ANCOC Score to Predict Mortality in Different SARS-CoV-2 Variants and Vaccination Status
Abstract
:1. Introduction
2. Materials and Methods
- -
- Demographic data (age; sex; body mass index (BMI); number and type of comorbidities);
- -
- COVID-19 infection and hospitalization data (first oropharyngeal swab positivity confirmed by PCR; cutoff index (COI) value of the rapid antigen test performed in the emergency department (ED); initiation of anticoagulant therapy at home or in the hospital; day of admission and duration of hospitalization; final outcome; intensity of hospitalization);
- -
- Therapy administered during hospitalization: pharmacologic therapy (corticosteroids, monoclonal antibodies, remdesivir, anti-interleukin 6 receptor) and oxygen therapy (high flow nasal therapy (HFNC); non-invasive ventilation (NIV); orotracheal intubation (OTI));
- -
- Laboratory data (blood urea nitrogen [BUN], CRP, procalcitonin, LDH, lymphocytes, eosinophils, D-dimer, fibrinogen);
- -
- FiO2, PaO2, PaO2/FiO2, SpO2 of the first blood gas analysis performed upon arrival at ED;
- -
- Immunization data: vaccinated (at least one booster) or not vaccinated;
- -
- We calculated the ANCOC score for all included patients (Table 1).
2.1. Statistical Analysis
2.2. Sample Size
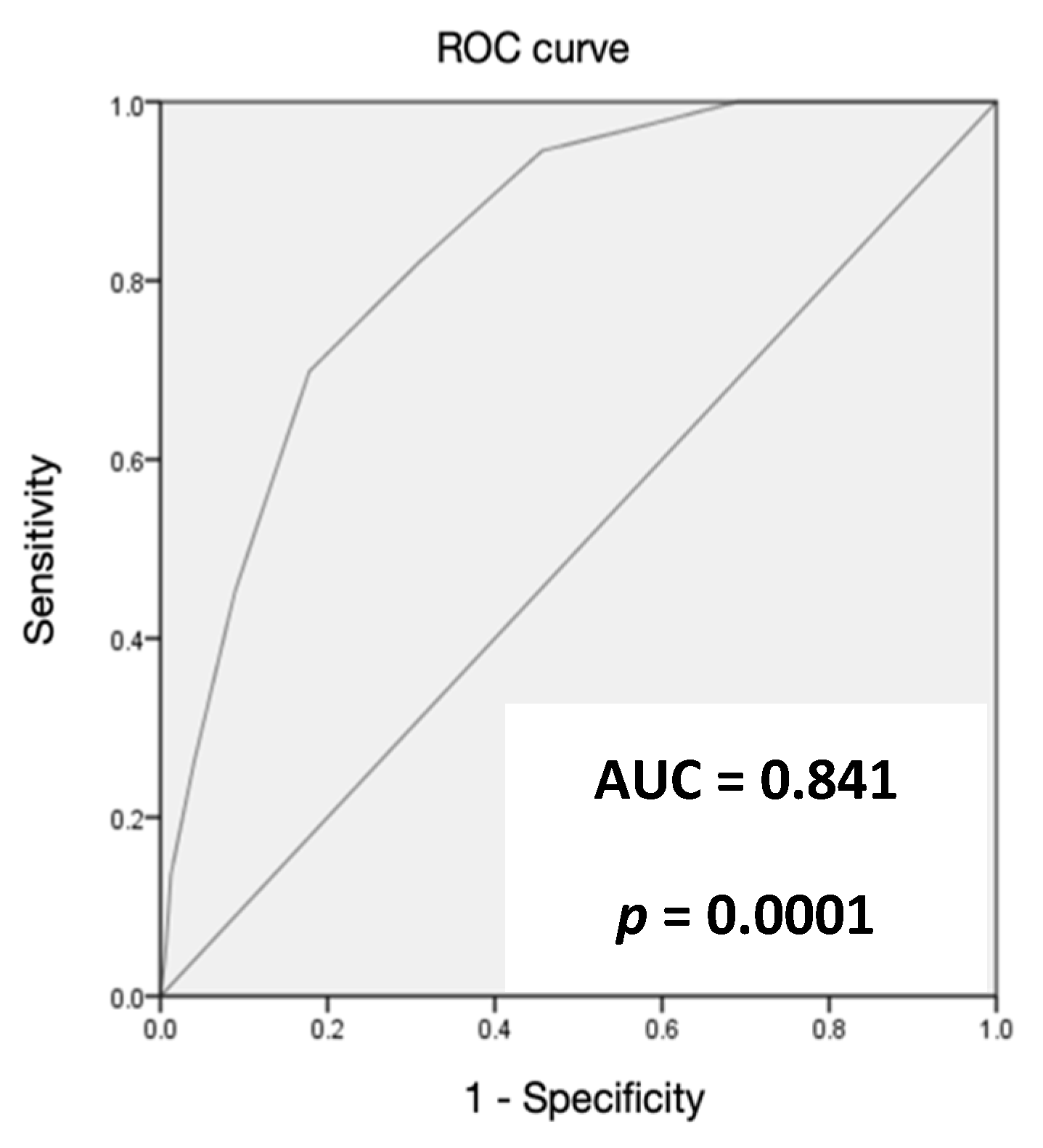
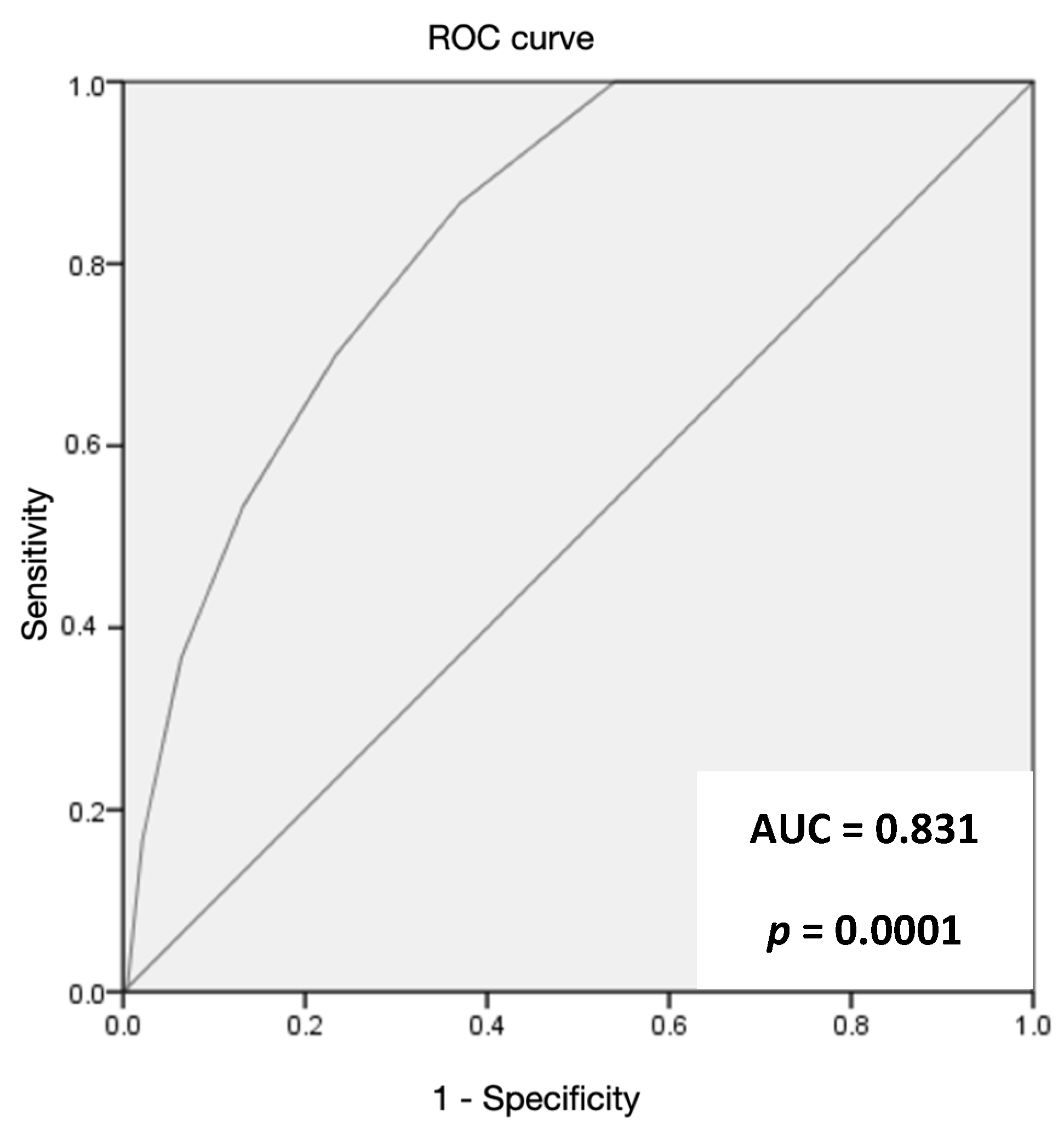
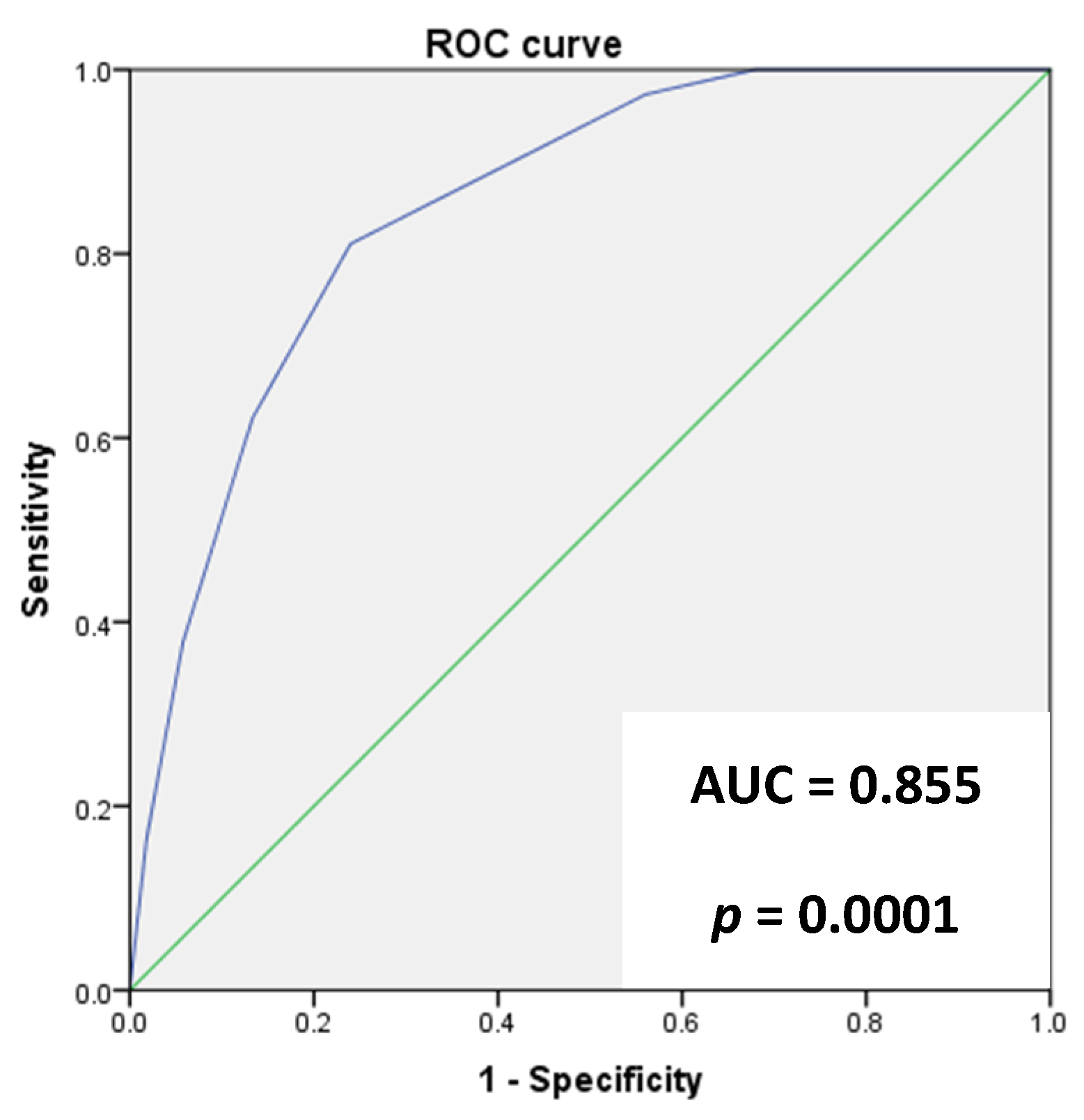
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhee, C.; Kanjilal, S.; Baker, M.; Klompas, M. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectivity: When Is It Safe to Discontinue Isolation? Clin. Infect. Dis. 2021, 72, 1467–1474. [Google Scholar] [CrossRef]
- Dixon, B.E.; Wools-Kaloustian, K.; Fadel, W.F.; Duszynski, T.J.; Yiannoutsos, C.; Halverson, P.K.; Menachemi, N. Symptoms and Symptom Clusters Associated with SARS-CoV-2 Infection in Community-Based Populations: Results from a Statewide Epidemiological Study. PLoS ONE 2021, 16, e0241875. [Google Scholar] [CrossRef]
- Nasir, A.; Aamir, U.B.; Kanji, A.; Bukhari, A.R.; Ansar, Z.; Ghanchi, N.K.; Masood, K.I.; Samreen, A.; Islam, N.; Ghani, S.; et al. Tracking SARS-CoV-2 variants through pandemic waves using RT-PCR testing in low-resource settings. PLoS Glob. Public Health 2023, 3, e0001896. [Google Scholar] [CrossRef]
- Lionte, C.; Sorodoc, V.; Haliga, R.E.; Bologa, C.; Ceasovschih, A.; Petris, O.R.; Coman, A.E.; Stoica, A.; Sirbu, O.; Puha, G.; et al. Inflammatory and Cardiac Biomarkers in Relation with Post-Acute COVID-19 and Mortality: What We Know after Successive Pandemic Waves. Diagnostics 2022, 12, 1373. [Google Scholar] [CrossRef]
- COVID-19 Omicron Delta Study Group. Clinical progression, disease severity, and mortality among adults hospitalized with COVID-19 caused by the Omicron and Delta SARS-CoV-2 variants: A population-based, matched cohort study. PLoS ONE 2023, 18, e0282806. [Google Scholar] [CrossRef]
- Esteban Ronda, V.; Ruiz Alcaraz, S.; Ruiz Torregrosa, P.; Giménez Suau, M.; Nofuentes Pérez, E.; León Ramírez, J.M.; Andrés, M.; Moreno-Pérez, Ó.; Candela Blanes, A.; Gil Carbonell, J.; et al. Application of Validated Severity Scores for Pneumonia Caused by SARS-CoV-2. Med. Clínica (Engl. Ed.) 2021, 157, 99–105. [Google Scholar] [CrossRef]
- Rauseo, M.; Perrini, M.; Gallo, C.; Mirabella, L.; Mariano, K.; Ferrara, G.; Santoro, F.; Tullo, L.; La Bella, D.; Vetuschi, P.; et al. Machine Learning and Predictive Models: 2 Years of SARS-CoV-2 Pandemic in a Single-Center Retrospective Analysis. J. Anesth. Analg. Crit. Care 2022, 2, 42. [Google Scholar] [CrossRef]
- Covino, M.; Sandroni, C.; Santoro, M.; Sabia, L.; Simeoni, B.; Bocci, M.G.; Ojetti, V.; Candelli, M.; Antonelli, M.; Gasbarrini, A.; et al. Predicting Intensive Care Unit Admission and Death for COVID-19 Patients in the Emergency Department Using Early Warning Scores. Resuscitation 2020, 156, 84–91. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk Stratification of Patients Admitted to Hospital with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol: Development and Validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Knight, S.R.; Gupta, R.K.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; et al. Prospective validation of the 4C prognostic models for adults hospitalised with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. Thorax 2022, 77, 606–615. [Google Scholar] [CrossRef]
- Candelli, M.; Pignataro, G.; Ferrigno, M.; Cicchinelli, S.; Torelli, E.; Gullì, A.; Sacco Fernandez, M.; Piccioni, A.; Ojetti, V.; Covino, M.; et al. Factors Associated with ICU Admission in Patients with COVID-19: The GOL2DS Score. Medicina 2021, 57, 1356. [Google Scholar] [CrossRef]
- Candelli, M.; Pignataro, G.; Fernandez, M.S.; Cicchinelli, S.; Gullì, A.; Torelli, E.; Gabrielli, M.; Piccioni, A.; Covino, M.; Ojetti, V.; et al. A New Simple Score to Predict Mortality of COVID-19 in the Emergency Department. Signa Vitae 2023, 19, 20–27. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; De Silva, T.I.; Peacock, S.J.; Barclay, W.S.; De Silva, T.I.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- AIFA (Drugs Italian Agency). Varianti del Virus. (Viral Variants). Available online: https://www.iss.it/en/cov19-cosa-fa-iss-varianti?p_p_id=com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_delta=20&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_year=2023&p_r_p_resetCur=false&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_yJS4xO2fauqM_cur=2 (accessed on 12 December 2022).
- ISS (Italian Institut of Health). EPICENTRO. Varianti Virali Prevalenti. (Prevalent Viral Variants). Available online: https://www.epicentro.iss.it/coronavirus/pdf/SARS-CoV-2-monitoraggio-varianti-rapporti-periodici-18-febbraio-2022.pdf (accessed on 12 December 2022).
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Uzun, O.; Akpolat, T.; Varol, A.; Turan, S.; Bektas, S.G.; Cetinkaya, P.D.; Dursun, M.; Bakan, N.; Ketencioglu, B.B.; Bayrak, M.; et al. COVID-19: Vaccination vs. Hospitalization. Infection 2022, 50, 747–752. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Camoin-Jau, L. An Update on Angiotensin-Converting Enzyme 2 Structure/Functions, Polymorphism, and Duplicitous Nature in the Pathophysiology of Coronavirus Disease 2019: Implications for Vascular and Coagulation Disease Associated with Severe Acute Respiratory Syndrome Coronavirus Infection. Front. Microbiol. 2022, 13, 1042200. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Fumagalli, L.A.M.; D’angelo, L.; Cerino, M.; Bonfanti, G.; Fumagalli, R.M.; Schiavo, G.; Lorini, C.; Lainu, E.; Terragni, S.; et al. Eosinopenia is a reliable marker of severe disease and unfavourable outcome in patients with COVID-19 pneumonia. Int. J. Clin. Pract. 2021, 75, e14047. [Google Scholar] [CrossRef]
- Olivieri, F.; Sabbatinelli, J.; Bonfigli, A.R.; Sarzani, R.; Giordano, P.; Cherubini, A.; Antonicelli, R.; Rosati, Y.; Del Prete, S.; Di Rosa, M.; et al. Routine laboratory parameters, including complete blood count, predict COVID-19 in-hospital mortality in geriatric patients. Mech. Ageing Dev. 2022, 204, 111674. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Marlow, R.; Nguyen, J.; Begier, E.; Southern, J.; King, J.; Morley, A.; Kinney, J.; Clout, M.; et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom. Lancet Reg. Health Eur. 2023, 25, 100556. [Google Scholar] [CrossRef]
- Macchia, I.; La Sorsa, V.; Urbani, F.; Moretti, S.; Antonucci, C.; Afferni, C.; Schiavoni, G. Eosinophils as potential biomarkers in respiratory viral infections. Front. Immunol. 2023, 14, 1170035. [Google Scholar] [CrossRef]
- Baicus, C. White blood cells, COVID-19, and mendelian randomization. J. Pers. Med. 2022, 12, 1425. [Google Scholar] [CrossRef]
- Ruangsomboon, O.; Phanprasert, N.; Jirathanavichai, S.; Puchongmart, C.; Boonmee, P.; Thirawattanasoot, N.; Dorongthom, T.; Praphruetkit, N.; Monsomboon, A. The utility of the Rapid Emergency Medicine Score (REMS) compared with three other early warning scores in predicting in-hospital mortality among COVID-19 patients in the emergency department: A multicenter validation study. BMC Emerg. Med. 2023, 23, 45. [Google Scholar] [CrossRef]
- Prasad, P.A.; Correia, J.; Fang, M.C.; Fisher, A.; Correll, M.; Oreper, S.; Auerbach, A. Performance of point-of-care severity scores to predict prognosis in patients admitted through the emergency department with COVID-19. J. Hosp. Med. 2023, 18, 413–423. [Google Scholar] [CrossRef]
- Jones, A.; Pitre, T.; Junek, M.; Kapralik, J.; Patel, R.; Feng, E.; Dawson, L.; Tsang, J.L.Y.; Duong, M.; Ho, T.; et al. External validation of the 4C mortality score among COVID-19 patients admitted to hospital in Ontario, Canada: A retrospective study. Sci. Rep. 2021, 11, 18638. [Google Scholar] [CrossRef]
- Häger, L.; Wendland, P.; Biergans, S.; Lederer, S.; de Arruda Botelho Herr, M.; Erhardt, C.; Schmauder, K.; Kschischo, M.; Malek, N.P.; Bunk, S.; et al. External Validation of COVID-19 Risk Scores during Three Waves of Pandemic in a German Cohort—A Retrospective Study. J. Pers. Med. 2022, 12, 1775. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Saderi, L.; Puci, M.; Zauli, B.; Meloni, M.C.; Fois, M.; Bitti, A.; Di Castri, C.; Fiore, V.; et al. Is the 4C Score Still a Valid Item to Predict In-Hospital Mortality in People with SARS-CoV-2 Infections in the Omicron Variant Era? Life 2023, 13, 183. [Google Scholar] [CrossRef]


| Criterion | Score |
|---|---|
| BUN > 35 | 1 |
| BUN < 15 | −1 |
| SO2 > 96 | −1 |
| SO2 ≤ 88 | 1 |
| Comorbidities < 2 | −1 |
| Comorbidities > 4 | 1 |
| Age < 55 | −2 |
| Age > 80 | 2 |
| CRP > 26 | −1 |
| CRP > 155 | 1 |
| Clinical Data—Mean ± SD or n (%) | Total Population (843) | Delta (515) | Omicron 1 (328) | p-Value |
|---|---|---|---|---|
| Age (years) | 62 ± 19 | 60 ± 18 | 64 ± 18 | 0.001 |
| Male | 481 (57) | 287 (56) | 194 (59) | 0.3 |
| Female | 362 (43) | 228 (44) | 134 (41) | 0.3 |
| BMI (kg/m2) | 27.3 ± 6 | 27.4 ± 7 | 27.1 ± 6 | 0.6 |
| No comorbidities | 265 (31) | 170 (33) | 95 (29) | 0.31 |
| One comorbidity | 218 (26) | 145 (28) | 73 (22) | 0.2 |
| ≥2 comorbidities | 360 (43) | 200 (39) | 160 (48) | 0.2 |
| COI | 64 ± 56 | 58 ± 54 | 72 ± 57 | 0.005 |
| Comorbidity n (%) | All Patients (843) | Delta Variant (515) | Omicron Variant (328) | p |
|---|---|---|---|---|
| Hypertension | 320 (38) | 175 (34) | 145 (44) | 0.003 |
| Obesity | 205 (24) | 141 (27) | 64 (20) | 0.01 |
| Diabetes | 122 (14) | 73 (14) | 49 (15) | 0.75 |
| Active neoplasm | 105 (12) | 57 (11) | 48 (15) | 0.12 |
| Atrial fibrillation | 82 (10) | 52 (10) | 30 (9) | 0.65 |
| COPD | 80 (9) | 42 (8) | 38 (12) | 0.01 |
| CHD | 79 (9) | 43 (8) | 36 (11) | 0.20 |
| CKD | 61 (7) | 25 (5) | 36 (11) | 0.001 |
| Heart failure | 59 (7) | 36 (7) | 23 (7) | 0.99 |
| Other chronic disease | 164 (19) | 92 (18) | 71 (22) | 0.17 |
| p-Value | Odds Ratio | 95% CI | |
|---|---|---|---|
| Obesity | 0.26 | 0.72 | 0.41–1.27 |
| Hypertension | 0.01 | 0.48 | 0.28–0.84 |
| COPD | 0.01 | 0.40 | 0.19–0.83 |
| CKD | 0.70 | 0.83 | 0.31–2.17 |
| Total Population (843) | Delta (515) | Omicron 1 (328) | p-Value | |
|---|---|---|---|---|
| O2 saturation (%) | 94 ± 6 | 93 ± 6 | 95 ± 5 | 0.0001 |
| PaO2/FiO2 (mmHg) | 306 ± 111 | 296 ± 109 | 324 ± 115 | 0.001 |
| Laboratory Parameters Mean ± SD | Total Population | Delta | Omicron 1 | p-Value |
|---|---|---|---|---|
| BUN (mg/dl) | 26 ± 24 | 24 ± 23 | 29 ± 25 | 0.005 |
| LDH (U/L) | 320 ± 198 | 340 ± 184 | 288 ± 214 | 0.005 |
| Eosinophils (cells × 109/L) | 0.05 ± 0,12 | 0.04 ± 0.12 | 0.07 ± 0.12 | 0.005 |
| Lymphocytes (cells × 109/L) | 3.1 ± 3.7 | 4.0 ± 4.7 | 1.6 ± 3.8 | 0.25 |
| Fibrinogen (mg/dl) | 478 ± 169 | 513 ± 171 | 421 ± 149 | <0.001 |
| Procalcitonin (ng/mL) | 1.8 ± 20 | 1.60 ± 22 | 2.10 ± 14 | 0.72 |
| D-dimer (ng/mL) | 2589 ± 5623 | 2488 ± 5788 | 2787 ± 5293 | 0.51 |
| CRP (mg/dL) | 67 ± 71 | 69 ± 69 | 64 ± 74 | 0.36 |
| Treatment n (%) | Total Population (843) | Delta (515) | Omicron 1 (328) | p-Value |
|---|---|---|---|---|
| Remdesivir | 194 (23) | 139 (27) | 55 (16) | 0.005 |
| Corticosteroids | 508 (60) | 361 (70) | 147 (45) | 0.0001 |
| Anti-IL6R | 105 (12) | 113 (22) | 13 (4) | 0.001 |
| Mab | 85 (10) | 48 (9) | 37 (11) | 0.35 |
| Anticoagulants | 697 (83) | 434 (84) | 263 (80) | 0.64 |
| HFNT | 117 (14) | 88 (17) | 29 (9) | 0.001 |
| NIV | 80 (9) | 60 (11) | 20 (6) | 0.01 |
| OTI/Tracheostomy | 65 (7) | 6 (7) | 29 (9) | 0.3 |
| Outcome n (%) | Total Population (843) | Delta Population (515) | Omicron 1 Population (328) | p-Value |
|---|---|---|---|---|
| Discharged from ED | 191 (23) | 79 (15) | 112 (34) | 0.0001 |
| Admitted in medical ward | 509 (60) | 336 (65) | 173 (53) | 0.0003 |
| Admitted in ICU | 143 (17) | 100 (20) | 43 (13) | 0.02 |
| Deceased | 114 (13) | 73 (14) | 41 (12) | 0.48 |
| Vaccination Status | Total Population | Delta Population | Omicron 1 Population | p-Value |
|---|---|---|---|---|
| Vaccinated n (%) | 357 (42) | 176 (34) | 183 (56) | 0.001 |
| Not vaccinated n (%) | 486 (58) | 339 (66) | 145 (44) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candelli, M.; Sacco Fernandez, M.; Pignataro, G.; Merra, G.; Tullo, G.; Bronzino, A.; Piccioni, A.; Ojetti, V.; Gasbarrini, A.; Franceschi, F. ANCOC Score to Predict Mortality in Different SARS-CoV-2 Variants and Vaccination Status. J. Clin. Med. 2023, 12, 5838. https://doi.org/10.3390/jcm12185838
Candelli M, Sacco Fernandez M, Pignataro G, Merra G, Tullo G, Bronzino A, Piccioni A, Ojetti V, Gasbarrini A, Franceschi F. ANCOC Score to Predict Mortality in Different SARS-CoV-2 Variants and Vaccination Status. Journal of Clinical Medicine. 2023; 12(18):5838. https://doi.org/10.3390/jcm12185838
Chicago/Turabian StyleCandelli, Marcello, Marta Sacco Fernandez, Giulia Pignataro, Giuseppe Merra, Gianluca Tullo, Alessandra Bronzino, Andrea Piccioni, Veronica Ojetti, Antonio Gasbarrini, and Francesco Franceschi. 2023. "ANCOC Score to Predict Mortality in Different SARS-CoV-2 Variants and Vaccination Status" Journal of Clinical Medicine 12, no. 18: 5838. https://doi.org/10.3390/jcm12185838
APA StyleCandelli, M., Sacco Fernandez, M., Pignataro, G., Merra, G., Tullo, G., Bronzino, A., Piccioni, A., Ojetti, V., Gasbarrini, A., & Franceschi, F. (2023). ANCOC Score to Predict Mortality in Different SARS-CoV-2 Variants and Vaccination Status. Journal of Clinical Medicine, 12(18), 5838. https://doi.org/10.3390/jcm12185838










