Can Non-Invasive Spectrophotometric Hemoglobin Replace Laboratory Hemoglobin Concentrations for Preoperative Anemia Screening? A Diagnostic Test Accuracy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Hemoglobin, Perfusion Index, and Pleth Variability Index Measurements
2.3. Statistical Analysis
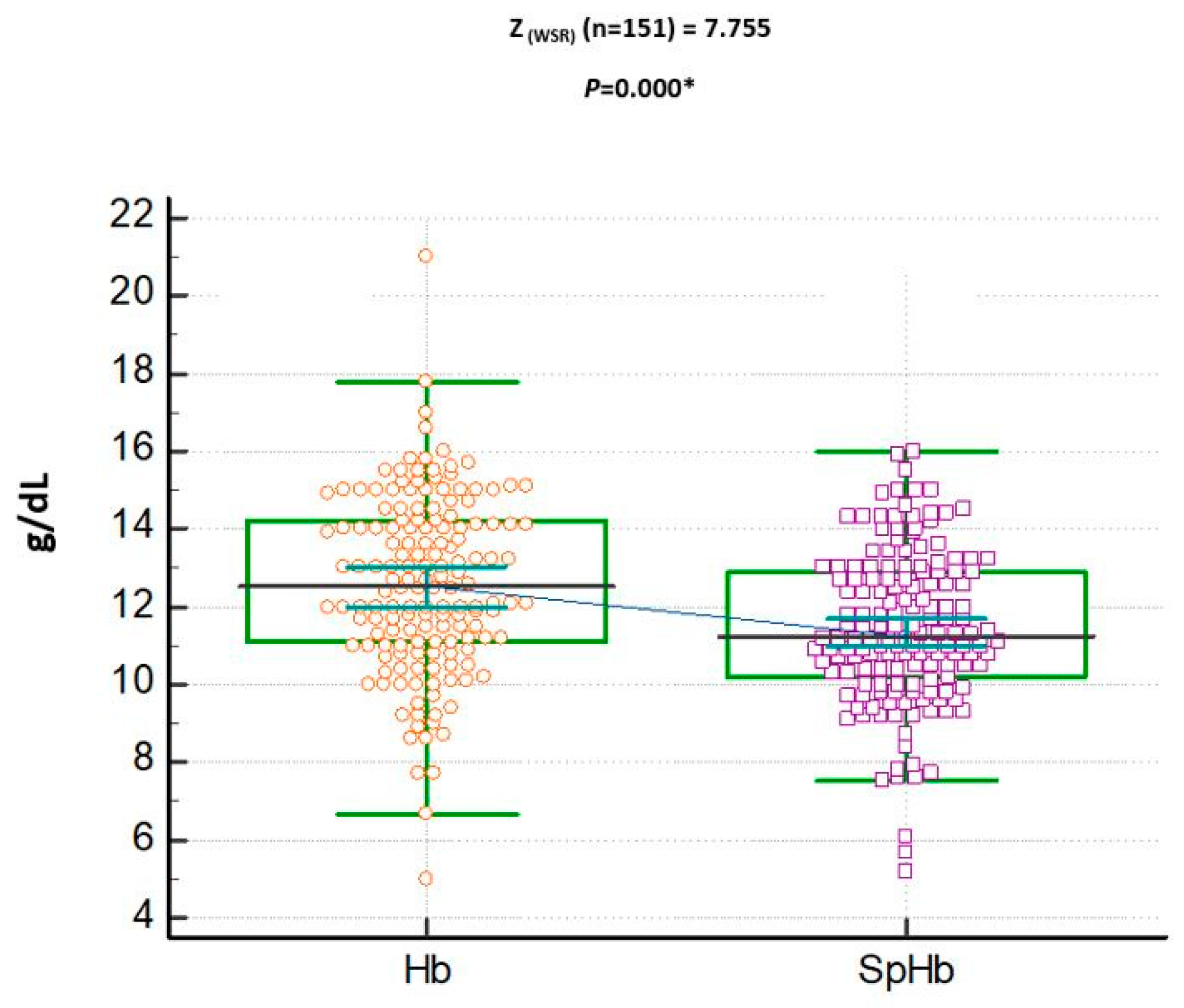
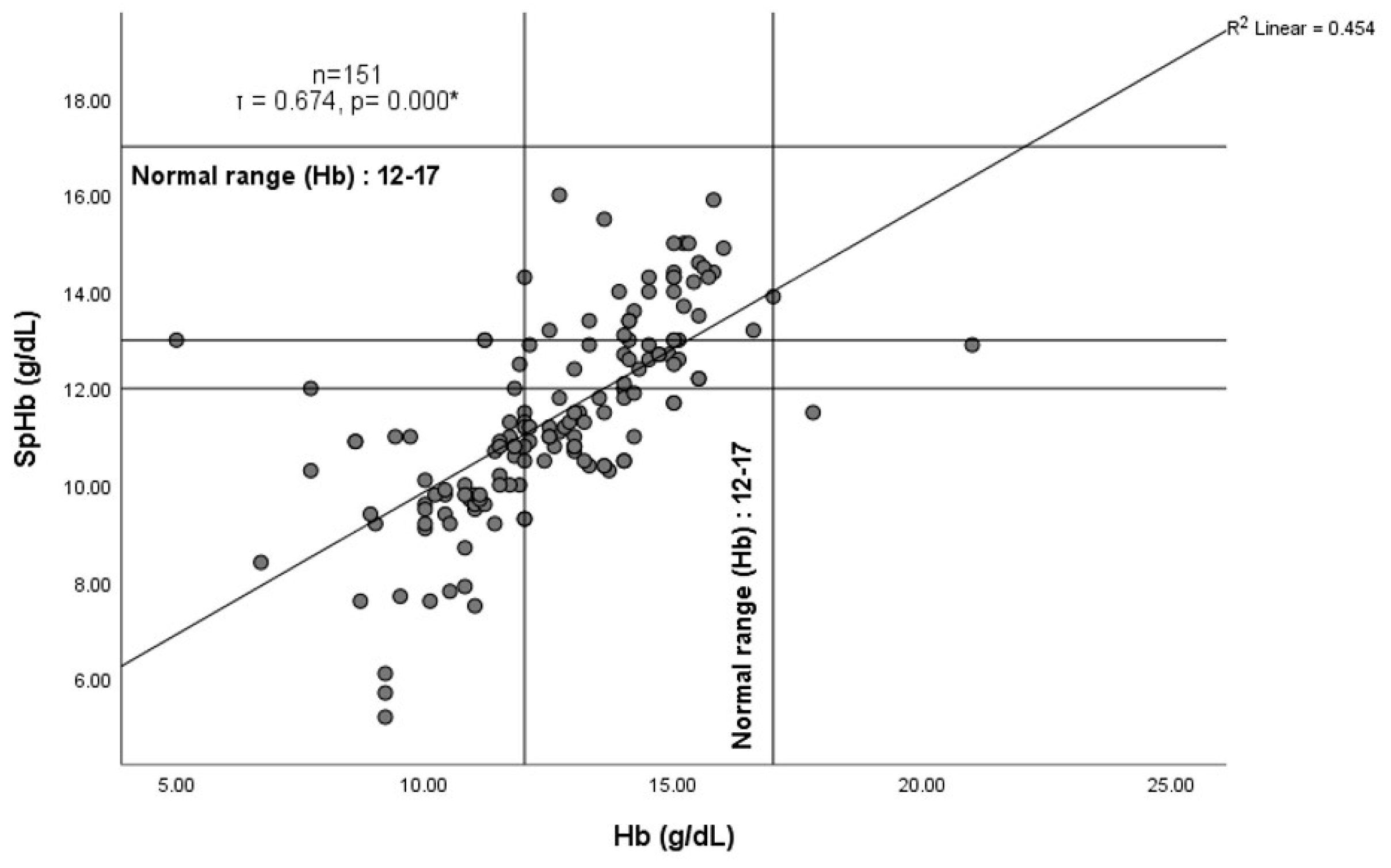
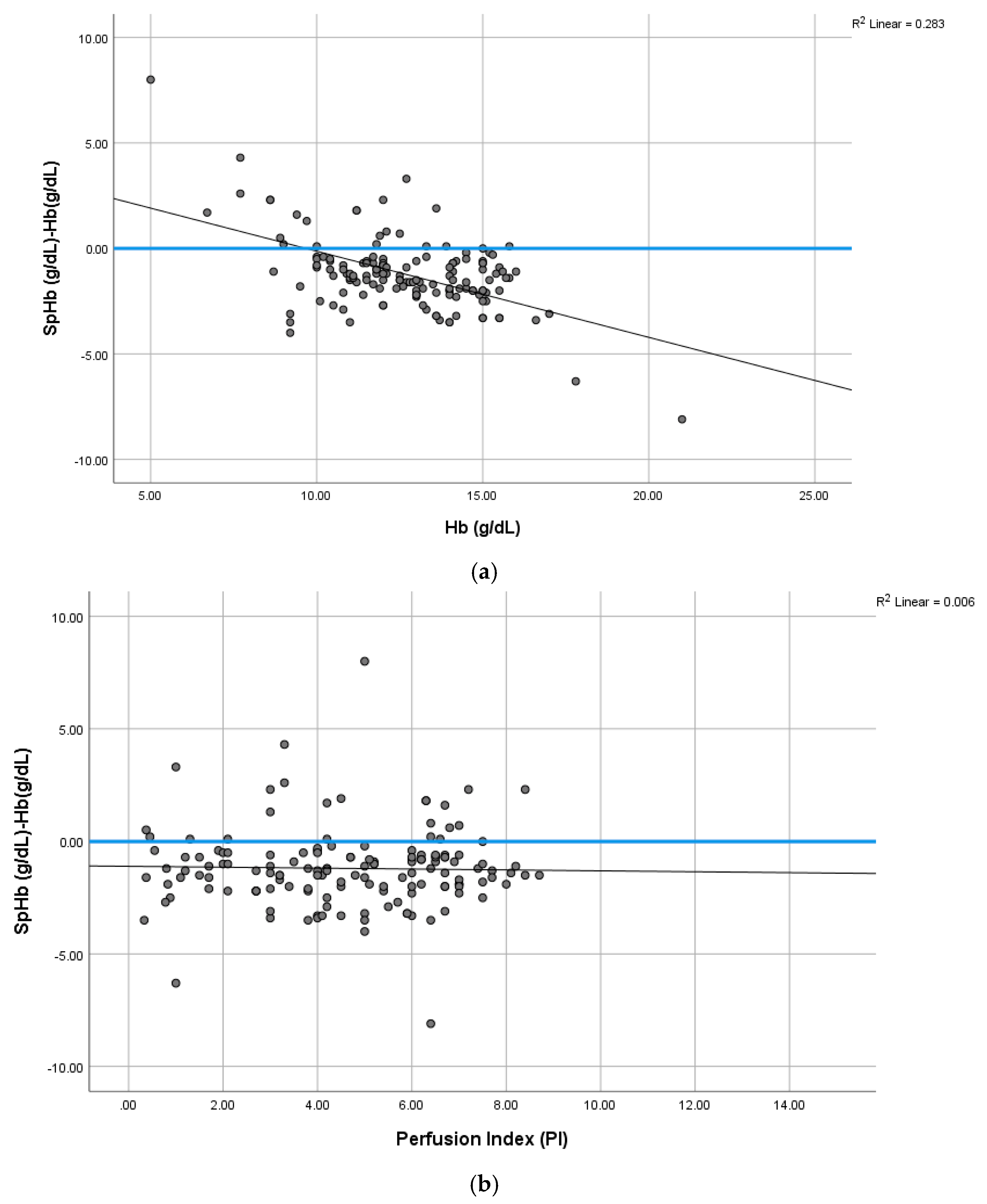
3. Results
4. Discussion
4.1. SpHb Agreement with CBC Total Hemoglobin
4.2. Total Hemoglobin (Hb) and SpHb–Hb Relationship
4.3. SpHb Monitoring and Anemia Screening
4.4. PI Effect on SpHb
4.5. Oxygen Effect on SpHb
5. Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, M.M.; Van Remoortel, H.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R.; Carson, J.L.; Cichutek, K.; De Buck, E.; Devine, D.; et al. Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. J. Am. Med. Assoc. 2019, 321, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Tamim, H.M.; Richards, T.; Spahn, D.R.; Rosendaal, F.R.; Habbal, A.; Khreiss, M.; Dahdaleh, F.S.; Khavandi, K.; Sfeir, P.M.; et al. Preoperative anemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet 2011, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.M.; Hochrieser, H.; Posch, M.; Metnitz, B.; Rhodes, A.; Moreno, R.P.; Pearse, R.M.; Metnitz, P. Preoperative anemia is associated with poor clinical outcome in non-cardiac surgery patients. Br. J. Anaesth. 2014, 113, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.D.; Brown, J.P.; Corwin, H.L.; Gross, I.; Ozawa, S.J.; Shander, A. Special report from the society for the Advancement of Blood Management: The choosing wisely campaign. Anesth. Analg. 2019, 129, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Gomez-Ramirez, S.; Kozek-Langeneker, S.; Shander, A.; Richards, T.; Pavía, J.; Kehlet, H.; Acheson, A.G.; Evans, C.; Raobaikady, R.; et al. ’Fit to fly’: Overcoming barriers to preoperative hemoglobin optimization in surgical patients. Br. J. Anaesth. 2015, 115, 15–24. [Google Scholar] [CrossRef]
- Qadri, M.I.; Islam, S.A. Hemoglobin H disease in the eastern region of Saudi Arabia. Saudi Med. J. 2000, 21, 666–671. [Google Scholar]
- Alzahrani, M.; Felimban, R.; Alzahrani, F.; Qadah, T. Hemoglobin Disorders Among Anemic Patients: A Cross-Sectional Study from Jeddah City, Western Saudi Arabia. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, E.S.; Farhat, G.N.; Assiri, A.M.; Memish, Z.; Ahmed, E.M.; Saeedi, M.Y.; Al-Dossary, M.F.; Bashawri, H. Distribution of hemoglobinopathy disorders in Saudi Arabia based on data from the premarital screening and genetic counseling program, 2011–2015. J. Epidemiol. Glob Health 2018, 7 (Suppl. S1), S41–S47. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Chilvers, C.R.; Thomas, M.; Chilvers, C.M.; Sexton, M.; Vialle, M.; Robertson, I.K. Usefulness of noninvasive spectrophotometric hemoglobin estimation for detecting low hemoglobin levels when compared with a standard laboratory assay for preoperative assessment. Br. J. Anaesth. 2015, 114, 669–676. [Google Scholar] [CrossRef]
- Honnef, G.; Auinger, D.; Eichinger, M.; Eichlseder, M.; Metnitz, P.G.H.; Rief, M.; Zajic, P.; Zoidl, P.; Bornemann-Cimenti, H. Evaluation of the usefulness of non-invasive serum hemoglobin measurement in a perioperative setting in a prospective observational study. Sci. Rep. 2022, 12, 9065. [Google Scholar] [CrossRef]
- Wittenmeier, E.; Paumen, Y.; Mildenberger, P.; Smetiprach, J.; Pirlich, N.; Griemert, E.V.; Kriege, M.; Engelhard, K. Non-invasive hemoglobin measurement as an index test to detect pre-operative anemia in elective surgery patients—A prospective study. Anaesthesia 2021, 76, 647–654. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, Y.H.; Kim, J.T. Current Use of Noninvasive Hemoglobin Monitoring in Anesthesia. Curr. Anesthesiol. Rep. 2014, 4, 233–241. [Google Scholar] [CrossRef][Green Version]
- Barker, S.J.; Badal, J.J. The measurement of dyshemoglobins and total hemoglobin by pulse oximetry. Curr. Opin. Anaesthesiol. 2008, 21, 805–810. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change With Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Perel, A. Iatrogenic hemodilution: A possible cause for avoidable blood transfusions? Crit. Care 2017, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, D.; Rodriquez, D.; Petro, M.; Blakeman, T.C.; Branson, R.D. Impact of Oxygenation Status on the Noninvasive Measurement of Hemoglobin. Mil. Med. 2017, 182, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.: Armonk, NY, USA, 2012.
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE Publications Ltd.: London, CA, USA; New Delhi, India, 2013. [Google Scholar]
- Friedman, M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Kendall, M.G. A new measure of rank correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Koch, G.G. Intraclass correlation coefficient. In Encyclopedia of Statistical Sciences; Kotz, S., Johnson, N.L., Read, C.B., Eds.; John Wiley & Sons.: Hoboken, NJ, USA, 1982; pp. 213–217. [Google Scholar]
- Mantha, S.; Roizen, M.F.; Fleisher, L.A.; Thisted, R.; Foss, J. Comparing methods of clinical measurement: Reporting standards for bland and altman analysis. Anesth. Analg. 2000, 90, 593–602. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Okazaki, K.; Okazaki, K.; Uesugi, M.; Matsusima, T.; Hataya, H. Evaluation of the accuracy of a non-invasive hemoglobin-monitoring device in schoolchildren. Pediatr Neonatol. 2022, 63, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.J. Sample size calculation for an agreement study. Pharm. Stat. 2010, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Wilkins, E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010, 126, 619–625. [Google Scholar] [CrossRef]
- Nikolopoulou, K. What Is Convenience Sampling?|Definition & Examples. 2022. Available online: https://www.scribbr.com/methodology/convenience-sampling/ (accessed on 20 October 2022).
- Czempik, P.F.; Pluta, M.P.; Krzych, Ł.J. Hemoglobin Determination Using Pulse Co-Oximetry and Reduced-Volume Blood Gas Analysis in the Critically Ill: A Prospective Cohort Study. Diagnostics 2022, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Dahyot-Fizelier, C.; Catherine, K.; Levrat, Q.; Debaene, B.; Mimoz, O. Accuracy of a continuous noninvasive hemoglobin monitor in intensive care unit patients. Crit. Care Med. 2011, 39, 2277–2782. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-González, K.D.; Jacob, A.K.; Burt, J.M.; Higgins, A.A.; Engel, E.M.; Hanson, A.C.; Belch, L.; Kor, D.J.; Warner, M.A. Non-invasive hemoglobin estimation for preoperative anemia screening. Transfusion 2023, 63, 315–322. [Google Scholar] [CrossRef]
- Gayat, E.; Aulagnier, J.; Matthieu, E.; Boisson, M.; Fischler, M. Noninvasive measurement of hemoglobin: Assessment of two different point-of-care technologies. PLoS ONE 2012, 7, e30065. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Raines, K.; Jameel, F.; Sidi, M.; Oliveira-Streiff, S.; Nwajei, P.; McGlamry, K.; Ou, J.; Oladele, A.; Suchdev, P.S. Non-invasive hemoglobin measurement devices require refinement to match diagnostic performance with their high level of usability and acceptability. PLoS ONE 2021, 16, e0254629. [Google Scholar] [CrossRef]
- Avcioglu, G.; Nural, C.; Yilmaz, F.M.; Baran, P.; Erel, Ö.; Yilmaz, G. Comparison of noninvasive and invasive point-of-care testing methods with reference method for hemoglobin measurement. J. Clin. Lab. Anal. 2018, 32, e22309. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, J.H.; Song, H.G.; Byon, H.J.; Kim, H.S.; Kim, J.T. The accuracy of noninvasive hemoglobin monitoring using the radical-7 pulse CO-oximeter in children undergoing neurosurgery. Anesth. Analg. 2012, 115, 1302–1307. [Google Scholar] [CrossRef]
- Applegate, R.L., 2nd; Barr, S.J.; Collier, C.E.; Rook, J.L.; Mangus, D.B.; Allard, M.W. Evaluation of pulse cooximetry in patients undergoing abdominal or pelvic surgery. Anesthesiology 2012, 116, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.J.; Kalmar, A.F.; Struys, M.M.; Porte, R.J.; Wietasch, J.K.; Scheeren, T.W.; Hendriks, H.G. Accuracy of non-invasive measurement of haemoglobin concentration by pulse co-oximetry during steady-state and dynamic conditions in liver surgery. Br. J. Anaesth. 2012, 109, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.D.; Ward, T.A.; Shiboski, S.C.; Cohen, N.H. A comparison of three methods of hemoglobin monitoring in patients undergoing spine surgery. Anesth. Analg. 2011, 112, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Lee, O.H.; Park, Y.H.; Shin, H.Y.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C. The changes of non-invasive hemoglobin and perfusion index of Pulse CO-Oximetry during induction of general anesthesia. Korean J. Anesthesiol. 2015, 68, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lilot, M.; Murphy, L.S.; Sidhu, K.S.; Yu, Z.; Rinehart, J.; Cannesson, M. Accuracy of continuous noninvasive hemoglobin monitoring: A systematic review and meta-analysis. Anesth. Analg. 2014, 119, 332–346. [Google Scholar] [CrossRef]
- Gayat, E.; Bodin, A.; Fischler, M. Instability in non-invasive haemoglobin measurement: A possible influence of oxygen administration. Acta Anaesthesiol. Scand. 2011, 55, 902. [Google Scholar] [CrossRef] [PubMed]
- Gayat, E.; Imbert, N.; Roujansky, A.; Lemasle, L.; Fischler, M. Influence of Fraction of Inspired Oxygen on Noninvasive Hemoglobin Measurement: Parallel Assessment of 2 Monitors. Anesth. Analg. 2017, 124, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 2020, 383, 2477–2478, Erratum in N. Engl. J. Med. 2021, 385, 2496. [Google Scholar] [CrossRef]

| Variable | n (%) | ||
|---|---|---|---|
| Gender | Male | 96 (62.2) | |
| Female | 58 (38.4) | ||
| American Society of Anesthesiology (ASA) Classification | 1 | 55 (36.4) | |
| 2 | 65 (43.04) | ||
| 3 | 31 (20.53) | ||
| Median | Mean | SD | |
| Age (years) | 40 | 41.29 | 15.67 |
| BMI (kg/m2) | 28 | 28.50 | 5.09 |
| SpHb (g/dL) | 11.2 | 11.43 | 2.02 |
| Hb (g/dL) | 12.7 | 12.65 | 2.30 |
| PVI (%) | 13 | 13.40 | 5.77 |
| PI | 5 | 5.46 | 7.03 |
| Oxygen Saturation (%) | 99 | 98.81 | 1.15 |
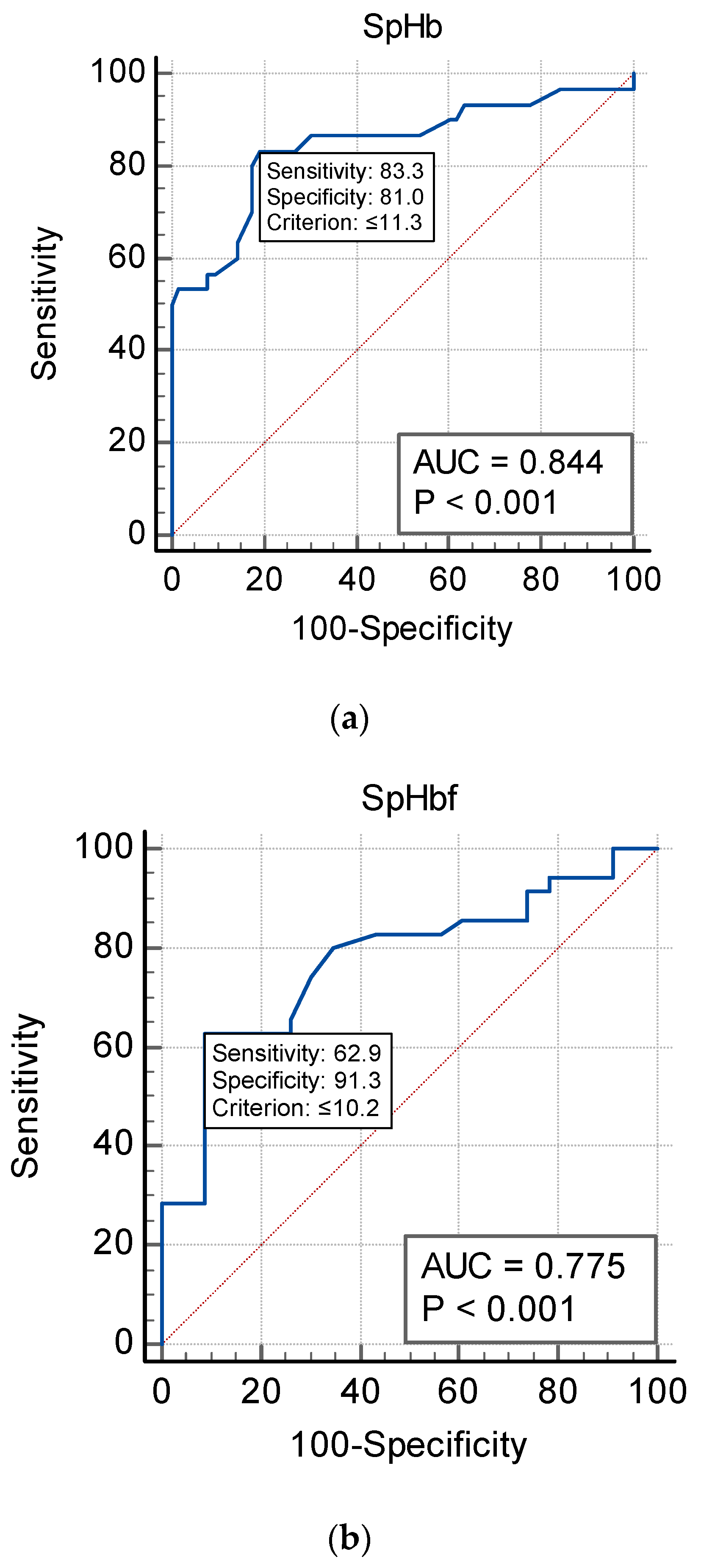
| Hb (g/dL) | SpHb | Sens | Spec | NPV | PPV | AUC |
|---|---|---|---|---|---|---|
| WHO (F) Hb < 12 | 10.2 | 62.9 | 91.3 | 68.4 | 73 | 0.775 |
| WHO (M) Hb < 13 | 11.3 | 83.3 | 81 | 73.9 | 76.1 | 0.844 |
| CBC (F + M) Hb < 12 | 12.9 | 62.9 | 52.2 | 76.8 | 51.5 | 0.54 |
| CBC (F + M) Hb < 13 | 11.3 | 83.3 | 81 | 84.3 | 78.9 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwabari, M.; Alhamad, F.; Alsahaf, F.; Al Amer, F.; Alniniya, F.; Alherz, I.; Omer, N.; Bushehab, A.; Yassen, K. Can Non-Invasive Spectrophotometric Hemoglobin Replace Laboratory Hemoglobin Concentrations for Preoperative Anemia Screening? A Diagnostic Test Accuracy Study. J. Clin. Med. 2023, 12, 5733. https://doi.org/10.3390/jcm12175733
Alwabari M, Alhamad F, Alsahaf F, Al Amer F, Alniniya F, Alherz I, Omer N, Bushehab A, Yassen K. Can Non-Invasive Spectrophotometric Hemoglobin Replace Laboratory Hemoglobin Concentrations for Preoperative Anemia Screening? A Diagnostic Test Accuracy Study. Journal of Clinical Medicine. 2023; 12(17):5733. https://doi.org/10.3390/jcm12175733
Chicago/Turabian StyleAlwabari, Maryam, Fatimah Alhamad, Fatimah Alsahaf, Fatima Al Amer, Fatma Alniniya, Imran Alherz, Nawal Omer, Abdulaziz Bushehab, and Khaled Yassen. 2023. "Can Non-Invasive Spectrophotometric Hemoglobin Replace Laboratory Hemoglobin Concentrations for Preoperative Anemia Screening? A Diagnostic Test Accuracy Study" Journal of Clinical Medicine 12, no. 17: 5733. https://doi.org/10.3390/jcm12175733
APA StyleAlwabari, M., Alhamad, F., Alsahaf, F., Al Amer, F., Alniniya, F., Alherz, I., Omer, N., Bushehab, A., & Yassen, K. (2023). Can Non-Invasive Spectrophotometric Hemoglobin Replace Laboratory Hemoglobin Concentrations for Preoperative Anemia Screening? A Diagnostic Test Accuracy Study. Journal of Clinical Medicine, 12(17), 5733. https://doi.org/10.3390/jcm12175733