Outcomes in Patients with Acute Myocardial Infarction and Known Sleep Apnea: A Nationwide Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Study Population
2.3. Outcomes
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Preexisting SA and Acute Coronary Syndrome
4.2. Screening of SA in Acute Coronary Syndrome
4.3. Atrial Fibrillation and SA Following Myocardial Infarction
4.4. SA and Heart Failure Following Myocardial Infarction
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 44, e56–e67. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; De La Cruz-Moron, I.; Almeida-Gozalez, C.; Catalan-Serra, P.; Montserrat, J.M. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: A cohort study. Ann. Intern. Med. 2012, 156, 115–122. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Catalan-Serra, P.; Soler-Cataluna, J.J.; Almeida-Gonzales, C.; De La Cruz-Moron, I.; Duran-Cantolla, J.; Montserrat, J.M. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am. J. Respir. Crit. Care Med. 2012, 186, 909–916. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.H.K.; Liu, P.Y.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep 2008, 31, 1079–1085. [Google Scholar] [PubMed]
- Chantry, A.A.; Deneux-Tharaux, C.; Cans, C.; Ego, A.; Quantin, C.; Bouvier-Colle, M.H.; GRACE Study Group. Hospital discharge data can be used for monitoring procedures and intensive care related to severe maternal morbidity. J. Clin. Epidemiol. 2011, 64, 1014–1022. [Google Scholar] [CrossRef]
- Djennaoui, M.; Ficheur, G.; Beuscart, R.; Chazard, E. Improvement of the quality of medical databases: Data-mining-based prediction of diagnostic codes from previous patient codes. Stud. Health Technol. Inform. 2015, 210, 419–423. [Google Scholar] [PubMed]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Saint-Etienne, C.; Grammatico-Guillon, L.; Porto, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Impact of Sapien 3 Balloon-Expandable versus Evolut R Self-Expandable Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis: Data from a Nationwide Analysis. Circulation 2020, 141, 260–268. [Google Scholar] [CrossRef]
- Lorgis, L.; Cottenet, J.; Molins, G.; Benzenine, E.; Zeller, M.; Aube, H.; Touzery, C.; Hamblin, J.; Gudjoncik, A.; Cottin, Y.; et al. Outcomes after acute myocardial infarction in HIV-infected patients: Analysis of data from a French nationwide hospital medical information database. Circulation 2013, 127, 1767–1774. [Google Scholar] [CrossRef]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y.H. Patients with Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef]
- Bezin, J.; Pariente, A.; Lassalle, R.; Dureau-Pournin, C.; Abouelfath, A.; Robinson, P.; Moore, N.; Droz-Perroteau, C.; Fourrier-Reglat, A. Use of the recommended drug combination for secondary prevention after a first occurrence of acute coronary syndrome in France. Eur. J. Clin. Pharmacol. 2014, 70, 429–436. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009, 108, 246–249. [Google Scholar] [PubMed]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef]
- Peker, Y.; Kraiczi, H.; Hedner, J.; Löth, S.; Johansson, A.; Bende, M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur. Respir. J. 1999, 14, 179–184. [Google Scholar] [CrossRef]
- Peker, Y.; Carlson, J.; Hedner, J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur. Respir. J. 2006, 28, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Peppard, P. Sleep-disordered breathing and cardiovascular disease: Epidemiologic evidence for a relationship. Sleep 2000, 23 (Suppl. S4), S122-6. [Google Scholar] [PubMed]
- Peker, Y.; Hedner, J.; Kraiczi, H.; Löth, S. Respiratory disturbance index: An independent predictor of mortality in coronary artery disease. Am. J. Respir. Crit. Care Med. 2000, 162, 81–86. [Google Scholar] [CrossRef]
- Loke, Y.K.; Brown, J.W.L.; Kwok, C.S.; Niruban, A.; Myint, P.K. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 720–728. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef]
- Buchner, N.J.; Sanner, B.M.; Borgel, J.; Rump, L.C. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am. J. Respir. Crit. Care Med. 2007, 176, 1274–1280. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Taylor, J.L.; Makarem, N.; Shimbo, D.; Aggarwal, B. Gender Differences in Associations between Stress and Cardiovascular Risk Factors and Outcomes. Gend. Genome 2018, 1, 111–122. [Google Scholar] [CrossRef]
- Leppänen, T.; Kulkas, A.; Duce, B.; Mervaala, E.; Töyräs, J. Severity of individual obstruction events is gender dependent in sleep apnea. Sleep Breath. 2017, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.Q.; Redline, S.; Claggett, B.; Bello, N.; Ballantyne, C.M.; Solomon, S.D.; Shah, A.M. Sex-Specific Association of Sleep Apnea Severity with Subclinical Myocardial Injury, Ventricular Hypertrophy, and Heart Failure Risk in a Community-Dwelling Cohort: The Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation 2015, 6, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, X.; Ma, X.; Somers, V.K.; Nie, S.; Wei, Y. Association of Obstructive Sleep Apnea with Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. J. Am. Heart Assoc. 2019, 22, e010826. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, J.; Guo, R.; Hao, W.; Gong, W.; Yan, Y.; Zheng, W.; Ai, H.; Que, B.; Hu, D.; et al. Association of obstructive sleep apnoea with cardiovascular events in women and men with acute coronary syndrome. Eur. Respir. J. 2023, 27, 2201110. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Sharafkhaneh, A.; Nambi, V.; Bahammam, A.; Razjouyan, J. Obstructive sleep apnea modulates clinical outcomes post-acute myocardial infarction: A large longitudinal veterans’ dataset report. Respir. Med. 2023, 211, 107214. [Google Scholar] [CrossRef]
- Mouhananey, D.; Villablanca, P.A.; Gupta, T.; Agrawal, S.; Faulx, M.; Menon, V.; Kapadia, S.R.; Griffin, B.P.; Ellis, S.G.; Desai, M.Y. Recognized Obstructive Sleep Apnea is Associated with Improved In-Hospital Outcomes after ST Elevation Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e006133. [Google Scholar] [CrossRef]
- Gupta, T.; Kolte, D.; Mohananey, D.; Khera, S.; Goel, K.; Mondal, P.; Aronow, W.S.; Jain, D.; Cooper, H.A.; Iwai, S.; et al. Relation of Obesity to Survival after In-Hospital Cardiac Arrest. Am. J. Cardiol. 2016, 118, 662–667. [Google Scholar] [CrossRef]
- Storza, E.; Roche, F. Chronic intermittent hypoxia and obstructive sleep apnea: An experimental and clinical approach. Hypoxia 2016, 4, 99–108. [Google Scholar]
- Summerer, V.; Arzt, M.; Fox, H.; Oldenburg, O.; Zeman, F.; Debl, K.; Buchner, S.; Stalder, S. Occurrence of Coronary Collaterals in Acute Myocardial Infarction and Sleep Apnea. J. Am. Heart Assoc. 2021, 10, e020340. [Google Scholar] [CrossRef] [PubMed]
- Özkan, E.; Celik, Y.; Yucel-Lindberg, T.; Peker, Y. Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. J. Clin. Med. 2023, 12, 4053. [Google Scholar] [CrossRef]
- Mooe, T.; Franklin, K.A.; Holmström, K.; Rabben, T.; Wiklund, U. Sleep-disordered breathing and coronary artery disease: Long-term prognosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Ostructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 6, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Bjerre, J.; Zareini, B.; Koch, H.; Tønnesen, P.; Gislason, G.H.; Nielsen, O.W.; Schou, M.; Lamberts, M. Sleep apnea, the risk of developing heart failure, and potential benefits of continuous positive airway pressure (CPAP) therapy. J. Am. Heart Asscoc. 2018, 7, e008684. [Google Scholar] [CrossRef]
- Youssef, I.; Kamran, H.; Yacoub, M.; Patel, N.; Goulbourne, C.; Kumar, S.; Kane, J.; Hoffner, H.; Salifu, M.; McFarlane, S.I. Obstructive Sleep Apnea as a Risk Factor for Atrial Fibrillation: A Meta-Analysis. J. Sleep Disord. Ther. 2018, 7, 282. [Google Scholar] [CrossRef]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.M.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef]
- Monahan, K.; Brewster, J.; Wang, L.; Parvez, B.; Goyal, S.; Roden, D.M.; Darbar, D. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am. J. Cardiol. 2012, 1, 369–372. [Google Scholar] [CrossRef]
- Chen, H.; Cade, B.E.; Gleason, K.J.; Bjonnes, A.C.; Stilp, A.M.; Sofer, T.; Conomos, M.P.; Ancoli-Israel, S.; Arens, R.; Azarbarzin, A.; et al. Multiethnic Meta-Analysis Identifies RAI1 as a Possible Obstructive Sleep Apnea-related Quantitative Trait Locus in Men. Am. J. Respir. Cell Mol. Biol. 2018, 58, 391–401. [Google Scholar] [CrossRef]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Levy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea with Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 1, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Petrie, M.C.; Anker, S.D.; Bhatt, D.L.; Jones, W.S.; Udell, J.A.; Hernandez, A.F.; Butler, J. Evaluating the Application of Chronic Heart Failure Therapies and Developing Treatments in Individuals with Recent Myocardial Infarction: A Review. JAMA Cardiol. 2022, 7, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Desta, L.; Jernberg, T.; Spaak, J.; Hofman-Bang, C.; Persson, H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: A Swedish nationwide observational study. Int. J. Cardiol. 2017, 248, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Claggett, B.; Lewis, E.F.; Granger, C.B.; Kober, L.; Maggioni, A.P.; Mann, D.L.; McMurray, J.J.V.; Rouleau, J.L.; Solomon, S.D.; et al. PARADISE-MI Investigators and Committees. Angiotensin receptor-neprilysin inhibition in acutemyocardial infarction. N. Engl. J. Med. 2021, 385, 1845–1855. [Google Scholar] [CrossRef]
- Khot, U.N.; Jia, G.; Moliterno, D.J.; Lincoff, A.M.; Khot, M.B.; Harrington, R.A.; Topol, E.J. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: Theenduring value of Killip classification. JAMA 2003, 290, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-myocardial infarction heart failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef]
- Hall, T.S.; von Lueder, T.G.; Zannad, F.; Rossignol, P.; Duarte, K.; Chouihed, T.; Solomon, S.D.; Dickstein, K.; Atar, D.; Agewall, S.; et al. High-Risk Myocardial Infarction Database Initiative investigators. Left ventricular ejection fraction and adjudicated, cause-specific hospitalizations after myocardial infarction complicated by heart failure or left ventricular dysfunction. Am. Heart J. 2019, 215, 83–90. [Google Scholar] [CrossRef]
- Wu, A.H.; Parsons, L.; Every, N.R.; Bates, E.R.; Second National Registry of Myocardial Infarction. Hospital outcomes in patients presenting with congestiveheart failure complicating acute myocardial infarction: A report from the Second National Registry of Myocardial Infarction (NRMI-2). J. Am. Coll. Cardiol. 2002, 40, 1389–1394. [Google Scholar] [CrossRef]

| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| Total | No Sleep Apnea Syndrome | Sleep Apnea Syndrome | p | No Sleep Apnea Syndrome | Sleep Apnea Syndrome | p | |
| (n = 797,212) | (n = 499,443) | (n = 28,908) | (n = 260,694) | (n = 8167) | |||
| Age (years), mean ± SD | 69.1 ± 14.9 | 65.8 ± 14.3 | 68.2 ± 11.7 | <0.0001 | 75.5 ± 14.3 | 72.4 ± 11.7 | <0.0001 |
| Sex (male), n (%) | 528,351 (66.3) | 499,443 (100.0) | 28,908 (100.0) | - | 0 (0.0) | 0 (0.0) | - |
| Charlson comorbidity index, mean ± SD | 3.8 ± 2.9 | 3.6 ± 2.9 | 5.2 ± 3.4 | <0.0001 | 3.9 ± 2.7 | 5.7 ± 3.3 | <0.0001 |
| Frailty index, mean ± SD | 6.2 ± 8.1 | 5.1 ± 7.3 | 7.6 ± 8.6 | <0.0001 | 8.0 ± 8.9 | 10.0 ± 9.6 | <0.0001 |
| Obesity, n (%) | 132,312 (16.6) | 72,554 (14.5) | 15,222 (52.7) | <0.0001 | 39,261 (15.1) | 5275 (64.6) | <0.0001 |
| Dyslipidemia, n (%) | 267,840 (33.6) | 169,163 (33.9) | 15,694 (54.3) | <0.0001 | 78,662 (30.2) | 4321 (52.9) | <0.0001 |
| Diabetes mellitus, n (%) | 192,456 (24.1) | 110,902 (22.2) | 13,276 (45.9) | <0.0001 | 63,771 (24.5) | 4507 (55.2) | <0.0001 |
| Hypertension, n (%) | 440,432 (55.2) | 246,164 (49.3) | 22,404 (77.5) | <0.0001 | 164,801 (63.2) | 7063 (86.5) | <0.0001 |
| Smoker, n (%) | 173,728 (21.8) | 132,245 (26.5) | 8398 (29.1) | <0.0001 | 31,802 (12.2) | 1283 (15.7) | <0.0001 |
| Alcohol-related diagnoses, n (%) | 42,217 (5.3) | 32,860 (6.6) | 2889 (10.0) | <0.0001 | 6170 (2.4) | 298 (3.6) | <0.0001 |
| Coronary artery disease, n (%) | 167,855 (21.1) | 103,965 (20.8) | 12,663 (43.8) | <0.0001 | 48,093 (18.4) | 3134 (38.4) | <0.0001 |
| Previous PCI, n (%) | 38,369 (4.8) | 24,993 (5.0) | 3427 (11.9) | <0.0001 | 9189 (3.5) | 760 (9.3) | <0.0001 |
| Dilated cardiomyopathy, n (%) | 43,528 (5.5) | 23,898 (4.8) | 3052 (10.6) | <0.0001 | 15,612 (6.0) | 966 (11.8) | <0.0001 |
| Heart failure with congestion, n (%) | 82,752 (10.4) | 40,088 (8.0) | 6498 (22.5) | <0.0001 | 33,695 (12.9) | 2471 (30.3) | <0.0001 |
| Ischemic stroke, n (%) | 30,326 (3.8) | 15,993 (3.2) | 1631 (5.6) | <0.0001 | 12,192 (4.7) | 510 (6.2) | <0.0001 |
| Atrial fibrillation, n (%) | 146,922 (18.4) | 78,378 (15.7) | 8252 (28.5) | <0.0001 | 57,870 (22.2) | 2422 (29.7) | <0.0001 |
| Previous pacemaker or ICD, n (%) | 25,346 (3.2) | 14,923 (3.0) | 2364 (8.2) | <0.0001 | 7552 (2.9) | 507 (6.2) | <0.0001 |
| Vascular disease, n (%) | 103,933 (13.0) | 64,816 (13.0) | 8743 (30.2) | <0.0001 | 28,350 (10.9) | 2024 (24.8) | <0.0001 |
| Abnormal renal function, n (%) | 53,847 (6.8) | 28,237 (5.7) | 4077 (14.1) | <0.0001 | 20,066 (7.7) | 1467 (18.0) | <0.0001 |
| Liver disease, n (%) | 26,589 (3.3) | 16,242 (3.3) | 1984 (6.9) | <0.0001 | 7748 (3.0) | 615 (7.5) | <0.0001 |
| Lung disease, n (%) | 107,049 (13.4) | 61,142 (12.2) | 9776 (33.8) | <0.0001 | 32,897 (12.6) | 3234 (39.6) | <0.0001 |
| COPD, n (%) | 61,950 (7.8) | 37,275 (7.5) | 7142 (24.7) | <0.0001 | 15,658 (6.0) | 1875 (23.0) | <0.0001 |
| Anemia, n (%) | 92,307 (11.6) | 45,950 (9.2) | 4790 (16.6) | <0.0001 | 39,531 (15.2) | 2036 (24.9) | <0.0001 |
| Previous cancer, n (%) | 87,988 (11.0) | 56,631 (11.3) | 4715 (16.3) | <0.0001 | 25,664 (9.8) | 978 (12.0) | <0.0001 |
| History of metastasis, n (%) | 16,432 (2.1) | 9885 (2.0) | 701 (2.4) | <0.0001 | 5658 (2.2) | 188 (2.3) | 0.42 |
| Thyroid diseases, n (%) | 52,495 (6.6) | 15,039 (3.0) | 2104 (7.3) | <0.0001 | 33,358 (12.8) | 1994 (24.4) | <0.0001 |
| HIV infection, n (%) | 2521 (0.3) | 2119 (0.4) | 73 (0.3) | <0.0001 | 322 (0.1) | 7 (0.1) | 0.34 |
| Illicit drug use, n (%) | 4328 (0.5) | 3596 (0.7) | 139 (0.5) | <0.0001 | 561 (0.2) | 32 (0.4) | 0.001 |
| Cognitive impairment, n (%) | 54,422 (6.8) | 22,118 (4.4) | 1923 (6.7) | <0.0001 | 29,471 (11.3) | 910 (11.1) | 0.65 |
| Depression, n (%) | 58,668 (7.4) | 23,209 (4.6) | 3030 (10.5) | <0.0001 | 30,443 (11.7) | 1986 (24.3) | <0.0001 |
| Poor nutrition, n (%) | 48,781 (6.1) | 21,696 (4.3) | 1943 (6.7) | <0.0001 | 24,180 (9.3) | 962 (11.8) | <0.0001 |
| STEMI, n (%) | 520,258 (65.3) | 332,503 (66.6) | 15,425 (53.4) | <0.0001 | 168,133 (64.5) | 4197 (51.4) | <0.0001 |
| NSTEMI, n (%) | 276,954 (34.7) | 166,940 (33.4) | 13,483 (46.6) | <0.0001 | 92,561 (35.5) | 3970 (48.6) | <0.0001 |
| Anterior MI, n (%) | 221,344 (27.8) | 140,515 (28.1) | 6216 (21.5) | <0.0001 | 72,884 (28.0) | 1729 (21.2) | <0.0001 |
| Inferior MI, n (%) | 173,162 (21.7) | 119,107 (23.8) | 5180 (17.9) | <0.0001 | 47,700 (18.3) | 1175 (14.4) | <0.0001 |
| MI with other location, n (%) | 402,706 (50.5) | 239,821 (48.0) | 17,512 (60.6) | <0.0001 | 140,110 (53.7) | 5263 (64.4) | <0.0001 |
| HF at the acute phase, n (%) | 199,601 (25.0) | 117,246 (23.5) | 5564 (19.2) | <0.0001 | 75,083 (28.8) | 1708 (20.9) | <0.0001 |
| Pulm. edema/shock at the acute phase, n (%) | 48,129 (6.0) | 28,426 (5.7) | 1532 (5.3) | 0.01 | 17,703 (6.8) | 468 (5.7) | 0.0002 |
| PCI at the acute phase, n (%) | 241,387 (30.3) | 175,495 (35.1) | 7096 (24.5) | <0.0001 | 57,412 (22.0) | 1384 (16.9) | <0.0001 |
| PCI during first 8 days, n (%) | 423,932 (53.2) | 298,199 (59.7) | 14,176 (49.0) | <0.0001 | 108,414 (41.6) | 3143 (38.5) | <0.0001 |
| CABG at the acute phase, n (%) | 14,723 (1.8) | 10,873 (2.2) | 790 (2.7) | <0.0001 | 2961 (1.1) | 99 (1.2) | 0.52 |
| CABG during first 8 days, n (%) | 29,880 (3.7) | 22,316 (4.5) | 1494 (5.2) | <0.0001 | 5864 (2.2) | 206 (2.5) | 0.1 |
| Death at 1 month, n (%) | 78,826 (9.9) | 41,005 (8.2) | 2271 (7.9) | 0.03 | 34,742 (13.3) | 808 (9.9) | <0.0001 |
| Cardiovascular death at 1 month, n (%) | 56,582 (7.1) | 29,083 (5.8) | 1531 (5.3) | 0.0002 | 25,421 (9.8) | 547 (6.7) | <0.0001 |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Incidence, %/Year (95% CI) | No SA | SA | p | No SA | SA | p |
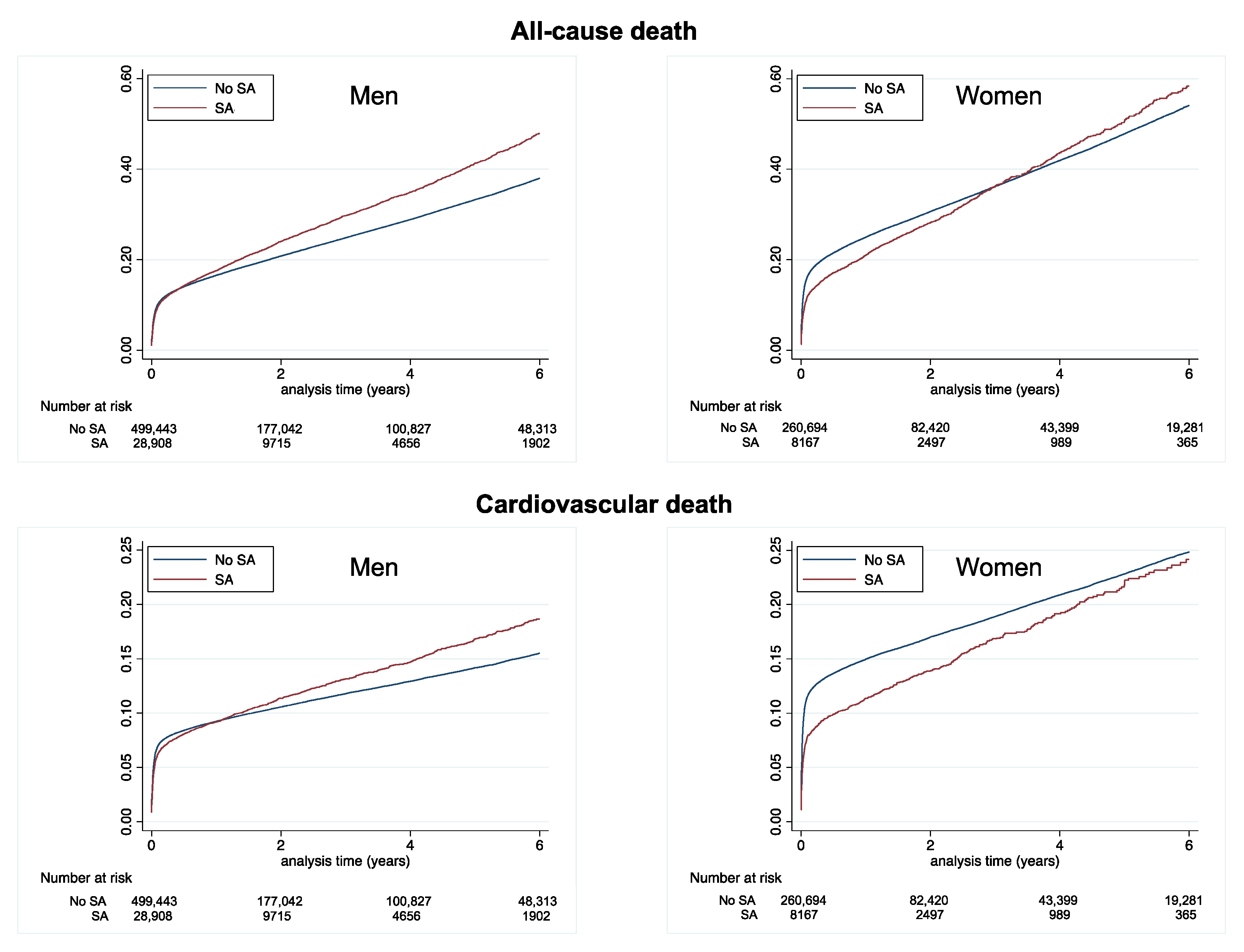
| All-cause death | 9.39 (9.33–9.45) | 11.45 (11.16–11.75) | <0.0001 | 14.88 (14.76–14.99) | 14.65 (14.00–15.32) | 0.51 |
| Cardiovascular death | 4.68 (4.63–4.72) | 5.39 (5.20–5.60) | <0.0001 | 8.32 (8.24–8.41) | 7.31 (6.86–7.79) | 0.0001 |
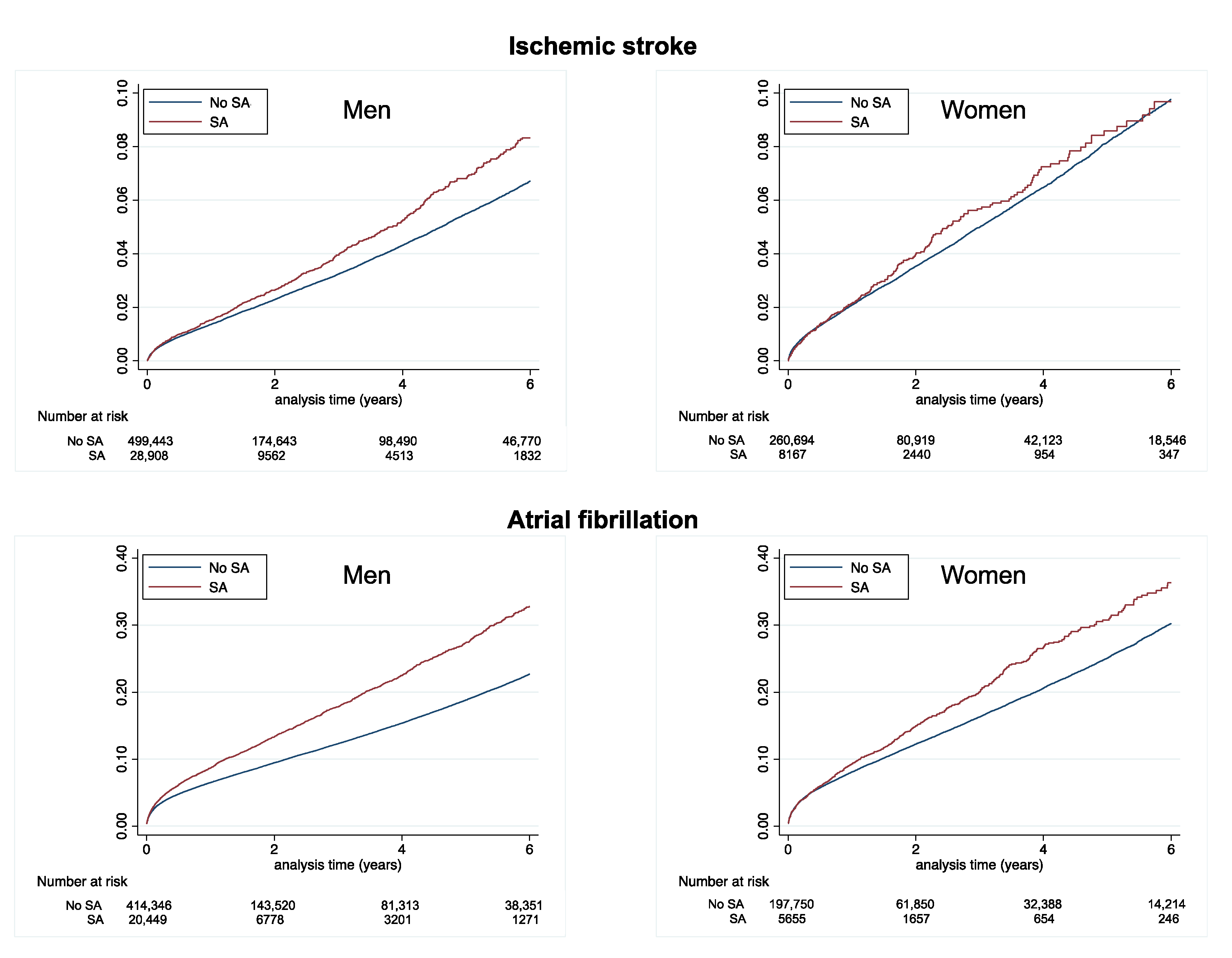
| Ischemic stroke | 1.21 (1.18–1.23) | 1.42 (1.32–1.53) | <0.0001 | 1.80 (1.76–1.84) | 1.89 (1.67–2.15) | 0.42 |
| New-onset AF | 4.56 (4.52–4.61) | 6.52 (6.26–6.79) | <0.0001 | 6.04 (5.96–6.13) | 7.42 (6.87–8.01) | <0.0001 |
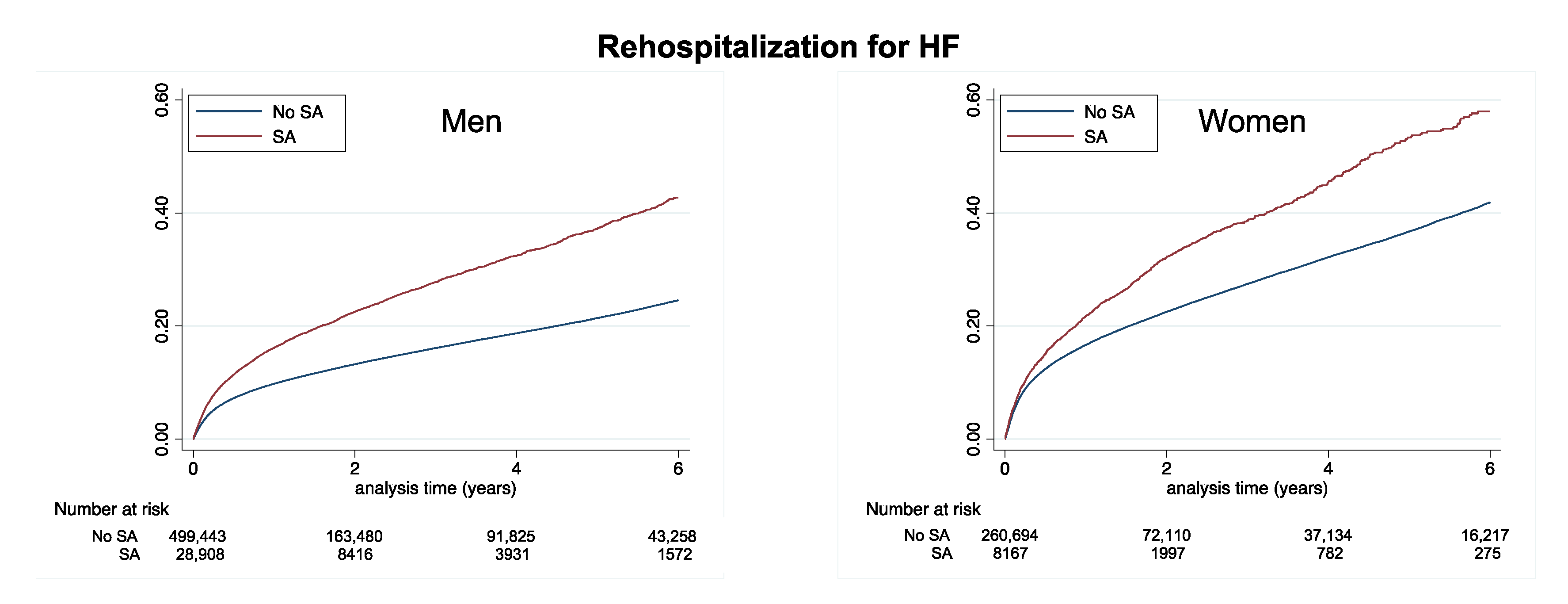
| Rehospitalization for HF | 5.42 (5.38–5.47) | 9.97 (9.68–10.27) | <0.0001 | 9.72 (9.63–9.82) | 14.59 (13.88–15.32) | <0.0001 |
| HR (95% CI) | Model A | Model B | Model C | Model D |
|---|---|---|---|---|
| Men | ||||
| All-cause death | 1.142 (1.112–1.172) | 1.121 (1.092–1.151) | 1.092 (1.063–1.123) | 0.901 (0.876–0.926) |
| Cardiovascular death | 1.055 (1.015–1.096) | 1.029 (0.990–1.069) | 1.037 (0.997–1.079) | 0.923 (0.887–0.961) |
| Ischemic stroke | 1.183 (1.097–1.276) | 1.149 (1.066–1.239) | 1.053 (0.974–1.138) | 0.978 (0.904–1.059) |
| New-onset AF | 1.393 (1.336–1.453) | 1.374 (1.318–1.433) | 1.169 (1.119–1.221) | 1.059 (1.013–1.107) |
| Rehospitalization for HF | 1.684 (1.633–1.736) | 1.659 (1.609–1.710) | 1.296 (1.255–1.338) | 1.038 (1.004–1.072) |
| Women | ||||
| All-cause death | 0.916 (0.875–0.958) | 1.146 (1.095–1.200) | 1.111 (1.059–1.164) | 0.967 (0.922–1.014) |
| Cardiovascular death | 0.804 (0.754–0.858) | 1.018 (0.954–1.086) | 1.025 (0.959–1.095) | 0.985 (0.921–1.053) |
| Ischemic stroke | 1.046 (0.919–1.189) | 1.219 (1.071–1.387) | 1.105 (0.968–1.262) | 1.071 (0.937–1.225) |
| New-onset AF | 1.191 (1.101–1.288) | 1.479 (1.367–1.600) | 1.256 (1.158–1.362) | 1.147 (1.057–1.245) |
| Rehospitalization for HF | 1.351 (1.284–1.421) | 1.695 (1.611–1.783) | 1.244 (1.180–1.310) | 1.030 (0.977–1.086) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabec, C.; Sombrun, C.; Bentounes, S.A.; Georges, M.; Bisson, A.; Bichat, F.; Bodin, A.; Herbert, J.; Zeller, M.; Cottin, Y.; et al. Outcomes in Patients with Acute Myocardial Infarction and Known Sleep Apnea: A Nationwide Analysis. J. Clin. Med. 2023, 12, 5924. https://doi.org/10.3390/jcm12185924
Rabec C, Sombrun C, Bentounes SA, Georges M, Bisson A, Bichat F, Bodin A, Herbert J, Zeller M, Cottin Y, et al. Outcomes in Patients with Acute Myocardial Infarction and Known Sleep Apnea: A Nationwide Analysis. Journal of Clinical Medicine. 2023; 12(18):5924. https://doi.org/10.3390/jcm12185924
Chicago/Turabian StyleRabec, Claudio, Chan Sombrun, Sid Ahmed Bentounes, Marjolaine Georges, Arnaud Bisson, Florence Bichat, Alexandre Bodin, Julien Herbert, Marianne Zeller, Yves Cottin, and et al. 2023. "Outcomes in Patients with Acute Myocardial Infarction and Known Sleep Apnea: A Nationwide Analysis" Journal of Clinical Medicine 12, no. 18: 5924. https://doi.org/10.3390/jcm12185924
APA StyleRabec, C., Sombrun, C., Bentounes, S. A., Georges, M., Bisson, A., Bichat, F., Bodin, A., Herbert, J., Zeller, M., Cottin, Y., & Fauchier, L. (2023). Outcomes in Patients with Acute Myocardial Infarction and Known Sleep Apnea: A Nationwide Analysis. Journal of Clinical Medicine, 12(18), 5924. https://doi.org/10.3390/jcm12185924






