis, leading to RPL.
Our study is the first to investigate ANRIL gene polymorphisms in women with RPL. The number of pregnancy losses, age, and BMI have been found to be correlated to RPL [2,48] and adjusting for them enhances the validity of our results. Nevertheless, there were no available demographic data for women or their partners in the control group, restricting relevant analyses. Additionally, we do not have consistent data available on other factors that might also contribute to RPL development, such as the presence of thrombophilia, chromosomal abnormalities, or autoimmune diseases.
Future studies could aim at investigating the underlying molecular mechanisms that might explain a potential ANRIL and RPL correlation. In addition to utilizing next-generation sequencing, including more baseline demographic characteristics of the couples could help establish useful RPL biomarkers.
5. Conclusions
In summary, this study provides the first possible association between ANRIL gene polymorphism and recurrent abortions. Our results suggest that rs4977574 is associated with RPL prevalence, while an association with the number of pregnancy losses cannot be excluded. Although the mechanisms underlying the aforementioned association are not clarified, the present study proposes that a shared pathophysiological mechanism is possible for both RPL and cardiovascular disease, potentially through atheromatosis and arterial thrombosis as genetic variants of ANRIL influence atherosclerosis mechanisms such as thrombogenesis, vascular repair, and plaque stability by altering ANRIL expression and cell proliferation. Consequently, the studied polymorphism could be proposed as a possible biomarker of recurrent abortions, and additionally, future studies may focus on the genes interfering with ANRIL and also on genes in linkage disequilibrium with the studied polymorphism.
Author Contributions
Conceptualization, P.C., E.D. (Ekaterini Domali) and S.S.; methodology, D.M. and C.S.; validation, D.M. and A.Z.; formal analysis, M.P.; data curation, K.K.; writing—original draft preparation, P.C. and E.D. (Eirini Drakaki); writing—review and editing, A.P., N.M. and T.K.; visualization, A.P.; supervision, N.V., E.D. (Ekaterini Domali) and P.D.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study was conducted according to the Declaration of Helsinki for Medical Research involving Human Subjects and approved by the Ethics and Scientific Committee of Alexandra University Hospital, Medical School of the National and Kapodistrian University of Athens with protocol identification number 91970.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all the members of the First Department of Obstetrics and Gynecology in Alexandra University Hospital for their help, as well as all the women and their partners who took part in our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rasmark Roepke, E.; Matthiesen, L.; Rylance, R.; Christiansen, O.B. Is the incidence of recurrent pregnancy loss increasing? A retrospective register-based study in Sweden. Acta Obstet. Gynecol. Scand. 2017, 96, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Wilcox, A.J.; Morken, N.H.; Weinberg, C.R.; Haberg, S.E. Role of maternal age and pregnancy history in risk of miscarriage: Prospective register based study. BMJ 2019, 364, l869. [Google Scholar] [CrossRef]
- The ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: A guideline. Fertil. Steril. 2020, 113, 305–322. [Google Scholar] [CrossRef]
- Preston, F.E.; Rosendaal, F.R.; Walker, I.D.; Briet, E.; Berntorp, E.; Conard, J.; Fontcuberta, J.; Makris, M.; Mariani, G.; Noteboom, W.; et al. Increased fetal loss in women with heritable thrombophilia. Lancet 1996, 348, 913–916. [Google Scholar] [CrossRef]
- Kutteh, W.H.; Triplett, D.A. Thrombophilias and recurrent pregnancy loss. Semin. Reprod. Med. 2006, 24, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef]
- Jauniaux, E.; Farquharson, R.G.; Christiansen, O.B.; Exalto, N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum. Reprod. 2006, 21, 2216–2222. [Google Scholar] [CrossRef]
- Murugappan, G.; Leonard, S.A.; Farland, L.V.; Lau, E.S.; Shadyab, A.H.; Wild, R.A.; Schnatz, P.; Carmichael, S.L.; Stefanick, M.L.; Parikh, N.I. Association of infertility with atherosclerotic cardiovascular disease among postmenopausal participants in the Women’s Health Initiative. Fertil. Steril. 2022, 117, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; Erdmann, J.; Hall, A.S.; Hengstenberg, C.; Mangino, M.; Mayer, B.; Dixon, R.J.; Meitinger, T.; Braund, P.; Wichmann, H.E.; et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007, 357, 443–453. [Google Scholar] [CrossRef]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef]
- Hubberten, M.; Bochenek, G.; Chen, H.; Hasler, R.; Wiehe, R.; Rosenstiel, P.; Jepsen, S.; Dommisch, H.; Schaefer, A.S. Linear isoforms of the long noncoding RNA CDKN2B-AS1 regulate the c-myc-enhancer binding factor RBMS1. Eur. J. Hum. Genet. 2019, 27, 80–89. [Google Scholar] [CrossRef]
- Rivera, N.V.; Carreras-Torres, R.; Roncarati, R.; Viviani-Anselmi, C.; De Micco, F.; Mezzelani, A.; Koch, W.; Hoppmann, P.; Kastrati, A.; Stewart, A.F.; et al. Assessment of the 9p21.3 locus in severity of coronary artery disease in the presence and absence of type 2 diabetes. BMC Med. Genet. 2013, 14, 11. [Google Scholar] [CrossRef]
- Broadbent, H.M.; Peden, J.F.; Lorkowski, S.; Goel, A.; Ongen, H.; Green, F.; Clarke, R.; Collins, R.; Franzosi, M.G.; Tognoni, G.; et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum. Mol. Genet. 2008, 17, 806–814. [Google Scholar] [CrossRef]
- Burdon, K.P.; Macgregor, S.; Hewitt, A.W.; Sharma, S.; Chidlow, G.; Mills, R.A.; Danoy, P.; Casson, R.; Viswanathan, A.C.; Liu, J.Z.; et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011, 43, 574–578. [Google Scholar] [CrossRef]
- Cugino, D.; Gianfagna, F.; Santimone, I.; de Gaetano, G.; Donati, M.B.; Iacoviello, L.; Di Castelnuovo, A. Type 2 diabetes and polymorphisms on chromosome 9p21: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Lista, S.; Ghidoni, R.; Binetti, G.; Cereda, C.; Benussi, L.; Maletta, R.; Bruni, A.C.; Politi, P. Chromosome 9p21.3 genotype is associated with vascular dementia and Alzheimer’s disease. Neurobiol. Aging 2011, 32, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Hsieh, C.H.; Alonso, L.C. ANRIL: A lncRNA at the CDKN2A/B Locus With Roles in Cancer and Metabolic Disease. Front. Endocrinol. 2018, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D.; van Koolwijk, L.M.; Lemij, H.G.; Pasutto, F.; Cree, A.J.; Thorleifsson, G.; Janssen, S.F.; Jacoline, T.B.; Amin, N.; Rivadeneira, F.; et al. Common genetic variants associated with open-angle glaucoma. Hum. Mol. Genet. 2011, 20, 2464–2471. [Google Scholar] [CrossRef]
- Schaefer, A.S.; Richter, G.M.; Groessner-Schreiber, B.; Noack, B.; Nothnagel, M.; El Mokhtari, N.E.; Loos, B.G.; Jepsen, S.; Schreiber, S. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009, 5, e1000378. [Google Scholar] [CrossRef]
- Uno, S.; Zembutsu, H.; Hirasawa, A.; Takahashi, A.; Kubo, M.; Akahane, T.; Aoki, D.; Kamatani, N.; Hirata, K.; Nakamura, Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat. Genet. 2010, 42, 707–710. [Google Scholar] [CrossRef]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Nanda, V.; Downing, K.P.; Ye, J.; Xiao, S.; Kojima, Y.; Spin, J.M.; DiRenzo, D.; Nead, K.T.; Connolly, A.J.; Dandona, S.; et al. CDKN2B Regulates TGFbeta Signaling and Smooth Muscle Cell Investment of Hypoxic Neovessels. Circ. Res. 2016, 118, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Papait, R.; Kunderfranco, P.; Stirparo, G.G.; Latronico, M.V.; Condorelli, G. Long noncoding RNA: A new player of heart failure? J. Cardiovasc. Transl. Res. 2013, 6, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zollbrecht, C.; Grassl, M.; Fenk, S.; Hocherl, R.; Hubauer, U.; Reinhard, W.; Esslinger, U.B.; Ebert, S.; Langmann, T.; Stark, K.; et al. Expression pattern in human macrophages dependent on 9p21.3 coronary artery disease risk locus. Atherosclerosis 2013, 227, 244–249. [Google Scholar] [CrossRef]
- Jin, W.; Wu, W.; Yang, K.; Shen, F.; Fu, N.; Feng, Y.; Fu, Y. The Single Nucleotide Polymorphisms of Chromosome 9p21 and CD147 Were Relevant with the Carotid Plaque Risk in Acute Cerebral Infarction Patients Among Chinese Han Population. J. Mol. Neurosci. 2020, 70, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Song, L.; Jiang, L.; Tang, X.; Xu, L.; Gao, Z.; Zhao, X.; Xu, J.; Gao, R.; Yuan, J. Susceptible gene polymorphism in patients with three-vessel coronary artery disease. BMC Cardiovasc. Disord. 2020, 20, 172. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Maimaiti, Y.; Feng, Y.; Sun, J.; Zhuang, J.; Zeng, L.; Fu, Y. Variants on Chromosome 9p21 Confer Risks of Noncardioembolic Cerebral Infarction and Carotid Plaque in the Chinese Han Population. J. Atheroscler. Thromb. 2015, 22, 1061–1070. [Google Scholar] [CrossRef]
- Sakalar, C.; Gurbuz, E.; Kalay, N.; Kaya, M.G. Higher frequency of rs4977574 (the G Allele) on chromosome 9p21.3 in patients with myocardial infarction as revealed by PCR-RFLP analysis. Tohoku J. Exp. Med. 2013, 230, 171–176. [Google Scholar] [CrossRef]
- Xu, B.; Fang, Z.; He, S.; Wang, J.; Yang, X. ANRIL polymorphism rs4977574 is associated with increased risk of coronary artery disease in Asian populations: A meta-analysis of 12,005 subjects. Medicine 2018, 97, e12641. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Kula, D.; Urbanek, T.; Puz, P.; Szymszal, J.; Jarzab, M.; Halczok, M.; Cyplinska, R.; Bal, W.; Labuz-Roszak, B.; et al. The Association of SNPs Located in the CDKN2B-AS1 and LPA Genes With Carotid Artery Stenosis and Atherogenic Stroke. Front. Neurol. 2019, 10, 1170. [Google Scholar] [CrossRef]
- Congrains, A.; Kamide, K.; Oguro, R.; Yasuda, O.; Miyata, K.; Yamamoto, E.; Kawai, T.; Kusunoki, H.; Yamamoto, H.; Takeya, Y.; et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis 2012, 220, 449–455. [Google Scholar] [CrossRef]
- Jarinova, O.; Stewart, A.F.; Roberts, R.; Wells, G.; Lau, P.; Naing, T.; Buerki, C.; McLean, B.W.; Cook, R.C.; Parker, J.S.; et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1671–1677. [Google Scholar] [CrossRef]
- Zhou, X.; Han, X.; Wittfeldt, A.; Sun, J.; Liu, C.; Wang, X.; Gan, L.M.; Cao, H.; Liang, Z. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-kappaB pathway. RNA Biol. 2016, 13, 98–108. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, S.; Jiang, G.; Zhou, Y.; Zhou, M.; Zhao, Y.; Li, S.; Wang, F.; Lv, Q.; Huang, Y.; et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke 2014, 45, 383–388. [Google Scholar] [CrossRef]
- Thomas, A.A.; Feng, B.; Chakrabarti, S. ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, F.; Di Cecilia, S.; Walsh, M.J. Long Non-coding RNA ANRIL and Polycomb in Human Cancers and Cardiovascular Disease. Curr. Top. Microbiol. Immunol. 2016, 394, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Tziomalos, K.; Chatzizisis, Y.; Elisaf, M.; Hatzitolios, A.I. Effect of HMG-CoA reductase inhibitors on vascular cell apoptosis: Beneficial or detrimental? Atherosclerosis 2010, 211, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, T.Y.; Guan, Y.F.; Zhao, Y.; Li, Z.Y.; Lan, X.H.; Wang, X.; Yang, P.Y.; Kang, Z.M.; Vanhoutte, P.M.; et al. Vascular smooth muscle cell apoptosis is an early trigger for hypothyroid atherosclerosis. Cardiovasc. Res. 2014, 102, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef]
- Rudiger, N.S.; Gregersen, N.; Kielland-Brandt, M.C. One short well conserved region of Alu-sequences is involved in human gene rearrangements and has homology with prokaryotic chi. Nucleic Acids Res. 1995, 23, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.S.; Li, J.Z.; Jia, J.J.; Zhang, T.; Liu, X.M.; Yi, L. Long non-coding RNA ANRIL in gene regulation and its duality in atherosclerosis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hueso, M.; Cruzado, J.M.; Torras, J.; Navarro, E. ALUminating the Path of Atherosclerosis Progression: Chaos Theory Suggests a Role for Alu Repeats in the Development of Atherosclerotic Vascular Disease. Int. J. Mol. Sci. 2018, 19, 1734. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, H.; Zhang, Y.Y. CDKN2B-AS1 gene rs4977574 A/G polymorphism and coronary heart disease: A meta-analysis of 40,979 subjects. J. Cell Mol. Med. 2021, 25, 8877–8889. [Google Scholar] [CrossRef] [PubMed]
- Kunnas, T.; Piesanen, J.; Nikkari, S.T. Association of a Chromosome Locus 9p21.3 CDKN2B-AS1 Variant rs4977574 with Hypertension: The TAMRISK Study. Genet. Test. Mol. Biomark. 2018, 22, 327–330. [Google Scholar] [CrossRef]
- Hindy, G.; Ericson, U.; Hamrefors, V.; Drake, I.; Wirfalt, E.; Melander, O.; Orho-Melander, M. The chromosome 9p21 variant interacts with vegetable and wine intake to influence the risk of cardiovascular disease: A population based cohort study. BMC Med. Genet. 2014, 15, 1220. [Google Scholar] [CrossRef]
- Hamrefors, V.; Hedblad, B.; Hindy, G.; Smith, J.G.; Almgren, P.; Engstrom, G.; Sjogren, M.; Gransbo, K.; Orho-Melander, M.; Melander, O. Smoking modifies the associated increased risk of future cardiovascular disease by genetic variation on chromosome 9p21. PLoS ONE 2014, 9, e85893. [Google Scholar] [CrossRef]
- Boots, C.; Stephenson, M.D. Does obesity increase the risk of miscarriage in spontaneous conception: A systematic review. Semin. Reprod. Med. 2011, 29, 507–513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).