Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Surgical Procedure
2.2.1. Fluoroscopy-Guided Transforaminal Approach
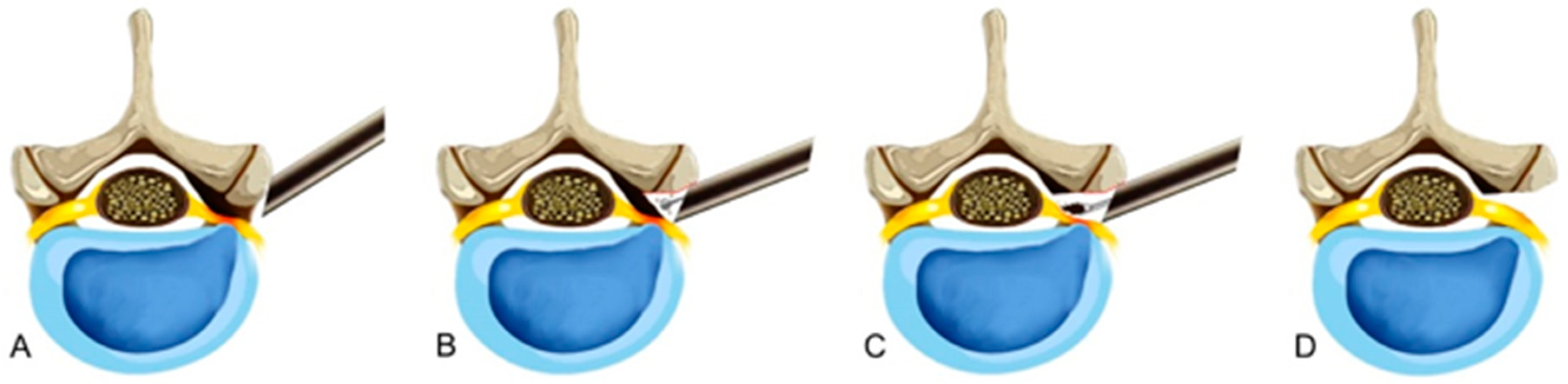
2.2.2. Bony Unroofing under Endoscopic Visualization
2.2.3. Soft Tissue Decompression under Endoscopic Visualization
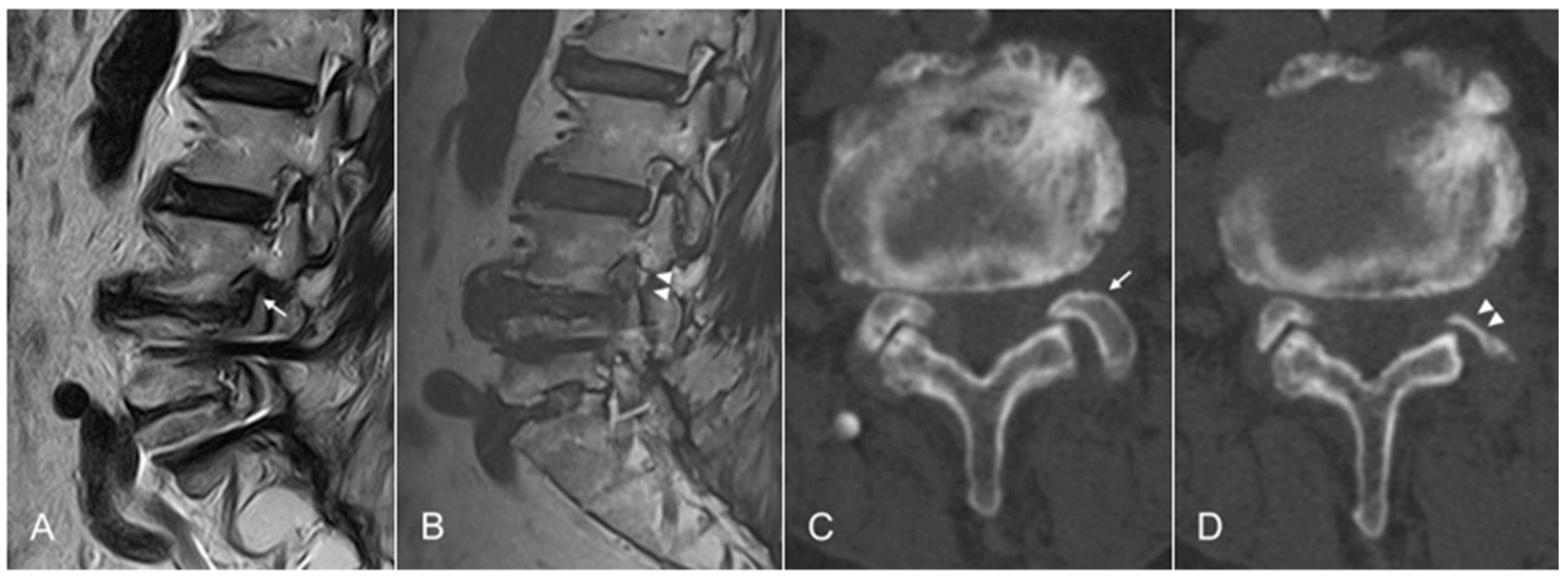
2.2.4. Endpoint and Postoperative Care
2.3. Outcome Evaluation
2.4. Statistical Analysis
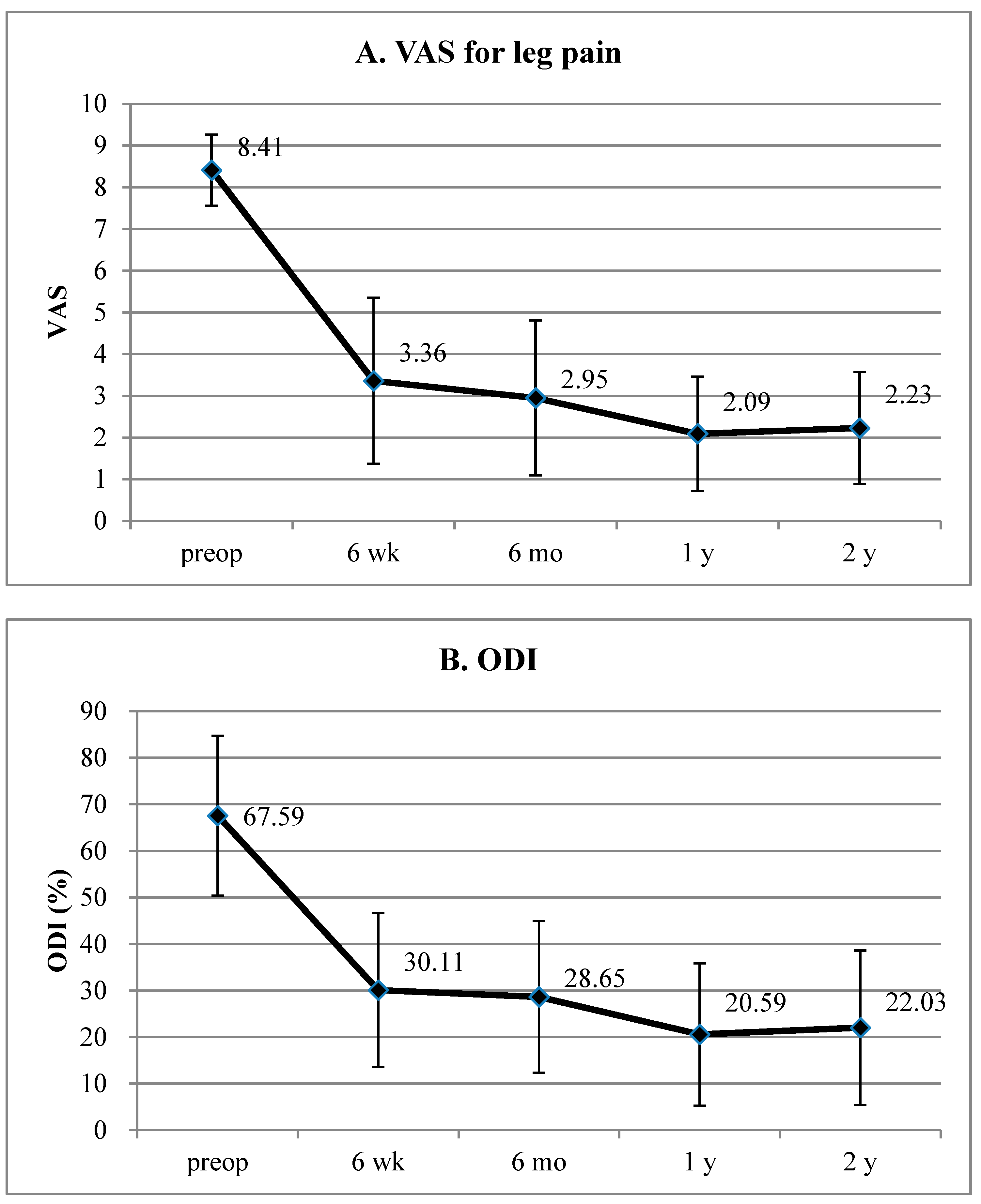
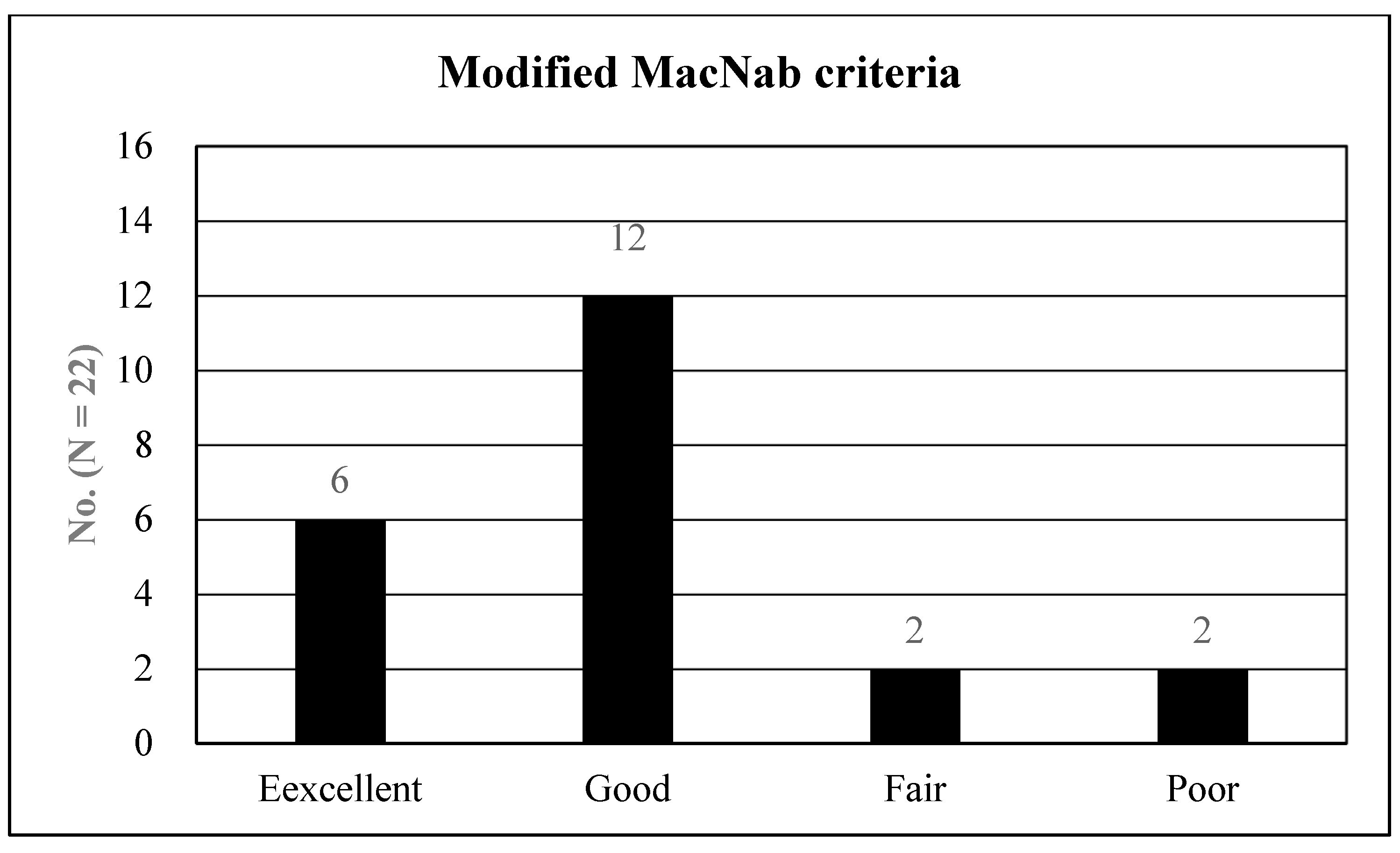
3. Results
4. Discussion
4.1. Data Interpretation
4.2. Conventional Surgery for Adjacent Level Foraminal Stenosis
4.3. TELF Technique for Juxta-Fusional Foraminal Stenosis
4.4. Pros and Cons of TELF
4.5. Technical Keys to Success
4.6. Limitations of the Study
4.7. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etebar, S.; Cahill, D.W. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J. Neurosurg. 1999, 90 (Suppl. 2), 163–169. [Google Scholar] [CrossRef]
- Park, P.; Garton, H.J.; Gala, V.C.; Hoff, J.T.; McGillicuddy, J.E. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine 2004, 29, 1938–1944. [Google Scholar] [CrossRef]
- Harrop, J.S.; Youssef, J.A.; Maltenfort, M.; Vorwald, P.; Jabbour, P.; Bono, C.M.; Goldfarb, N.; Vaccaro, A.R.; Hilibrand, A.S. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine 2008, 33, 1701–1707. [Google Scholar] [CrossRef]
- Orita, S.; Yamagata, M.; Ikeda, Y.; Nakajima, F.; Aoki, Y.; Nakamura, J.; Takahashi, K.; Suzuki, T.; Ohtori, S. Retrospective exploration of risk factors for L5 radiculopathy following lumbar floating fusion surgery. J. Orthop. Surg. Res. 2015, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Maruenda, J.I.; Barrios, C.; Garibo, F.; Maruenda, B. Adjacent segment degeneration and revision surgery after circumferential lumbar fusion: Outcomes throughout 15 years of follow-up. Eur. Spine J. 2016, 25, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Aiki, H.; Ohwada, O.; Kobayashi, H.; Hayakawa, M.; Kawaguchi, S.; Takebayashi, T.; Yamashita, T. Adjacent segment stenosis after lumbar fusion requiring second operation. J. Orthop. Sci. 2005, 10, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Leivseth, G.; Brox, J.I.; Fritzell, P.; Hägg, O.; Fairbank, J.C. ISSLS Prize winner: Long-term follow-up suggests spinal fusion is associated with increased adjacent segment disc degeneration but without influence on clinical outcome: Results of a combined follow-up from 4 randomized controlled trials. Spine 2014, 39, 1373–1383. [Google Scholar] [CrossRef]
- Irmola, T.M.; Häkkinen, A.; Järvenpää, S.; Marttinen, I.; Vihtonen, K.; Neva, M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine 2018, 43, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.P.; Jo, Y.H.; Jeong, H.W.; Choi, W.R.; Kang, C.N. Outcomes of Revision Surgery Following Instrumented Posterolateral Fusion in Degenerative Lumbar Spinal Stenosis: A Comparative Analysis between Pseudarthrosis and Adjacent Segment Disease. Asian Spine J. 2017, 11, 463–471. [Google Scholar] [CrossRef]
- Früh, A.; Leißa, P.; Tkatschenko, D.; Truckenmüller, P.; Wessels, L.; Vajkoczy, P.; Bayerl, S. Decompression with or without fusion in degenerative adjacent segment stenosis after lumbar fusions. Neurosurg. Rev. 2022, 45, 3739–3748. [Google Scholar] [CrossRef]
- Shimamura, Y.; Kanayama, M.; Oha, F.; Tsujimoto, T.; Takana, M.; Hasegawa, Y.; Endo, T.; Hashimoto, T. Pre-existing adjacent level foraminal stenosis does not affect the outcome of a single level lumbar interbody fusion. J. Orthop. Sci. 2023, 28, 719–723. [Google Scholar] [CrossRef]
- Lapp, M.A.; Bridwell, K.H.; Lenke, L.G.; Daniel Riew, K.; Linville, D.A.; Eck, K.R.; Ungacta, F.F. Long-term complications in adult spinal deformity patients having combined surgery a comparison of primary to revision patients. Spine 2001, 26, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cammisa, F.P., Jr.; Sandhu, H.S.; Girardi, F.P.; Khan, S.N. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine 2002, 27, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Basques, B.A.; Ibe, I.; Samuel, A.M.; Lukasiewicz, A.M.; Webb, M.L.; Bohl, D.D.; Grauer, J.N. Predicting Postoperative Morbidity and Readmission for Revision Posterior Lumbar Fusion. Clin. Spine Surg. 2017, 30, E770–E775. [Google Scholar] [CrossRef] [PubMed]
- Miscusi, M.; Trungu, S.; Ricciardi, L.; Forcato, S.; Piazza, A.; Ramieri, A.; Raco, A. Stand-Alone Oblique Lumbar Interbody Fusion (OLIF) for the Treatment of Adjacent Segment Disease (ASD) after Previous Posterior Lumbar Fusion: Clinical and Radiological Outcomes and Comparison with Posterior Revision Surgery. J. Clin. Med. 2023, 12, 2985. [Google Scholar] [CrossRef]
- Garrido, E.; Connaughton, P.N. Unilateral facetectomy approach for lateral lumbar disc herniation. J. Neurosurg. 1991, 74, 754–756. [Google Scholar] [CrossRef]
- Kunogi, J.; Hasue, M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine 1991, 16, 1312–1320. [Google Scholar] [CrossRef]
- Chang, S.B.; Lee, S.H.; Ahn, Y.; Kim, J.M. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine 2006, 31, 1163–1167. [Google Scholar] [CrossRef]
- Knight, M.; Goswami, A. Management of isthmic spondylolisthesis with posterolateral endoscopic foraminal decompression. Spine 2003, 28, 573–581. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.H.; Park, W.M.; Lee, H.Y. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J. Neurosurg. 2003, 99 (Suppl. 3), 320–323. [Google Scholar] [CrossRef]
- Yeung, A.T. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon’s Experience Incorporating Adjunctive Techologies. SAS J. 2007, 1, 108–117. [Google Scholar] [CrossRef]
- Yeung, A.; Gore, S. Endoscopic foraminal decompression for failed back surgery syndrome under local anesthesia. Int. J. Spine Surg. 2014, 8, 22. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.K.; Kim, H.; Lee, S.H.; Lee, H.N. Percutaneous endoscopic lumbar foraminotomy: An advanced surgical technique and clinical outcomes. Neurosurgery 2014, 75, 124–133. [Google Scholar] [CrossRef]
- Ahn, Y.; Park, H.B.; Yoo, B.R.; Jeong, T.S. Endoscopic lumbar foraminotomy for foraminal stenosis in stable spondylolisthesis. Front. Surg. 2022, 9, 1042184. [Google Scholar] [CrossRef]
- Telfeian, A.E.; Jasper, G.P.; Francisco, G.M. Transforaminal endoscopic treatment of lumbar radiculopathy after instrumented lumbar spine fusion. Pain Physician 2015, 18, 179–184. [Google Scholar] [CrossRef]
- Wu, J.J.; Chen, H.Z.; Zheng, C. Transforaminal Percutaneous Endoscopic Discectomy and Foraminoplasty after Lumbar Spinal Fusion Surgery. Pain Physician 2017, 20, E647–E651. [Google Scholar]
- Yamashita, K.; Higashino, K.; Sakai, T.; Takata, Y.; Hayashi, F.; Tezuka, F.; Morimoto, M.; Chikawa, T.; Nagamachi, A.; Sairyo, K. Percutaneous full endoscopic lumbar foraminoplasty for adjacent level foraminal stenosis following vertebral intersegmental fusion in an awake and aware patient under local anesthesia: A case report. J. Med. Investig. 2017, 64, 291–295. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Yeom, J.S.; Kim, K.J.; Kim, H.J.; Chung, S.K.; Kang, H.S. A practical MRI grading system for lumbar foraminal stenosis. Am. J. Roentgenol. 2010, 194, 1095–1098. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.S.; Lee, S.Y.; Park, N.H.; Rho, M.H.; Hong, H.P.; Kwag, H.J.; Kook, S.H.; Choi, S.H. Clinical correlation of a new MR imaging method for assessing lumbar foraminal stenosis. Am. J. Neuroradiol. 2012, 33, 818–822. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.H.; Lee, H.Y.; Lee, H.J.; Chang, S.B.; Chung, S.K.; Kim, H.J. Validation of the Korean version of the oswestry disability index. Spine 2005, 30, E123–E127. [Google Scholar] [CrossRef]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Ashton-James, C.E.; Skorpil, N.E.; Heymans, M.W.; Forouzanfar, T. What constitutes a clinically important pain reduction in patients after third molar surgery? Pain Res. Manag. 2013, 18, 319–322. [Google Scholar] [CrossRef]
- Ng, L.C.; Tafazal, S.; Sell, P. The effect of duration of symptoms on standard outcome measures in the surgical treatment of spinal stenosis. Eur. Spine J. 2007, 16, 199–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Keum, H.J.; Son, S. Percutaneous Endoscopic Lumbar Foraminotomy for Foraminal Stenosis with Postlaminectomy Syndrome in Geriatric Patients. World Neurosurg. 2019, 130, e1070–e1076. [Google Scholar] [CrossRef]
- Hussain, M.; Berger, M.; Eckenhoff, R.G.; Seitz, D.P. General anesthetic and the risk of dementia in elderly patients: Current insights. Clin. Interv. Aging 2014, 9, 1619–1628. [Google Scholar] [CrossRef][Green Version]
- Zorrilla-Vaca, A.; Healy, R.J.; Mirski, M.A. A Comparison of Regional Versus General Anesthesia for Lumbar Spine Surgery: A Meta-Analysis of Randomized Studies. J. Neurosurg. Anesthesiol. 2017, 29, 415–425. [Google Scholar] [CrossRef]
- Finsterwald, M.; Muster, M.; Farshad, M.; Saporito, A.; Brada, M.; Aguirre, J.A. Spinal versus general anesthesia for lumbar spine surgery in high risk patients: Perioperative hemodynamic stability, complications and costs. J. Clin. Anesth. 2018, 46, 3–7. [Google Scholar] [CrossRef]
- Knight, M.T.; Vajda, A.; Jakab, G.V.; Awan, S. Endoscopic laser foraminoplasty on the lumbar spine—Early experience. Minim. Invasive Neurosurg. 1998, 41, 5–9. [Google Scholar] [CrossRef]
- Schubert, M.; Hoogland, T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper. Orthop. Traumatol. 2005, 17, 641–661. [Google Scholar] [CrossRef]

| Variables (n = 22) | No. | % |
|---|---|---|
| Sex | ||
| Male | 10 | 45.5 |
| Female | 12 | 54.5 |
| Symptom-free period (primary fusion—radicular symptom) | 50.32 ± 20.45 (mo) | |
| Symptom duration (radicular symptom—endoscopic procedure) | 12.64 ± 7.77 (mo) | |
| Previous fusion level | ||
| L3-4 | 3 | 13.6 |
| L4-5 | 12 | 54.5 |
| L5-S1 | 5 | 22.7 |
| L3-4-5 | 1 | 4.5 |
| L4-5-S1 | 1 | 4.5 |
| Direction of adjacent disease | ||
| Upper | 13 | 59.1 |
| Lower | 9 | 40.9 |
| Operative level | ||
| L2-3 | 1 | 4.5 |
| L3-4 | 7 | 31.8 |
| L4-5 | 8 | 36.4 |
| L5-S1 | 6 | 27.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Park, H.-B. Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis. J. Clin. Med. 2023, 12, 5745. https://doi.org/10.3390/jcm12175745
Ahn Y, Park H-B. Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis. Journal of Clinical Medicine. 2023; 12(17):5745. https://doi.org/10.3390/jcm12175745
Chicago/Turabian StyleAhn, Yong, and Han-Byeol Park. 2023. "Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis" Journal of Clinical Medicine 12, no. 17: 5745. https://doi.org/10.3390/jcm12175745
APA StyleAhn, Y., & Park, H.-B. (2023). Transforaminal Endoscopic Lumbar Foraminotomy for Juxta-Fusional Foraminal Stenosis. Journal of Clinical Medicine, 12(17), 5745. https://doi.org/10.3390/jcm12175745






