Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Ambulatory Blood Pressure Monitoring (ABPM)
- -
- Heart rate [beats/min] for daytime (DTHR) and nighttime (NTHR);
- -
- Systolic blood pressure [mmHg] for the day (DTSBP) and night (NTSBP);
- -
- Diastolic blood pressure [mmHg] for the day (DTDBP) and night (NTDBP).
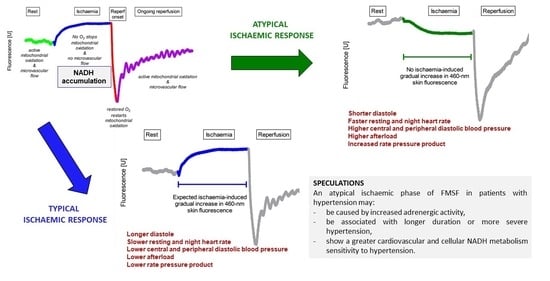
2.3. Flow-Mediated Skin Florescence (FMSF) and Division of the Study Group
- -
- Typical response—those with an expected increase and plateau in 460 nm skin fluorescence during ischaemia;
- -
- Atypical response—the remaining patients with a decrease, no increase, delayed and short-lived, or multimodal with brief increases and decreases in fluorescence.
2.4. Non-Invasive Assessment of the Radial and Central Arterial Pressure Waveforms
2.5. Ethics Approval Statement
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Study Limitations
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-Invasive Assessment of Vascular Circulation Based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef]
- Piotrowski, L.; Urbaniak, M.; Jedrzejczak, B.; Marcinek, A.; Gebicki, J. Note: Flow mediated skin fluorescence—A novel technique for evaluation of cutaneous microcirculation. Rev. Sci. Instrum. 2016, 87, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. Am. J. Physiol.—Cell Physiol. 2007, 292, C615–C640. [Google Scholar] [CrossRef] [PubMed]
- Bugaj, O.; Kusy, K.; Kantanista, A.; Korman, P.; Wieliński, D.; Zieliński, J. The effect of a 7-week training period on changes in skin NADH fluorescence in highly trained athletes. Appl. Sci. 2020, 10, 5133. [Google Scholar] [CrossRef]
- Tarnawska, M.; Dorniak, K.; Kaszubowski, M.; Dudziak, M.; Hellmann, M. A pilot study with flow mediated skin fluorescence: A novel device to assess microvascular endothelial function in coronary artery disease. Cardiol. J. 2018, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Borkowska, A.; Czajkowski, P.; Los, A.; Szczerbinski, L.; Milewska-Kranc, A.; Marcinek, A.; Kretowski, A.; Cypryk, K.; Gebicki, J. Flow Mediated Skin Fluorescence technique reveals remarkable effect of age on microcirculation and metabolic regulation in type 1 diabetes. Microvasc. Res. 2019, 124, 19–24. [Google Scholar] [CrossRef]
- Majewski, S.; Szewczyk, K.; Białas, A.J.; Miłkowska-Dymanowska, J.; Kurmanowska, Z.; Górski, P. Assessment of microvascular function in vivo using flow mediated skin fluorescence (FMSF) in patients with obstructive lung diseases: A preliminary study. Microvasc. Res. 2020, 127, 103914. [Google Scholar] [CrossRef]
- Bogaczewicz, J.; Tokarska, K.; Wozniacka, A. Changes of NADH Fluorescence from the Skin of Patients with Systemic Lupus Erythematosus. Biomed. Res. Int. 2019, 2019, 5897487. [Google Scholar] [CrossRef]
- Pawlak-Chomicka, R.; Chomicki, W.; Krauze, T.; Uruski, P.; Guzik, M.; Piskorski, J.; Tykarski, A.; Guzik, P. Investigating the Ischaemic Phase of Skin NADH Fluorescence Dynamics in Recently Diagnosed Primary Hypertension: A Time Series Analysis. J. Clin. Med. 2023, 12, 1247. [Google Scholar] [CrossRef]
- Pawlak-Chomicka, R.; Krauze, T.; Uruski, P.; Piskorski, J.; Wykretowicz, A.; Tykarski, A.; Guzik, P. The Effect of Antihypertensive Drugs on NADH in Newly Diagnosed Primary Hypertension. Cardiol. Res. Pract. 2022, 2022, 6159883. [Google Scholar] [CrossRef]
- Nizinski, J.; Kamieniarz, L.; Filberek, P.; Sibrecht, G.; Guzik, P. Monitoring the skin NADH changes during ischaemia and reperfusion in humans. J. Med. Sci. 2020, 89, e405. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESHGuidelines for themanagement of arterial hypertension. J. Hypertens. 2018, 36, 1956–2041. [Google Scholar] [CrossRef]
- Staessen, J.A.; Huang, Q.-F.; Yang, W.-Y.; Asayama, K.; Zhang, Z.-Y.; Thijs, L.; Li, Y.; O’Brien, E. Ambulatory Blood Pressure Monitoring to Diagnose and Manage Hypertension. Hypertension 2021, 77, 254–264. [Google Scholar] [CrossRef]
- Etyang, A.O.; Sigilai, A.; Odipo, E.; Oyando, R.; Ong’Ayo, G.; Muthami, L.; Munge, K.; Kirui, F.; Mbui, J.; Bukania, Z.; et al. Diagnostic accuracy of unattended automated office blood pressure measurement in screening for hypertension in Kenya. Hypertension 2019, 74, 1490–1498. [Google Scholar] [CrossRef]
- Hellmann, M.; Tarnawska, M.; Dudziak, M.; Dorniak, K.; Roustit, M.; Cracowski, J.L. Reproducibility of flow mediated skin fluorescence to assess microvascular function. Microvasc. Res. 2017, 113, 60–64. [Google Scholar] [CrossRef]
- Holowatz, L.A.; Thompson-Torgerson, C.S.; Kenney, W.L. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol. 2008, 105, 370–372. [Google Scholar] [CrossRef]
- Guzik, P.; Schneider, A.; Piskorski, J.; Klimas, K.; Krauze, T.; Marciniak, R.; Minczykowski, A.; Mihailidou, A.S.; Wykretowicz, A. Sex differences in excess and reservoir arterial blood pressures as markers of phenotype. J. Hypertens. 2019, 37, 2159–2167. [Google Scholar] [CrossRef]
- Armstrong, M.K.; Schultz, M.G.; Hughes, A.D.; Picone, D.S.; Sharman, J.E. Physiological and clinical insights from reservoir-excess pressure analysis. J. Hum. Hypertens. 2021, 35, 758–768. [Google Scholar] [CrossRef]
- Davies, J.E.; Lacy, P.; Tillin, T.; Collier, D.; Cruickshank, J.K.; Francis, D.P.; Malaweera, A.; Mayet, J.; Stanton, A.; Williams, B.; et al. Excess pressure integral predicts cardiovascular events independent of other risk factors in the conduit artery functional evaluation substudy of Anglo-Scandinavian Cardiac Outcomes Trial. Hypertension 2014, 64, 60–68. [Google Scholar] [CrossRef]
- Hametner, B.; Wassertheurer, S.; Hughes, A.D.; Parker, K.H.; Weber, T.; Eber, B. Reservoir and excess pressures predict cardiovascular events in high-risk patients. Int. J. Cardiol. 2014, 171, 31–36. [Google Scholar] [CrossRef]
- Narayan, O.; Davies, J.E.; Hughes, A.D.; Dart, A.M.; Parker, K.H.; Reid, C.; Cameron, J.D. Central aortic reservoir-wave analysis improves prediction of cardiovascular events in elderly hypertensives. Hypertension 2015, 65, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Sung, S.H.; Wang, J.J.; Wu, C.K.; Lin, L.Y.; Lee, J.C.; Cheng, H.M.; Chen, C.H. Excess Pressure Integral Predicts Long-Term All-Cause Mortality in Stable Heart Failure Patients. Am. J. Hypertens. 2017, 30, 271–278. [Google Scholar] [CrossRef]
- Davies, J.E.; Hadjiloizou, N.; Leibovich, D.; Malaweera, A.; Alastruey-Arimon, J.; Whinnett, Z.I.; Manisty, C.H.; Francis, D.P.; Aguado-Sierra, J.; Foale, R.A.; et al. Importance of the aortic reservoir in determining the shape of the arterial pressure waveform—The forgotten lessons of Frank. Artery Res. 2007, 1, 40–45. [Google Scholar] [CrossRef]
- Narayan, O.; Parker, K.H.; Davies, J.E.; Hughes, A.D.; Meredith, I.T.; Cameron, J.D. Reservoir pressure analysis of aortic blood pressure: An in-vivo study at five locations in humans. J. Hypertens. 2017, 35, 2025–2033. [Google Scholar] [CrossRef]
- Peng, X.; Schultz, M.G.; Picone, D.S.; Black, J.A.; Dwyer, N.; Roberts-Thomson, P.; Davies, J.E.; Sharman, J.E. Arterial reservoir characteristics and central-to-peripheral blood pressure amplification in the human upper limb. J. Hypertens. 2017, 35, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Sierra, J.; Alastruey, J.; Wang, J.J.; Hadjiloizou, N.; Davies, J.; Parker, K.H. Separation of the reservoir and wave pressure and velocity from measurements at an arbitrary location in arteries. Proc. Inst. Mech. Eng. H. 2008, 222, 403–416. [Google Scholar] [CrossRef]
- Maynard, A.G.; Kanarek, N. NADH Ties One-Carbon Metabolism to Cellular Respiration. Cell Metab. 2020, 31, 660–662. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions. Biomolecules 2019, 9, 332. [Google Scholar] [CrossRef]
- Ronquist, G.; Andersson, A.; Bendsoe, N.; Falck, B. Human epidermal energy metabolism is functionally anaerobic. Exp. Dermatol. 2003, 12, 572–579. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Eirin, A.; Lerman, A.; Lerman, L.O. Enhancing Mitochondrial Health to Treat Hypertension. Curr. Hypertens. Rep. 2018, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.C.; Amaral, F.G.; Campos, L.A.; Cipolla-Neto, J. Melatonin, mitochondria and hypertension. Cell. Mol. Life Sci. 2017, 74, 3955–3964. [Google Scholar] [CrossRef]
- Figueroa, M.A.; DeMeersman, R.E.; Manning, J. The autonomic and rate pressure product responses of Tai Chi practitioners. N. Am. J. Med. Sci. 2012, 4, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic Neural Mechanisms in Cardiovascular Function. Mayo Clin. Proc. 2009, 84, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Chemla, D.; Plamann, K.; Nitenberg, A. Towards new indices of arterial stiffness using systolic pulse contour analysis: A theoretical point of view. J. Cardiovasc. Pharmacol. 2008, 51, 111–117. [Google Scholar] [CrossRef]
- Baim, D.S.; Grossman, W. Grossman’s Cardiac Catheterization, Angiography, and Intervention, 6th ed. Circulation 2001, 104, E70. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am. J. Hypertens. 2004, 17, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Trammel, J.E.; Sapra, A. Physiology, Systemic Vascular Resistance. StatPearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556075/ (accessed on 19 June 2023).
- Butlin, M.; Qasem, A. Large Artery Stiffness Assessment Using SphygmoCor Technology. Pulse 2016, 4, 180–192. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538226/ (accessed on 19 June 2023).
- Sharman, J.E.; Davies, J.E.; Jenkins, C.; Marwick, T.H. Augmentation index, left ventricular contractility, and wave reflection. Hypertension 2009, 54, 1099–1105. [Google Scholar] [CrossRef]
- Gedikli, O.; Ozturk, S.; Yilmaz, H.; Baykan, M.; Kiris, A.; Durmus, I.; Baltaci, D.; Karahan, C.; Celik, S. Relationship between arterial stiffness and myocardial damage in patients with newly diagnosed essential hypertension. Am. J. Hypertens. 2008, 21, 989–993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salvi, P.; Parati, G. Aortic stiffness andmyocardial ischemia. J. Hypertens. 2015, 33, 1767–1771. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Explanation |
|---|---|
| HR | Heart rate [beats/min] |
| DD | Diastole duration [ms]—the duration of ventricular relaxation which reflects most of the coronary perfusion time |
| pSBP | Peripheral systolic blood pressure [mmHg] |
| pDBP | Peripheral diastolic blood pressure [mmHg] |
| cSBP | Central systolic blood pressure [mmHg] |
| cDBP | Central diastolic blood pressure [mmHg] |
| cTTI | Central tension time index—an index of the myocardial oxygen demand per beat. It is calculated by multiplying the central systolic blood pressure by the systolic time interval. It reflects the afterload, i.e., the effects of blood pressure during left ventricle systole. |
| cRPP | Central rate pressure product [mmHg x beats/min], cRPP = cSBPxHR. It is an indirect measure of myocardial oxygen consumption |
| EPTI | Excess pressure–time integral of central pulse wave—the area under the excess pressure curve |
| RPTI | Reservoir pressure–time integral of central pulse wave—the area under the reservoir pressure |
| Typical Pattern N = 44 | Atypical Pattern N = 21 | p-Value (Mann–Whitney Test) | |||
|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| DTSBP [mmHg] | 148.4 | 142–156.5 | 147.2 | 143–156.4 | 0.84 |
| DTDBP [mmHg] | 87.6 | 83.1–92.3 | 91.6 | 84.9–95.9 | 0.09 |
| DTHR [beats/min] | 81.7 | 76–89.9 | 83.1 | 76.4–89.7 | 0.95 |
| NTSBP [mmHg] | 122.7 | 114.1–130.1 | 119 | 110.3–140.7 | 0.83 |
| NTDBP [mmHg] | 70 | 65.4–75.9 | 70.5 | 65.5–82.4 | 0.44 |
| NTHR [beats/min] | 63.2 | 57.7–71.2 | 68.6 | 63.6–76.9 | 0.03 |
| HR [beats/min] | 69.5 | 61.6–80.9 | 75.3 | 71.2–85 | 0.03 |
| DD [ms] | 547.4 | 445.5–647 | 506.5 | 427–532.7 | 0.02 |
| pSBP [mmHg] | 129.7 | 120.3–137.1 | 129.5 | 124.8–139.4 | 0.5 |
| pDBP [mmHg] | 78.8 | 74.7–85.1 | 86.6 | 78.8–92 | 0.03 |
| cSBP [mmHg] | 113.3 | 107.3–126.1 | 119.2 | 112.1–126.6 | 0.23 |
| cDBP [mmHg] | 80.5 | 75.9–86.6 | 87.2 | 79.7–93.3 | 0.03 |
| cTTI [mmHg × s−1 × min−1] | 2320.1 | 2118.1–2653.1 | 2524.7 | 2341.5–2764 | 0.02 |
| cRPP [mmHg × beats/min] | 7805.5 | 7111.9–9553.1 | 8801.2 | 8188.3–10,196.4 | 0.02 |
| EPTI [mmHg × ms] | 4.6 | 4.2–5.2 | 4.3 | 3.8–4.8 | 0.05 |
| RPTI [mmHg × ms] | 10.3 | 7.7–11.4 | 8 | 7–10.1 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak-Chomicka, R.; Uruski, P.; Krauze, T.; Piskorski, J.; Tykarski, A.; Guzik, P. Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia. J. Clin. Med. 2023, 12, 5886. https://doi.org/10.3390/jcm12185886
Pawlak-Chomicka R, Uruski P, Krauze T, Piskorski J, Tykarski A, Guzik P. Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia. Journal of Clinical Medicine. 2023; 12(18):5886. https://doi.org/10.3390/jcm12185886
Chicago/Turabian StylePawlak-Chomicka, Regina, Paweł Uruski, Tomasz Krauze, Jarosław Piskorski, Andrzej Tykarski, and Przemysław Guzik. 2023. "Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia" Journal of Clinical Medicine 12, no. 18: 5886. https://doi.org/10.3390/jcm12185886
APA StylePawlak-Chomicka, R., Uruski, P., Krauze, T., Piskorski, J., Tykarski, A., & Guzik, P. (2023). Arterial Blood Pressure Features of Hypertensive Patients with Typical and Atypical 460 nm Skin Fluorescence Response to Transient Ischaemia. Journal of Clinical Medicine, 12(18), 5886. https://doi.org/10.3390/jcm12185886