Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
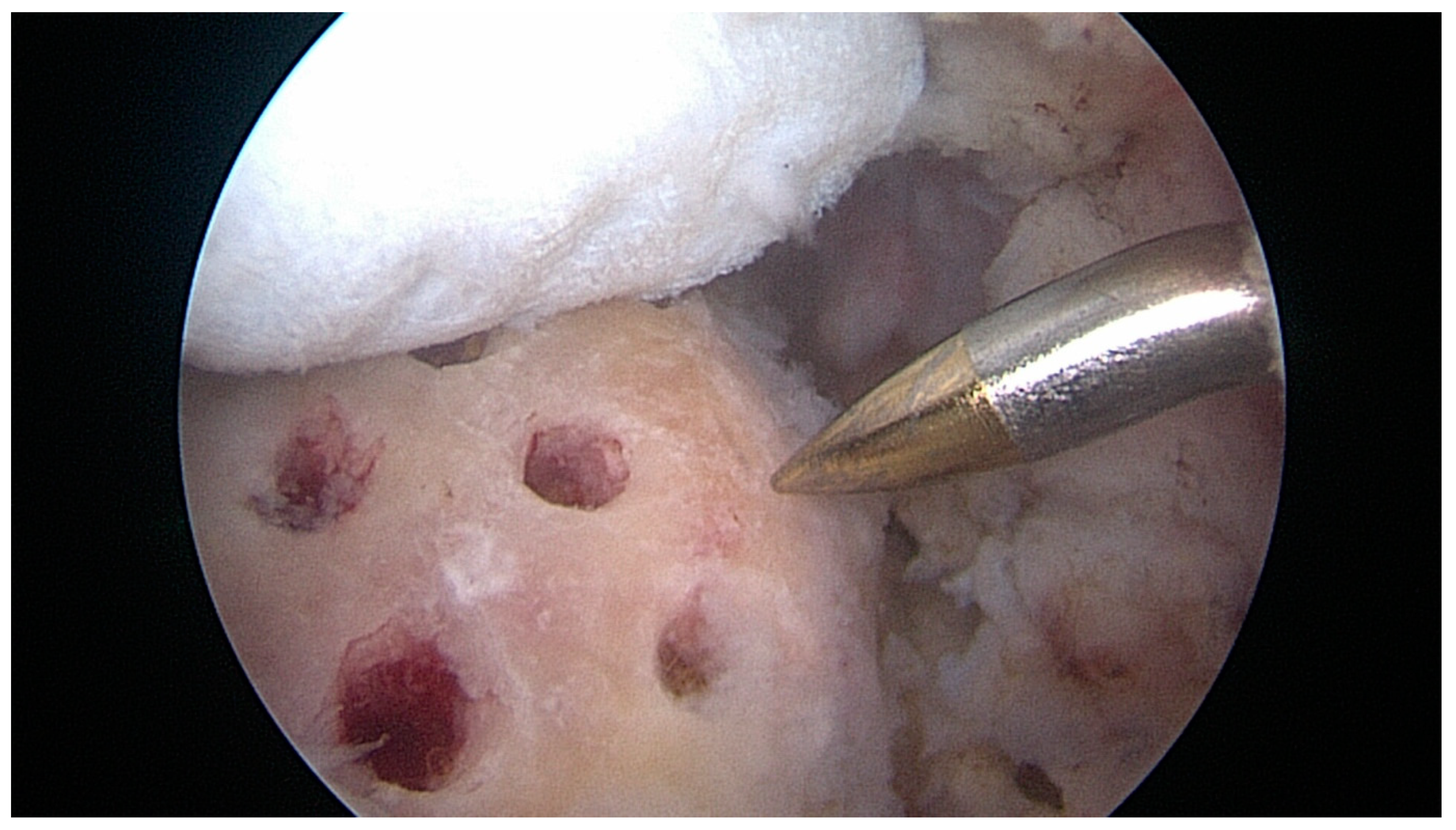
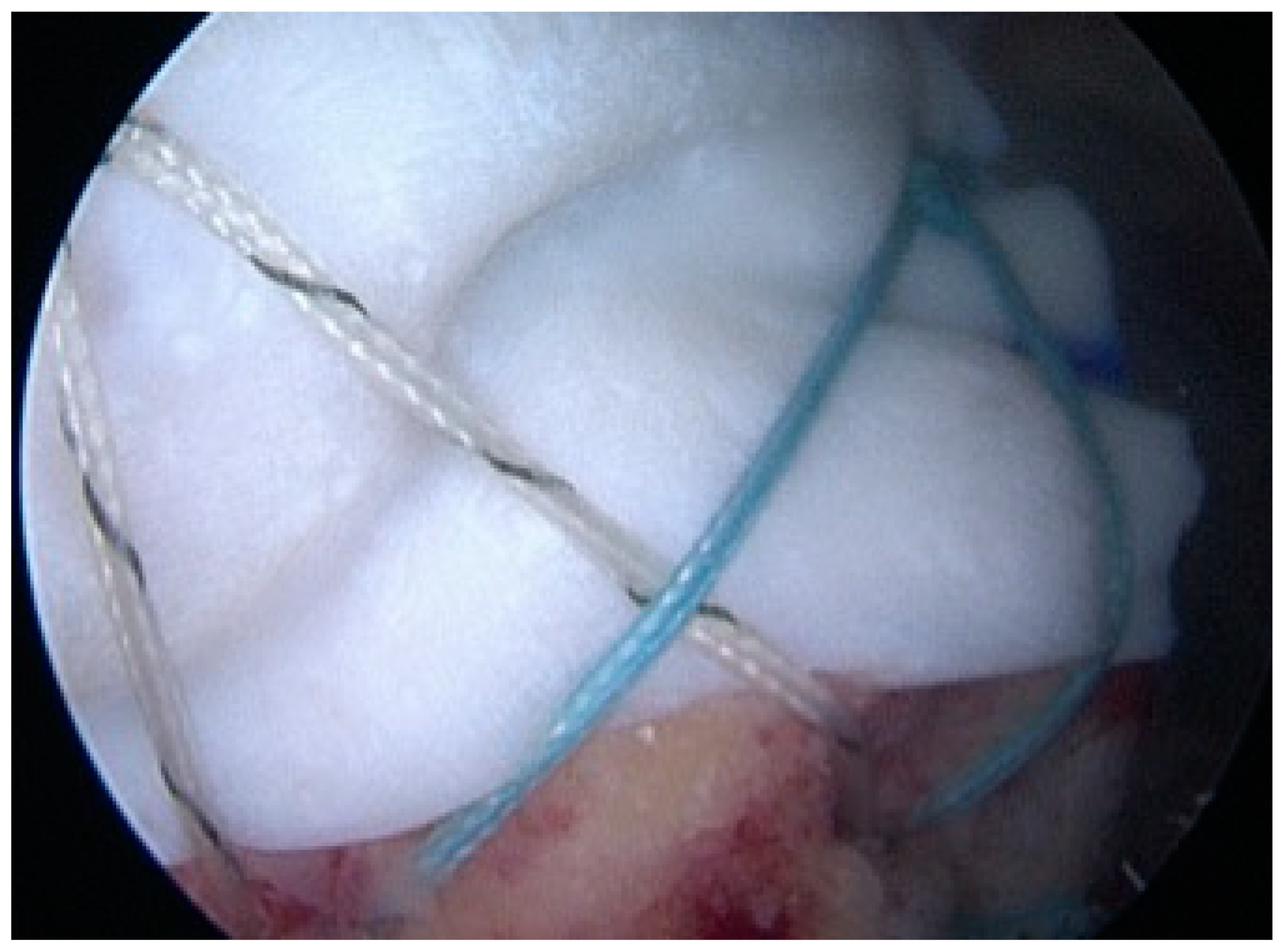
2.3. Treatment
2.4. Outcome Measurements
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, B.Y.; Diaz, M.; Binkley, M.; McFarland, E.G.; Srikumaran, U. Biomechanical Strength of Rotator Cuff Repairs: A Systematic Review and Meta-Regression Analysis of Cadaveric Studies. Am. J. Sports Med. 2019, 47, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.E.; Figueroa, N.M.; Gilot, G.J. Biomechanical Analysis of Rotator Cuff Repairs with Extracellular Matrix Graft Augmentation. Orthopedics 2014, 37, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Omae, H.; Steinmann, S.P.; Zhao, C.; Zobitz, M.E.; Wongtriratanachai, P.; Sperling, J.W.; An, K.-N. Biomechanical Effect of Rotator Cuff Augmentation with an Acellular Dermal Matrix Graft: A Cadaver Study. Clin. Biomech. Bristol. Avon. 2012, 27, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Ang, S.; Rushton, N.; Harvey, A.; Akhtar, K.; Dawson-Bowling, S.; Noorani, A. Platelet-Rich Plasma Augmentation of Arthroscopic Rotator Cuff Repair Lowers Retear Rates and Improves Short-Term Postoperative Functional Outcome Scores: A Systematic Review of Meta-Analyses. Arthrosc. Sports Med. Rehabil. 2022, 4, e823–e833. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, P.N.; Tashjian, R.Z. Patch Augmentation in Rotator Cuff Repair. Curr. Rev. Musculoskelet. Med. 2020, 13, 561–571. [Google Scholar] [CrossRef]
- Ajrawat, P.; Dwyer, T.; Almasri, M.; Veillette, C.; Romeo, A.; Leroux, T.; Theodoropoulos, J.; Nauth, A.; Henry, P.; Chahal, J. Bone Marrow Stimulation Decreases Retear Rates after Primary Arthroscopic Rotator Cuff Repair: A Systematic Review and Meta-Analysis. J. Shoulder Elbow Surg. 2019, 28, 782–791. [Google Scholar] [CrossRef]
- Peng, Y.; Du, L.; Yang, B.; Fan, D.; Jia, S.; Zheng, C. Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Systematic Review and Meta-Analysis. PM R 2023. [Google Scholar] [CrossRef]
- Sánchez-Losilla, C.; Ferré-Aniorte, A.; Álvarez-Díaz, P.; Barastegui-Fernández, D.; Cugat, R.; Alentorn-Geli, E. Efficacy of Platelet-Rich Plasma in Rotator Cuff Repair: Systematic Review and Meta-Analysis. Rev. Espanola Cirugia Ortop. Traumatol. 2023. [Google Scholar] [CrossRef]
- Muench, L.N.; Kia, C.; Berthold, D.P.; Uyeki, C.; Otto, A.; Cote, M.P.; McCarthy, M.B.; Beitzel, K.; Arciero, R.A.; Mazzocca, A.D. Preliminary Clinical Outcomes Following Biologic Augmentation of Arthroscopic Rotator Cuff Repair Using Subacromial Bursa, Concentrated Bone Marrow Aspirate, and Platelet-Rich Plasma. Arthrosc. Sports Med. Rehabil. 2020, 2, e803–e813. [Google Scholar] [CrossRef]
- Berthold, D.P.; Garvin, P.; Mancini, M.R.; Uyeki, C.L.; LeVasseur, M.R.; Mazzocca, A.D.; Voss, A. Arthroscopic Rotator Cuff Repair with Biologically Enhanced Patch Augmentation. Oper. Orthopadie Traumatol. 2022, 34, 4–12. [Google Scholar] [CrossRef]
- Voss, A.; McCarthy, M.B.; Bellas, N.; Kellner, R.; Beitzel, K.; Dyrna, F.; Imhoff, A.B.; Mazzocca, A.D.; Muench, L.N.; Berthold, D.P. Significant Improvement in Shoulder Function and Pain in Patients Following Biologic Augmentation of Revision Arthroscopic Rotator Cuff Repair Using an Autologous Fibrin Scaffold and Bone Marrow Aspirate Derived From the Proximal Humerus. Arthrosc. Sports Med. Rehabil. 2021, 3, e1819–e1825. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, H.; Maeda, K.; Matsuki, K.; Moriishi, J. Functional and Structural Outcome after Arthroscopic Full-Thickness Rotator Cuff Repair: Single-Row versus Dual-Row Fixation. Arthroscopy 2005, 21, 1307–1316. [Google Scholar] [CrossRef]
- Hamada, K.; Fukuda, H.; Mikasa, M.; Kobayashi, Y. Roentgenographic Findings in Massive Rotator Cuff Tears. A Long-Term Observation. Clin. Orthop. 1990, 254, 92–96. [Google Scholar] [CrossRef]
- Snow, M.; Hussain, F.; Pagkalos, J.; Kowalski, T.; Green, M.; Massoud, S.; James, S. The Effect of Delayed Injection of Leukocyte-Rich Platelet-Rich Plasma Following Rotator Cuff Repair on Patient Function: A Randomized Double-Blind Controlled Trial. Arthroscopy 2020, 36, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Murley, A.H. A Clinical Method of Functional Assessment of the Shoulder. Clin. Orthop. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Yian, E.H.; Ramappa, A.J.; Arneberg, O.; Gerber, C. The Constant Score in Normal Shoulders. J. Shoulder Elbow Surg. 2005, 14, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Padua, R.; Padua, L.; Ceccarelli, E.; Romanini, E.; Zanoli, G.; Amadio, P.C.; Campi, A. Italian Version of the Disability of the Arm, Shoulder and Hand (DASH) Questionnaire. Cross-Cultural Adaptation and Validation. J. Hand Surg. Edinb. Scotl. 2003, 28, 179–186. [Google Scholar] [CrossRef]
- Neyton, L.; Godenèche, A.; Nové-Josserand, L.; Carrillon, Y.; Cléchet, J.; Hardy, M.B. Arthroscopic Suture-Bridge Repair for Small to Medium Size Supraspinatus Tear: Healing Rate and Retear Pattern. Arthroscopy 2013, 29, 10–17. [Google Scholar] [CrossRef]
- Piasecki, D.P.; Verma, N.N.; Nho, S.J.; Bhatia, S.; Boniquit, N.; Cole, B.J.; Nicholson, G.P.; Romeo, A.A. Outcomes after Arthroscopic Revision Rotator Cuff Repair. Am. J. Sports Med. 2010, 38, 40–46. [Google Scholar] [CrossRef]
- Kukkonen, J.; Kauko, T.; Vahlberg, T.; Joukainen, A.; Aärimaa, V. Investigating Minimal Clinically Important Difference for Constant Score in Patients Undergoing Rotator Cuff Surgery. J. Shoulder Elbow Surg. 2013, 22, 1650–1655. [Google Scholar] [CrossRef]
- Cvetanovich, G.L.; Gowd, A.K.; Liu, J.N.; Nwachukwu, B.U.; Cabarcas, B.C.; Cole, B.J.; Forsythe, B.; Romeo, A.A.; Verma, N.N. Establishing Clinically Significant Outcome after Arthroscopic Rotator Cuff Repair. J. Shoulder Elbow Surg. 2019, 28, 939–948. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, A.L.L.; Garcia, T.A.; Brandão, H.d.S.; Sardeli, A.V.; Mouraria, G.G.; Belangero, W.D. Benefits of Patch Augmentation on Rotator Cuff Repair: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2022, 10, 23259671211071144. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, G.-H.; Cha, D.-H.; Lee, J.-H.; Lee, Y.-B. A Comparison of Clinical Outcomes in Rotator Cuff Re-Tear Patients Who Had Either an Arthroscopic Primary Repair or Arthroscopic Patch Augmentation for Large-to-Massive Rotator Cuff Tears. Diagn. Basel Switz. 2023, 13, 1961. [Google Scholar] [CrossRef]
- Sclamberg, S.G.; Tibone, J.E.; Itamura, J.M.; Kasraeian, S. Six-Month Magnetic Resonance Imaging Follow-up of Large and Massive Rotator Cuff Repairs Reinforced with Porcine Small Intestinal Submucosa. J. Shoulder Elbow Surg. 2004, 13, 538–541. [Google Scholar] [CrossRef]
- Gupta, A.K.; Hug, K.; Berkoff, D.J.; Boggess, B.R.; Gavigan, M.; Malley, P.C.; Toth, A.P. Dermal Tissue Allograft for the Repair of Massive Irreparable Rotator Cuff Tears. Am. J. Sports Med. 2012, 40, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Consigliere, P.; Bernasconi, A.; Dimock, R.; Narvani, A.A. Clinical Outcomes and Structural Integrity Rate of Arthroscopic Augmented Rotator Cuff Repairs Using Extracellular Porcine Matrix Patch. Shoulder Elb. 2022, 14, 38–51. [Google Scholar] [CrossRef]
- Karuppaiah, K.; Sinha, J. Scaffolds in the Management of Massive Rotator Cuff Tears: Current Concepts and Literature Review. EFORT Open Rev. 2019, 4, 557–566. [Google Scholar] [CrossRef]
- Quigley, R.; Verma, N.; Evuarherhe, A.; Cole, B.J. Rotator Cuff Repair with Graft Augmentation Improves Function, Decreases Revisions, and Is Cost-Effective. Arthroscopy 2022, 38, 2166–2174. [Google Scholar] [CrossRef]
- Milano, G.; Saccomanno, M.F.; Careri, S.; Taccardo, G.; De Vitis, R.; Fabbriciani, C. Efficacy of Marrow-Stimulating Technique in Arthroscopic Rotator Cuff Repair: A Prospective Randomized Study. Arthroscopy 2013, 29, 802–810. [Google Scholar] [CrossRef]
- Ruiz Ibán, M.A.; Sanchez Alepuz, E.; Diaz Heredia, J.; Hachem, A.-I.; Ezagüi Bentolila, L.; Calvo, A.; Verdú, C.; de Rus Aznar, I.; Soler Romagosa, F. Footprint Preparation with Nanofractures in a Supraspinatus Repair Cuts in Half the Retear Rate at 1-Year Follow-up. A Randomized Controlled Trial. Knee Surg. Sports Traumatol. 2021, 29, 2249–2256. [Google Scholar] [CrossRef]
- Wang, A.; McCann, P.; Colliver, J.; Koh, E.; Ackland, T.; Joss, B.; Zheng, M.; Breidahl, B. Do Postoperative Platelet-Rich Plasma Injections Accelerate Early Tendon Healing and Functional Recovery after Arthroscopic Supraspinatus Repair? A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Fu, S.C.; Qin, L.; Rolf, C.; Chan, K.M. Supplementation-Time Dependence of Growth Factors in Promoting Tendon Healing. Clin. Orthop. 2006, 448, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Morihara, T.; Sakamoto, H.; Matsuda, K.; Oshima, Y.; Yoshida, A.; Nagae, M.; Arai, Y.; Kawata, M.; Kubo, T. Platelet-Rich Plasma Enhances the Initial Mobilization of Circulation-Derived Cells for Tendon Healing. J. Cell. Physiol. 2008, 215, 837–845. [Google Scholar] [CrossRef]
- Galatz, L.M.; Sandell, L.J.; Rothermich, S.Y.; Das, R.; Mastny, A.; Havlioglu, N.; Silva, M.J.; Thomopoulos, S. Characteristics of the Rat Supraspinatus Tendon during Tendon-to-Bone Healing after Acute Injury. J. Orthop. Res. 2006, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.; Van der Meijden, O.; Pireau, N.; Chelli, M.; Gonzalez, J.-F.; Boileau, P. Arthroscopic Revision Cuff Repair: Do Tendons Have a Second Chance to Heal? J. Shoulder Elbow Surg. 2022, 31, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Shim, S.B.; Kim, W.J.; Yoo, J.C. Preoperative Rotator Cuff Tendon Integrity, Tear Size, and Muscle Atrophy and Fatty Infiltration Are Associated with Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2029–2038. [Google Scholar] [CrossRef]
- Skoff, H. Follow-up on a Biological Revision Technique for Large, Recurrent, Retracted, Rotator Cuff Tears. J. Am. Acad. Orthop. Surg. 2022, 30, e487–e494. [Google Scholar] [CrossRef]
- Lacheta, L.; Siebenlist, S.; Scheiderer, B.; Beitzel, K.; Woertler, K.; Imhoff, A.B.; Buchmann, S.; Willinger, L. Intact Revision Rotator Cuff Repair Stabilizes Muscle Atrophy and Fatty Infiltration after Minimum Follow up of Two Years. BMC Musculoskelet. Disord. 2023, 24, 515. [Google Scholar] [CrossRef]
- Milano, G.; Marchi, G.; Bertoni, G.; Vaisitti, N.; Galli, S.; Scaini, A.; Saccomanno, M.F. Augmented Repair of Large to Massive Delaminated Rotator Cuff Tears with Autologous Long Head of the Biceps Tendon Graft: The Arthroscopic “Cuff-Plus” Technique. Arthrosc. Tech. 2020, 9, e1683–e1688. [Google Scholar] [CrossRef]
- Gyftopoulos, S.; Cardoso, M.D.S.; Rodrigues, T.C.; Qian, K.; Chang, C.Y. Postoperative Imaging of the Rotator Cuff: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2022, 219, 717–723. [Google Scholar] [CrossRef]
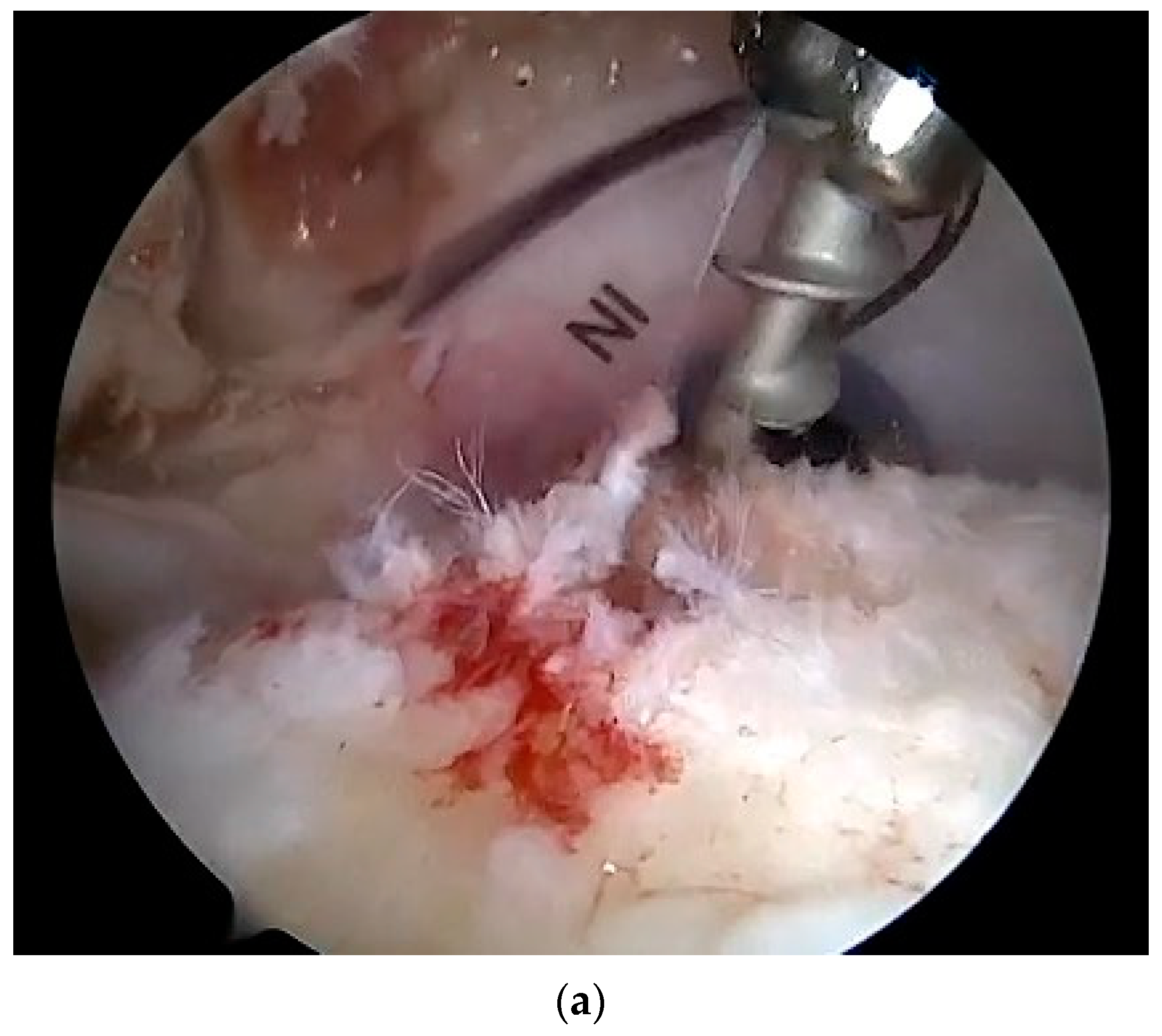
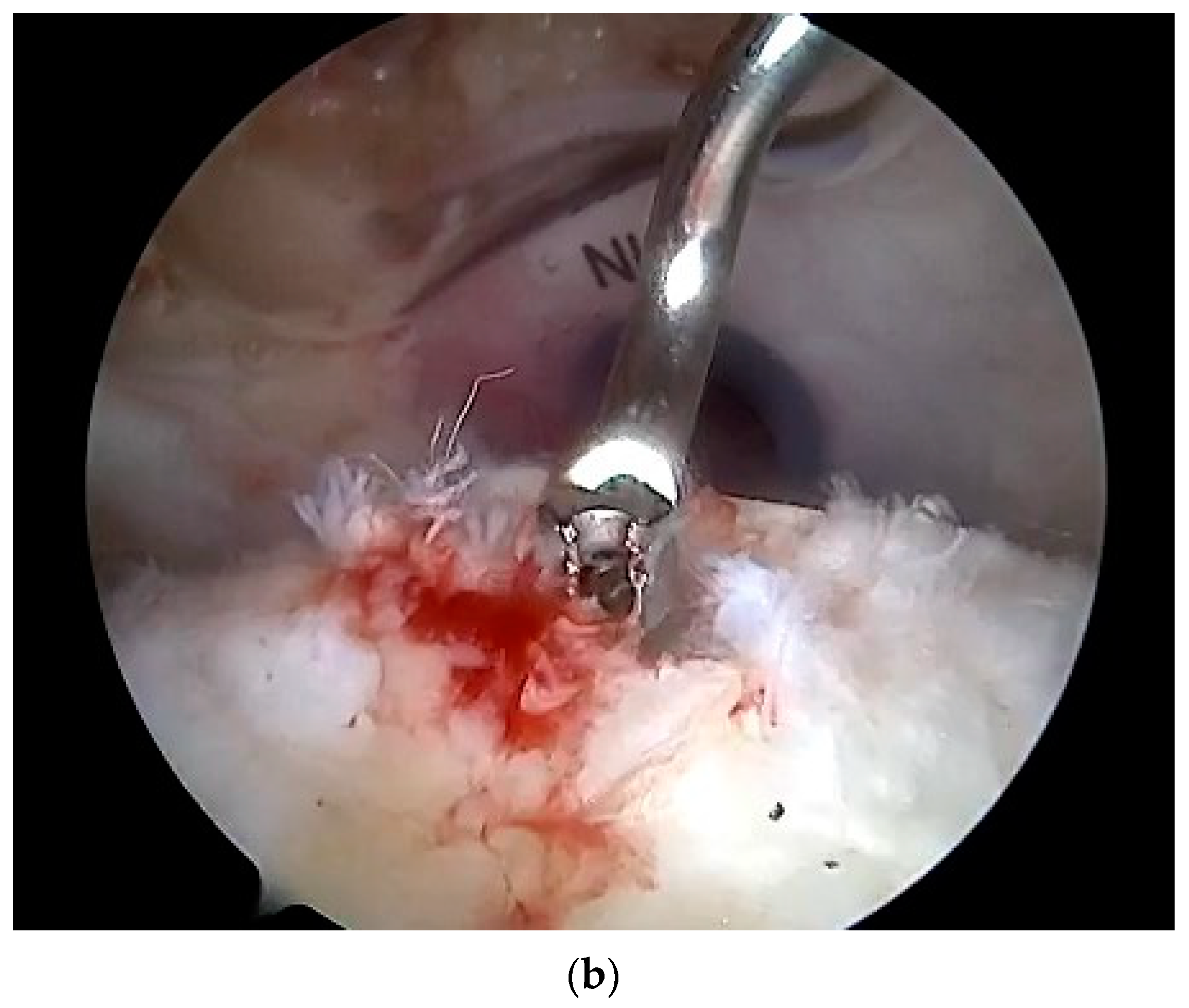
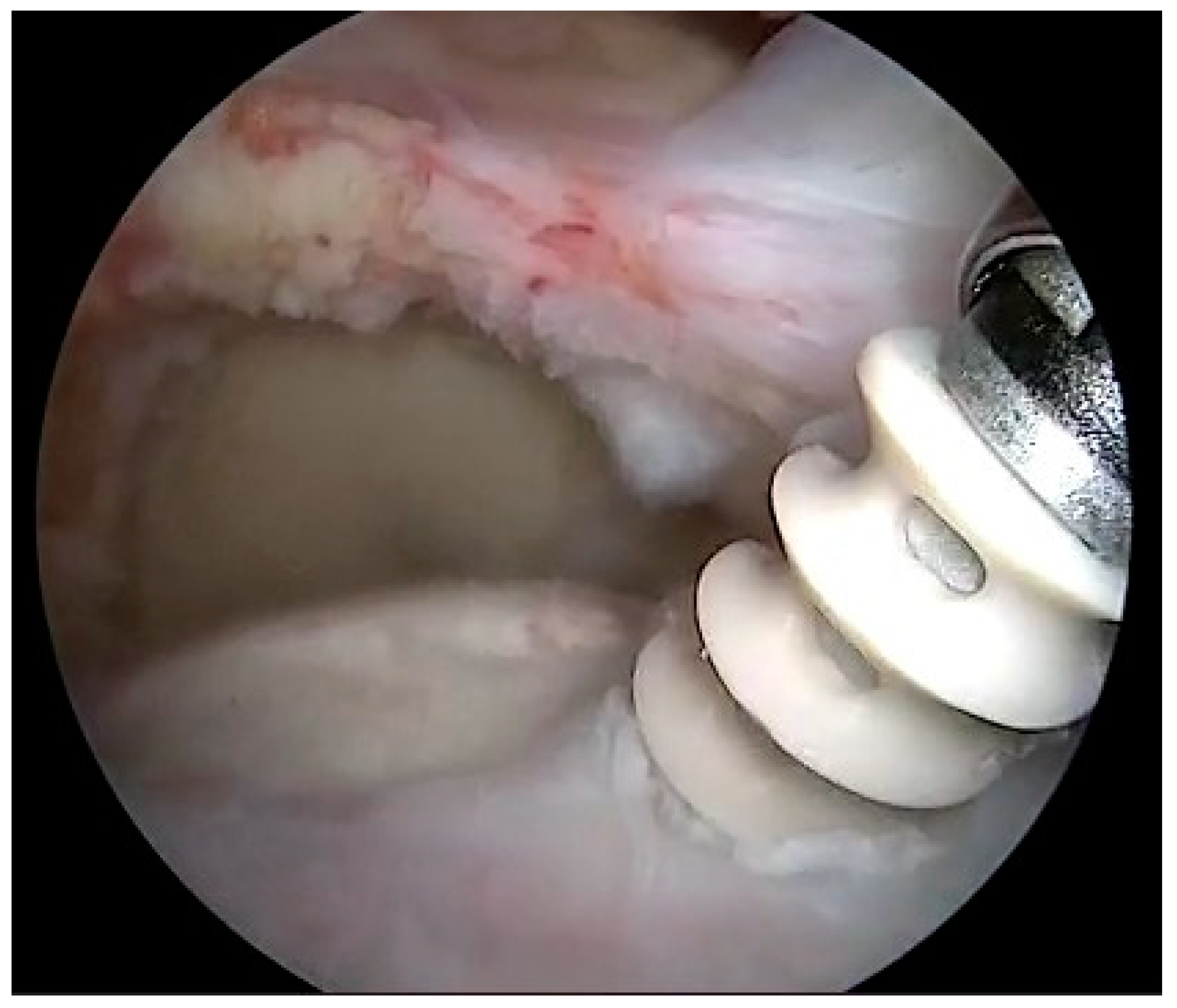
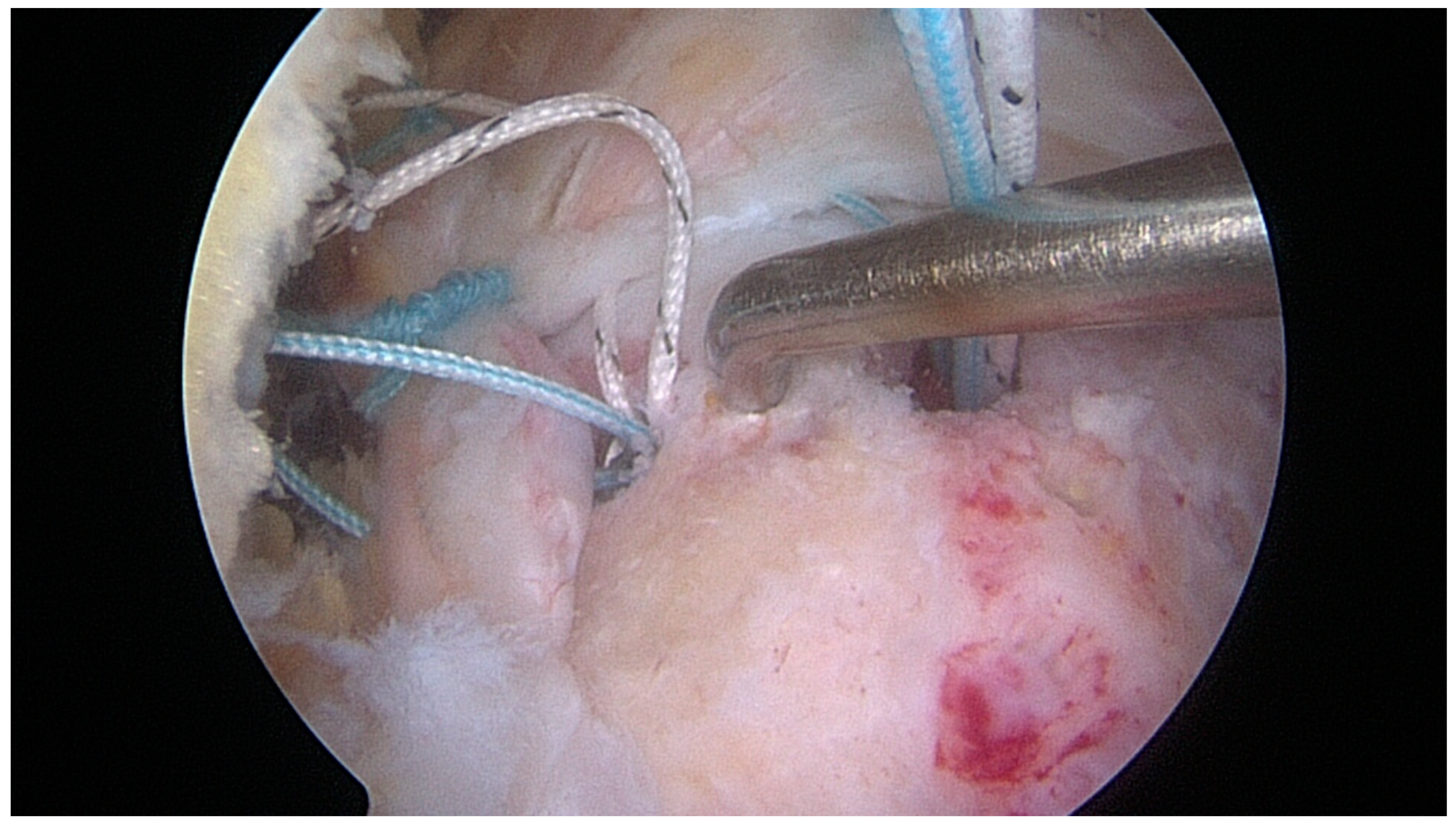
| Variables | Overall | |
|---|---|---|
| N = 40 | ||
| Gender | Male, N (%) | 18 (45%) |
| Female, N (%) | 22 (55%) | |
| Dominance | No, N (%) | 10 (25%) |
| Yes, N (%) | 30 (75%) | |
| Type of work | Manual, N (%) | 18 (45%) |
| Sedentary, N (%) | 22 (55%) | |
| Tear size | Large, N (%) | 29 (72.5%) |
| Massive, N (%) | 11 (27.5%) | |
| Variables | Controls | Augment | p | |
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| Age (years) | Mean ± SD | 63.8 ± 7.3 | 63.3 ± 7.5 | 0.832 |
| Gender | Male, N (%) | 10 (50%) | 8 (40%) | 0.376 |
| Female, N (%) | 10 (50%) | 12 (60%) | ||
| Dominance | No, N (%) | 4 (20%) | 6 (30%) | 0.358 |
| Yes, N (%) | 16 (80%) | 14 (70%) | ||
| Type of work | Manual, N (%) | 7 (35%) | 11 (55%) | 0.170 |
| Sedentary, N (%) | 13 (65%) | 9 (45%) | ||
| Tear size | Large, N (%) | 16 (80%) | 13 (65%) | 0.240 |
| Massive, N (%) | 4 (20%) | 7 (35%) | ||
| Follow-up (months) | Mean ± SD | 36.2 ± 9.1 | 36.1 ± 8.6 | 0.986 |
| Constant score | Mean ± SD | 47.9 ± 12.7 | 48.7 ± 18.6 | 0.873 |
| Quick-DASH | Mean ± SD | 59.8 ± 18.3 | 57.4 ± 9.9 | 0.610 |
| Variables | Controls | Augment | p | |
|---|---|---|---|---|
| N = 20 | N = 20 | |||
| Constant score | Mean ± SD | 80.7 ± 16.6 | 91.5 ± 11.5 | 0.022 * |
| Quick-DASH | Mean ± SD | 28.6 ± 21.6 | 20.1 ± 17.4 | 0.178 |
| Tendon healing | Yes, N (%) | 6 (30%) | 16 (80%) | 0.002 * |
| No, N (%) | 14 (70%) | 4 (20%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosio, A.; Bergomi, A.; Pratobevera, A.; Paderno, M.; Saccomanno, M.F.; Milano, G. Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair. J. Clin. Med. 2023, 12, 5694. https://doi.org/10.3390/jcm12175694
Colosio A, Bergomi A, Pratobevera A, Paderno M, Saccomanno MF, Milano G. Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair. Journal of Clinical Medicine. 2023; 12(17):5694. https://doi.org/10.3390/jcm12175694
Chicago/Turabian StyleColosio, Alessandro, Andrea Bergomi, Andrea Pratobevera, Marco Paderno, Maristella Francesca Saccomanno, and Giuseppe Milano. 2023. "Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair" Journal of Clinical Medicine 12, no. 17: 5694. https://doi.org/10.3390/jcm12175694
APA StyleColosio, A., Bergomi, A., Pratobevera, A., Paderno, M., Saccomanno, M. F., & Milano, G. (2023). Combined Biologic Augmentation Strategies with Collagen Patch Graft, Microfractures, and Platelet Concentrate Injections Improve Functional and Structural Outcomes of Arthroscopic Revision Rotator Cuff Repair. Journal of Clinical Medicine, 12(17), 5694. https://doi.org/10.3390/jcm12175694






