Abstract
Background: the prognosis of patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) is not benign; thus, prompting the need to validate prognostic scoring systems for this population. Aim: to evaluate and compare the prognostic performance of GRACE, TIMI, HEART, and ACEF scores in MINOCA patients. Methods: A total of 250 MINOCA patients from January 2017 to September 2021 were included. For each patient, the four scores at admission were retrospectively calculated. The primary outcome was a composite of all-cause death and acute myocardial infarction (AMI) at 1-year follow-up. The ability to predict 1-year all-cause death was also tested. Results: Overall, the tested scores presented a sub-optimal performance in predicting the composite major adverse event in MINOCA patients, showing an AUC ranging between 0.7 and 0.8. Among them, the GRACE score appeared to be the best in predicting all-cause death, reaching high specificity with low sensitivity. The best cut-off identified for the GRACE score was 171, higher compared to the cut-off of 140 generally applied to identify high-risk patients with obstructive AMI. When the scores were tested for prediction of 1-year all-cause death, the GRACE and the ACEF score showed very good accuracy (AUC = 0.932 and 0.828, respectively). Conclusion: the prognostic scoring tools, validated in AMI cohorts, could be useful even in MINOCA patients, although their performance appeared sub-optimal, prompting the need for risk assessment tools specific to MINOCA patients.
1. Introduction
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a heterogeneous clinical condition, representing 6–11% of the total population with AMI [1]. It is more prevalent in young, non-white, and female patients [2,3,4,5]. Since MINOCA prognosis is not as benign as traditionally assumed, the need to develop and validate prognostic scoring systems to stratify individual risk has emerged [4,5,6].
In acute coronary syndromes (ACSs), the most widespread tool to predict high-risk patients with worse outcomes is the Global Registry of Acute Coronary Events (GRACE) score [7]. Being initially conceived to identify patients at high risk of in-hospital mortality, it was then validated to predict the risk of all-cause death at long-term follow-up, up to 5 years [8,9,10,11,12,13]. Similarly, the Thrombolysis in Myocardial Infarction (TIMI) score is a scoring tool developed in non-STE-segment elevation ACS (NSTE-ACS) patients and validated in predicting 5-year all-cause mortality [14,15]. The HEART score was developed specifically for risk stratification in chest pain patients with suspected NSTE-ACS at the emergency department, and then it was shown to also be accurate when predicting worse outcomes at long-term follow-up [15,16,17,18,19]. Conversely, the ACEF score was originally proposed and validated to assess mortality risk in elective cardiac surgery, being then applied to also predict outcomes in patients with ACSs [20,21,22]. However, the accuracy of the mentioned prognostic scores in predicting outcomes in a population of solely MINOCA patients and a direct comparison among them remains to be evaluated [15].
Thus, our study aimed to evaluate and compare the prognostic performance of GRACE, TIMI NSTE-ACS, HEART, and ACEF scores in MINOCA patients.
2. Materials and Methods
2.1. Study Population
In this multicenter, observational study, all patients from the AMIPE Registry (“Acute Myocardial Infarction, Prognostic, and Therapeutic Evaluation”; ClinicalTrials.gov number: NCT03883711) admitted with AMI at S. Orsola-Malpighi and Maggiore Hospitals, from January 2017 to September 2021, were screened. Patients with unknown coronary anatomy, incomplete clinical data to assess all the scores, and missing follow-up data were excluded. Diagnosis of STEMI and NSTEMI, referral, and timing of invasive coronary angiography (ICA) were managed according to the current European Society of Cardiology (ESC) Guidelines [1,23,24]. The diagnosis of MINOCA was made according to the ESC Guidelines [1,25]. Other specific causes of acute myocardial injury were ruled out through cardiac magnetic resonance, pulmonary, and/or vascular computed tomography when clinically indicated. GRACE, TIMI NSTE-ACS, HEART, and ACEF scores at admission were retrospectively calculated according to the validated criteria [7,14,19,20]. The variables included in each score are shown in Table 1.

Table 1.
Summary of GRACE, TIMI NSTE-ACS, HEART, and ACEF scores.
2.2. Study Endpoints
The primary endpoint of the study was to evaluate and compare the prognostic performance of GRACE, TIMI NSTE-ACS, HEART, and ACEF scores in MINOCA patients. The primary outcome was a composite of all-cause death and AMI [“major adverse event” (MAE)] at 1-year follow-up. In addition, the ability to predict 1-year all-cause death was also tested.
2.3. Statistical Analysis
The normality distribution of continuous variables was assessed visually using histograms and Q-Q plots. Continuous variables with normal distribution were expressed as the mean ± standard deviation and non-normally distributed variables as the median [interquartile range]. Categorical variables were expressed as counts and percentages. Differences between groups were analyzed using the t-test and the Mann–Whitney U-test for continuous variables, when appropriate. The χ2 test or Fisher’s Exact test was used to compare group differences, as appropriate. The performance of prognostic scores in predicting long-term outcomes was assessed with the area under the curve (AUC) of the receiver operating characteristic (ROC) curves. Comparison among AUC was tested with the DeLong test. The Youden index was used to identify the best cut-off of each score for the prediction of the primary endpoint. The population was divided according to these cut-offs, and survival was estimated with Kaplan–Meier curves and compared using the log-rank test. The correlation among scores was assessed with the Spearman rank test. All analyses were performed using the Statistical Package for Social Sciences version 28.0 (SPSS, PC version, Chicago, IL, USA) and Stata version SE 18.0 (Stata Corp LLC, College Station, TX, USA). Statistical significance was defined as two-tailed p-values < 0.05.
3. Results
The study population eligible for the study consisted of 255 consecutive MINOCA patients with at least 1-year follow-up available. Five patients were excluded for insufficient data for prognostic risk score calculation. Thus, the final study population included 250 patients with MINOCA. Overall, the mean age was 68 [53–78] years, and 163 (65.2%) were females. Only one patient died (0.4%) during the index hospitalization, while three (1.2%) patients had a recurrent AMI within 30 days of follow-up. At the 1-year follow-up, 11 patients (4.4%) had died, of which 6/11 (54%) were cardiovascular deaths, and 8 (3.2%) presented a recurrent AMI. Accordingly, patients were divided into two groups: patients who experienced MAEs (composite endpoint of all-cause death and AMI) at the 1-year follow-up (n = 19, 7.6%) and patients who did not (n = 231). The flowchart of the study is shown in Figure 1.
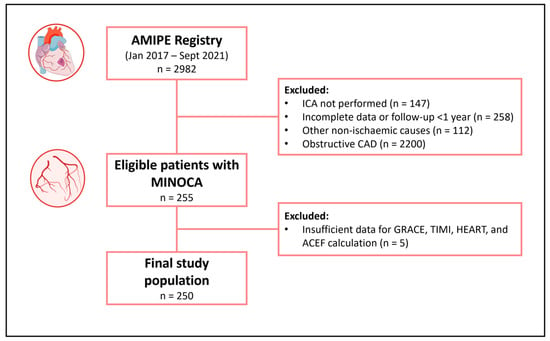
Figure 1.
Flowchart of the study. Abbreviations: AMIPE = Acute Myocardial Infarction, Prognostic, and Therapeutic Evaluation; CAD = coronary artery disease; ICA = invasive coronary angiography; MINOCA = myocardial infarction with non-obstructive coronary arteries.
3.1. Admission Clinical Features Included in the Prognostic Scores
Clinical, laboratory, and instrumental characteristics at admission are presented in Table 2. Compared to patients who did not experience MAE, patients who experienced it were older (p < 0.001), more frequently females (p = 0.021), with a higher prevalence of type-2 diabetes mellitus (p = 0.034), arterial hypertension (p = 0.029), and chronic obstructive pulmonary disease (p = 0.040). Patients reporting the composite endpoint presented lower systolic blood pressure (p = 0.008), a higher rate of atrial fibrillation (AF) (p = 0.008), with overall higher Killip class (p = 0.029) at admission compared to patients with better prognosis. Finally, patients presenting MAE had higher levels of inflammatory markers and lower levels of hemoglobin compared to patients who did not experience the composite endpoint (p < 0.05 for all). Therapy at discharge included dual antiplatelet therapy in 113 patients (45.2%), beta-blockers in 187 (74.8%), inhibitors of the renin–angiotensin–aldosterone system in 158 (63.2%), and statins in 185 patients (74%).

Table 2.
Baseline characteristics and comorbidities stratified by the occurrence of major adverse events (the composite endpoint) at 1 year of follow-up.
3.2. Prognostic Scoring Systems’ Performance
In our cohort of MINOCA, the median scores were 128 [105–157] for the GRACE, 2 [1–3] for the TIMI NSTE-ACS, 7 [5–7] for the HEART, and 1.11 [0.90–1.34] for the ACEF. ROC curves of GRACE, TIMI NSTE-ACS, HEART, and ACEF scores in predicting 1-year outcomes are displayed in Figure 2.
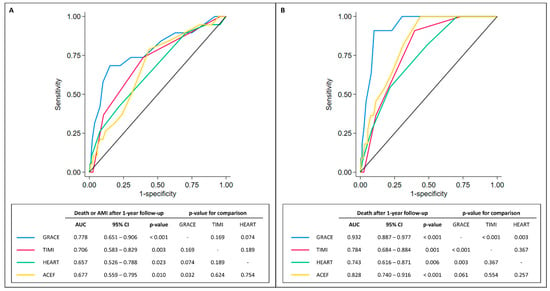
Figure 2.
ROC curves. Panel (A). ROC curves comparing GRACE, TIMI, HEART, and ACEF scores in predicting long-term all-cause death or AMI (after 1 year). Panel (B). ROC curves comparing GRACE, TIMI, HEART, and ACEF scores in predicting long-term all-cause death. Abbreviations: 95% CI = 95% confidence interval; AMI = acute myocardial infarction; AUC = area under the curve.
Interestingly, all the presented scores showed sub-optimal performance in predicting MAE in MINOCA patients with an AUC < 0.8. Among them, the GRACE score appeared to be superior to ACEF (p for comparison = 0.032) in predicting MAE. When the scores were tested for 1-year all-cause death, only the GRACE and the ACEF scores showed very good performance (>0.80) in predicting a worse prognosis (AUC = 0.932, 95% CI 0.887–0.977 for GRACE; AUC = 0.828, 95% CI 0.740–0.961 for ACEF). Similarly, the GRACE score was superior to the HEART and TIMI NSTE-ACS scores in predicting the 1-year all-cause death (p = 0.003 and p < 0.001, respectively). Of note, no other significant differences among the other scores were noted, with only a moderate correlation (0.3 < r < 0.7) among them (Table 3).

Table 3.
Correlations among GRACE, TIMI NSTE-ACS, HEART, and ACEF scores.
As shown in the alluvial plot (Figure 3), TIMI, HEART, and ACEF scores included in the high-risk category a considerable proportion of patients that the GRACE score categorizes as low-risk. The HEART score is the one in which a larger proportion of patients included in the low-risk category turn out to be at high-risk with the GRACE score. In contrast, for both TIMI and ACEF, only a small proportion of patients defined as low-risk turn out to be at high-risk for the GRACE score.
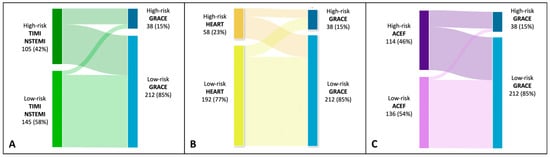
Figure 3.
Alluvial plot showing mutual relationships among risk class identified by GRACE, TIMI, HEART, and ACEF scores. Panel (A). High-risk class by TIMI if >2; high-risk class by GRACE if >171. Panel (B). High-risk class by HEART if >5. Panel (C). High-risk class by ACEF if >1.16.
3.3. Best Cut-Offs and Survival Analyses
Using the Youden index, the best cut-offs of each score to predict 1-year all-cause death and AMI prediction were identified. The accuracy indicators of each score are reported in Table 4.

Table 4.
Cut-offs and diagnostic performance of scores.
The cut-offs for each score were the following: 171 for the GRACE score, 2 for the TIMI NSTE-ACS, 1.16 for the ACEF, and 7 for the HEART score. Interestingly, the best cut-off identified for the GRACE score was 171, which is higher compared to the cut-off of 140 generally applied for the identification of high-risk patients with obstructive NSTE-ACS. These cut-offs were used for dichotomizing the study population into high and low risk for each prognostic score; thus, calculating the survival curves presented in Figure 4. For all the scores, high-risk patients presented statistically significantly lower survival at 1-year follow-up compared to low-risk patients (log-rank p values < 0.001 for the GRACE; p = 0.004 for the NSTE-ACS; p = 0.046 for the HEART score, and p = 0.003 for the ACEF score).
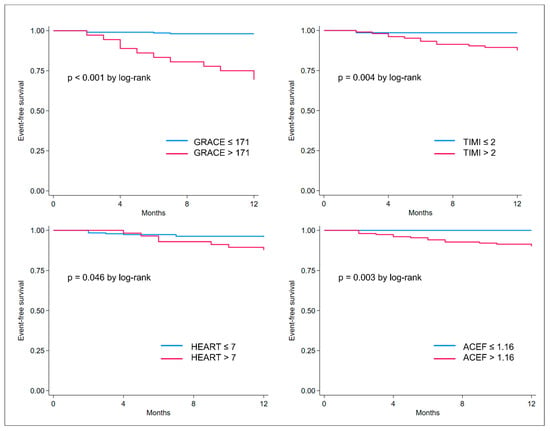
Figure 4.
Kaplan–Meier curves showing event-free survival (death or acute myocardial infarction) in risk categories as defined per the best cut-off identified for GRACE, TIMI, HEART, and ACEF scores.
4. Discussion
In this study, for the first time, we compared, in a population of solely MINOCA patients, the accuracy of the most commonly used scores predicting prognosis in ACSs. The main findings were as follows: (i) overall, the scores showed sub-optimal performance in predicting the composite MAE in MINOCA patients with an AUC ranging between 0.7 and 0.8; (ii) the GRACE score appeared to be the best prognostic score in predicting all-cause death in MINOCA, reaching high specificity with low sensitivity; (iii) the best cut-off identified for the GRACE score was 171, higher compared to the cut-off of 140 generally applied for the identification of high-risk patients with obstructive NSTE-ACS; (iv) when the scores were tested for 1-year all-cause death, only the GRACE and the ACEF scores showed very good performance (>0.80) in predicting the worse prognosis.
4.1. Prognostic Scores in MINOCA
To the best of our knowledge, this is the first study comparing the most used prognostic scores in a population of solely MINOCA patients. So far, only the GRACE score and the ACEF score have been tested in MINOCA [29,30]; however, a direct comparison of the prognostic accuracy of the available scores in the same MINOCA population has not been reported yet. In our study, the GRACE and ACEF scores showed a very good performance in predicting 1-year all-cause death. Nonetheless, their AUC in identifying patients at high risk of death and AMI at 1-year follow-up appeared sub-optimal (AUC < 0.80). Indeed, the prognostic performance of these scores in patients with ACSs was reported as very good [15,17,26,27,29].
These findings might be explained by the following considerations: (i) the prognostic scores were originally designed to stratify the patients’ risk at short-term and then validated at long-term follow-up; in our study, we only evaluated the composite endpoint at the l-year follow-up, due to the relatively low event rate at 30 days from the index hospitalization; (ii) the GRACE score itself was originally designed to predict the risk of mortality and then proven to accurately predict mortality and AMI at the 1-year follow-up; (iii) the heterogeneity of MINOCA patients, presenting different etiopathology of the acute event, with some sub-groups being at higher risk (e.g., young females [31]); (iv) these scores were tested mainly in patients with obstructive AMI (due to the high prevalence of obstructive AMI in the scenario of ACSs), who have a different cardiovascular profile compared to patients with MINOCA. Among all the scores tested, the GRACE score was the best in predicting 1-year all-cause death in the MINOCA population. This is probably due to the high “weight” attributed to the “Age” variable, which also represents one of the strongest independent predictors of all-cause death in MINOCA patients [31,32] (Figure 5).
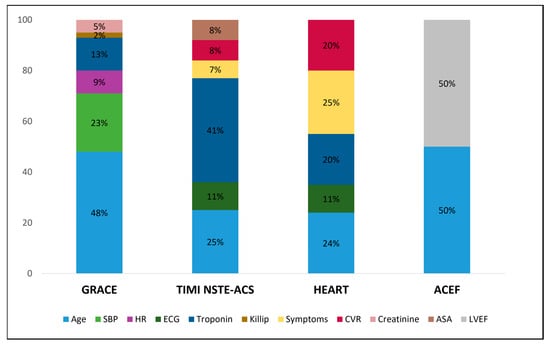
Figure 5.
Stacked bar plot showing the relative mean weight of each item in determining the maximum GRACE, TIMI, HEART, and ACEF score per patient. Of note, the weight of items included in the ACEF score was estimated to be 50% for either age or LVEF since it was calculated as age/LVEF (+1 if serum creatinine >2 mg/dL) and only a small percentage of patients had serum creatinine >2 mg/dL. Abbreviations: ASA = acetylsalicylic acid; CVR = cardiovascular risk factors; HR = heart rate; LVEF = left ventricle ejection fraction; NSTE-ACS = non-ST-elevation acute coronary syndrome; SBP = systolic blood pressure.
4.2. Risk Categories in MINOCA
In the present study, the best cut-offs to predict all-cause death and re-AMI at the 1-year follow-up were identified for all the prognostic scores tested. Interestingly, the best cut-off for the GRACE score in our cohort was 171, higher compared to the cut-off of 140 generally applied to identify high-risk patients with obstructive NSTE-ACS [33]. Similarly, Yin et al. reported the cut-off of 159 for the GRACE score to predict a composite of cardiac death, non-fatal AMI, stroke, heart failure, and cardiovascular rehospitalization in the MINOCA population [34]. This higher cut-off might reflect the overall better prognosis of MINOCA patients compared to MIOCA, with a lower but not negligible risk of cardiovascular adverse events at long-term follow-up [3,4,35,36,37]. This appears to be even more relevant, considering the overall lower GRACE score reported in MINOCA patients compared to MIOCA ones [38]. A GRACE score >171 reached high specificity (89%) with low sensitivity (68%). Among the scores tested in our study, the ACEF score with a cut-off of >1.16 showed the highest sensitivity (79%). The latter was already tested in MINOCA patients; however, a lower cut-off (1.02) was proposed to identify high-risk patients [30]. Applying the same cut-off in our cohort enhanced sensitivity to 90%, but PPV was 11%, and accuracy was 44%. This discrepancy might be due to the longer follow-up period (3.5 years) with more adverse events considered by Gao et al. in their study [30]. Overall, the GRACE score seems to perform better than the other tested scores, with a higher accuracy in predicting all-cause death compared to the composite outcome. Thus, our results confirm that the applicability of this score is feasible also in MINOCA patients.
4.3. Clinical Implications
The identification of patients with high-risk of adverse events at short- and long-term follow-up might be helpful in guiding a rational resource allocation. Our study confirmed that in patients with lower-risk scores, adverse events are particularly rare. Nonetheless, our data showed that MINOCA patients labeled as “high risk” by prognostic scoring systems could experience MAE (all-cause death and AMI) even at 1-year follow-up. Thus, these risk assessment tools might be applied to select patients who might benefit the most from (i) early invasive management (as also proved by the GRACE score in obstructive AMI), including the use of intravascular imaging and/or functional tests in the MINOCA setting [39,40,41,42]; (ii) more “aggressive”/adequate optimization of the medical therapy with an antiplatelet agent, statins, and renin–angiotensin–aldosterone system inhibitors, which seem to be effective also in patients with MINOCA [5,43,44,45,46]; and (iii) a closer follow-up after discharge.
However, it is crucial to highlight that none of the presented scores showed very good performance, with AUC > 0.80 in predicting MAE in MINOCA patients. Thus, a new and more targeted prognostic scoring system, including variables shown to be independent predictors of adverse outcomes in MINOCA patients, should be developed, including (i) clinical features (e.g., female sex, diabetes mellitus, ST-deviation at ECG) [47,48,49,50] and (ii) instrumental findings (presence of fibrosis at late gadolinium enhancement at cardiac magnetic resonance) or high-risk plaque features identified at intravascular imaging [51,52,53,54,55,56,57,58,59,60].
4.4. Limitations of the Study
Our study has some limitations. First, the relatively small sample size and low incidence of MAE might affect the statistical power. Nevertheless, our population of MINOCA patients was cautiously selected with the exclusion of possible non-cardiac causes of myocardial injury, myocarditis, cardiomyopathies, or takotsubo syndrome. Furthermore, the pathological mechanism of MINOCA was not systematically evaluated with intracoronary imaging and functional coronary tests. For these reasons, our findings should be interpreted as hypothesis-generating and further prospective and adequately powered studies are needed to confirm our findings.
5. Conclusions
Since the rate of MAEs in MINOCA patients is not negligible, prognostic scoring systems, validated in wide AMI cohorts, could also be useful in MINOCA patients. In particular, the GRACE score showed the best performance, although the cut-off to identify high-risk patients was higher compared to one used in patients with ACSs. Overall, the performance of these prognostic scores was sub-optimal in the setting of MINOCA, prompting the need for tailored risk-stratification tools for MINOCA patients.
Author Contributions
D.F. and L.B. carried out the statistical analysis. D.F., L.C., F.B. and N.S. wrote the first draft of the manuscript. F.P.T., A.I., O.D.I., S.A., K.R., D.B., D.C. and M.C. collected data. A.R., M.A., A.S. (Angelo Sansonetti), and A.S. (Andrea Stefanizzi) carried out a critical revision of the manuscript. A.F., L.L., P.P., G.C., F.A., E.G., M.B. and C.P. corrected and approved the revisions and definitive version of the manuscript. P.P., D.F. and C.P. are responsible for the conception and design of the study. C.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Data were collected as part of an approved multicenter observational study “AMIPE: Acute Myocardial Infarction, Prognostic, and Therapeutic Evaluation” (ClinicalTrials.gov Identifier: NCT03883711). The present study was conducted according to the principles of the Declaration of Helsinki.
Informed Consent Statement
All patients were informed about their participation in the registry and provided informed consent for the anonymous publication of scientific data.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting with Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Lindahl, B.; Litwin, P.; Tavella, R.; Williams, M.J.A.; Air, T.; Zeitz, C.; Smilowitz, N.R.; Reynolds, H.R.; Eggers, K.M.; et al. Survival in Patients with Suspected Myocardial Infarction with Nonobstructive Coronary Arteries: A Comprehensive Systematic Review and Meta-Analysis from the MINOCA Global Collaboration. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007880. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Chapman, A.R.; Dweck, M.R.; Mills, N.L.; Newby, D.E. MINOCA: A heterogenous group of conditions associated with myocardial damage. Heart. 2021, 107, 1458–1464. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef]
- Bassand, J.-P.; Hamm, C.W.; Ardissino, D.; Boersma, E.; Budaj, A.; Fernandez-Aviles, F.; Fox, K.A.A.; Hasdai, D.; Ohman, E.M.; Wallentin, L.; et al. Guidelines for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes: The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur. Heart J. 2007, 28, 1598–1660. [Google Scholar] [CrossRef]
- Granger, C.B. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M.; et al. A Validated Prediction Model for All Forms of Acute Coronary Syndrome. JAMA 2004, 291, 2727–2733. [Google Scholar] [CrossRef]
- Yusufali, A.; Zubaid, M.; Al-Zakwani, I.; Alsheikh-Ali, A.A.; Al-Mallah, M.H.; Al Suwaidi, J.; AlMahmeed, W.; Rashed, W.; Sulaiman, K.; Amin, H. Validation of the GRACE Risk Score for Hospital Mortality in Patients with Acute Coronary Syndrome in the Arab Middle East. Angiology 2011, 62, 390–396. [Google Scholar] [CrossRef]
- Elbarouni, B.; Goodman, S.G.; Yan, R.T.; Welsh, R.C.; Kornder, J.M.; DeYoung, J.P.; Wong, G.C.; Rose, B.; Grondin, F.R.; Gallo, R.; et al. Validation of the Global Registry of Acute Coronary Event (GRACE) Risk Score for in-Hospital Mortality in Patients with Acute Coronary Syndrome in Canada. Am. Heart. J. 2009, 158, 392–399. [Google Scholar] [CrossRef]
- Yan, A.T.; Yan, R.T.; Tan, M.; Eagle, K.A.; Granger, C.B.; Dabbous, O.H.; Fitchett, D.; Grima, E.; Langer, A.; Goodman, S.G. In-Hospital Revascularization and One-Year Outcome of Acute Coronary Syndrome Patients Stratified by the GRACE Risk Score. Am. J. Cardiol. 2005, 96, 913–916. [Google Scholar] [CrossRef]
- Tang, E.W.; Wong, C.-K.; Herbison, P. Global Registry of Acute Coronary Events (GRACE) Hospital Discharge Risk Score Accurately Predicts Long-Term Mortality Post Acute Coronary Syndrome. Am. Heart J. 2007, 153, 29–35. [Google Scholar] [CrossRef]
- Reaney, P.D.W.; Elliott, H.I.; Noman, A.; Cooper, J.G. Risk Stratifying Chest Pain Patients in the Emergency Department Using HEART, GRACE and TIMI Scores, with a Single Contemporary Troponin Result, to Predict Major Adverse Cardiac Events. Emerg. Med. J. 2018, 35, 420–427. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.L.M.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI Risk Score for Unstable Angina/Non–ST Elevation MI. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef]
- Yang, B.; Bai, L.; Zhang, Y.; Cheng, Y.; Zhao, C.; Huang, B.; Chen, M. The Value of Different Short-Term Risk Scoring Models in Predicting Long-Term Death of Acute Myocardial Infarction. J. Clin. Med. 2022, 11, 5054. [Google Scholar] [CrossRef] [PubMed]
- Backus, B.E.; Six, A.J.; Kelder, J.C.; Bosschaert, M.A.R.; Mast, E.G.; Mosterd, A.; Veldkamp, R.F.; Wardeh, A.J.; Tio, R.; Braam, R.; et al. A Prospective Validation of the HEART Score for Chest Pain Patients at the Emergency Department. Int. J. Cardiol. 2013, 168, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Pinto, G.; Preda, A.; Rampa, L.; Gaspardone, C.; Oppizzi, M.; Slavich, M.; Di Napoli, D.; Bianchi, G.; Etteri, M.; et al. Performances of HEART Score to Predict 6-Month Prognostic of Emergency Department Patients with Chest Pain: A Retrospective Cohort Analysis. Eur. J. Emerg. Med. 2023, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.; Akula, A.; De Michieli, L.; Knott, J.D.; Lobo, R.; Mehta, R.A.; Hodge, D.O.; Gulati, R.; Sandoval, Y.; Jaffe, A.S. Use of the HEAR Score for 30-Day Risk-Stratification in Emergency Department Patients. Am. J. Med. 2023, 136, 918–926.e5. [Google Scholar] [CrossRef] [PubMed]
- Six, A.J.; Backus, B.E.; Kelder, J.C. Chest Pain in the Emergency Room: Value of the HEART Score. Neth. Heart J. 2008, 16, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Castelvecchio, S.; Menicanti, L.; Frigiola, A.; Pelissero, G. Risk of Assessing Mortality Risk in Elective Cardiac Operations. Circulation 2009, 119, 3053–3061. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, M.H.; Yang, D.H.; Park, H.S.; Cho, Y.; Jeong, M.H.; Kim, Y.J.; Kim, K.-S.; Hur, S.H.; Seong, I.W.; et al. Prognostic Value of the Age, Creatinine, and Ejection Fraction Score for 1-Year Mortality in 30-Day Survivors Who Underwent Percutaneous Coronary Intervention After Acute Myocardial Infarction. Am. J. Cardiol. 2015, 115, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Stähli, B.E.; Wischnewsky, M.B.; Jakob, P.; Klingenberg, R.; Obeid, S.; Heg, D.; Räber, L.; Windecker, S.; Roffi, M.; Mach, F.; et al. Predictive Value of the Age, Creatinine, and Ejection Fraction (ACEF) Score in Patients with Acute Coronary Syndromes. Int. J. Cardiol. 2018, 270, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC Working Group Position Paper on Myocardial Infarction with Non-Obstructive Coronary Arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Meune, C.; Drexler, B.; Haaf, P.; Reichlin, T.; Reiter, M.; Meissner, J.; Twerenbold, R.; Stelzig, C.; Freese, M.; Winkler, K.; et al. The GRACE Score’s Performance in Predicting in-Hospital and 1-Year Outcome in the Era of High-Sensitivity Cardiac Troponin Assays and B-Type Natriuretic Peptide. Heart 2011, 97, 1479–1483. [Google Scholar] [CrossRef]
- Khand, A.U.; Backus, B.; Campbell, M.; Frost, F.; Mullen, L.; Fisher, M.; Theodoropoulos, K.C.; Obeidat, M.; Batouskaya, K.; Carlton, E.W.; et al. HEART Score Recalibration Using Higher Sensitivity Troponin T. Ann. Emerg. Med. 2023, in press. [Google Scholar] [CrossRef]
- Ma, C.-P.; Wang, X.; Wang, Q.-S.; Liu, X.-L.; He, X.-N.; Nie, S.-P. A Modified HEART Risk Score in Chest Pain Patients with Suspected Non-ST-Segment Elevation Acute Coronary Syndrome. J. Geriatr. Cardiol. 2016, 13, 64–69. [Google Scholar]
- Eggers, K.M.; Baron, T.; Hjort, M.; Nordenskjöld, A.M.; Tornvall, P.; Lindahl, B. GRACE 2.0 Score for Risk Prediction in Myocardial Infarction with Nonobstructive Coronary Arteries. J. Am. Heart Assoc. 2021, 10, e021374. [Google Scholar] [CrossRef]
- Gao, S.; Ma, W.; Huang, S.; Lin, X.; Yu, M. Predictive Value of the Age, Creatinine, and Ejection Fraction Score in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries. Clin. Cardiol. 2021, 44, 1011–1018. [Google Scholar] [CrossRef]
- Canton, L.; Fedele, D.; Bergamaschi, L.; Foà, A.; Di Iuorio, O.; Tattilo, F.P.; Rinaldi, A.; Angeli, F.; Armillotta, M.; Sansonetti, A.; et al. Sex- and Age-Related Differences in Outcomes of Patients with Acute Myocardial Infarction: MINOCA vs. MIOCA. Eur. Heart J. Acute Cardiovasc. Care 2023, zuad059, in press. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Foà, A.; Bergamaschi, L.; Angeli, F.; Fabrizio, M.; Donati, F.; Toniolo, S.; Chiti, C.; Rinaldi, A.; Stefanizzi, A.; et al. Impact of Admission Hyperglycemia on Short and Long-Term Prognosis in Acute Myocardial Infarction: MINOCA versus MIOCA. Cardiovasc. Diabetol. 2021, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Abdu, F.A.; Liu, L.; Xu, S.; Xu, B.; Luo, Y.; Lv, X.; Fan, R.; Che, W. Prognostic Value of GRACE Risk Scores in Patients with Non-ST-Elevation Myocardial Infarction with Non-Obstructive Coronary Arteries. Front. Cardiovasc. Med. 2021, 8, 582246. [Google Scholar] [CrossRef]
- Eggers, K.M.; Hjort, M.; Baron, T.; Jernberg, T.; Nordenskjöld, A.M.; Tornvall, P.; Lindahl, B. Morbidity and Cause-specific Mortality in First-time Myocardial Infarction with Nonobstructive Coronary Arteries. J. Intern. Med. 2019, 285, 419–428. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Mahajan, A.M.; Roe, M.T.; Hellkamp, A.S.; Chiswell, K.; Gulati, M.; Reynolds, H.R. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry–GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry–Get with the Guidelines). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003443. [Google Scholar] [CrossRef]
- Abdu, F.A.; Liu, L.; Mohammed, A.-Q.; Luo, Y.; Xu, S.; Auckle, R.; Xu, Y.; Che, W. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA) in Chinese Patients: Clinical Features, Treatment and 1 year Follow-Up. Int. J. Cardiol. 2019, 287, 27–31. [Google Scholar] [CrossRef]
- Rao, K.; De Silva, K.; Sood, A.; Denniss, A.R.; Hsu, C.-J. Predicting Patients with Troponin Positive Chest Pain and Unobstructed Coronary Arteries with Electrocardiogram, Troponin Kinetics and GRACE Score. Heart Lung Circ. 2022, 31, 1219–1227. [Google Scholar] [CrossRef]
- Jobs, A.; Mehta, S.R.; Montalescot, G.; Vicaut, E.; van’t Hof, A.W.J.; Badings, E.A.; Neumann, F.-J.; Kastrati, A.; Sciahbasi, A.; Reuter, P.-G.; et al. Optimal Timing of an Invasive Strategy in Patients with Non-ST-Elevation Acute Coronary Syndrome: A Meta-Analysis of Randomised Trials. Lancet 2017, 390, 737–746. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Clayton, T.C.; Damman, P.; Pocock, S.J.; de Winter, R.J.; Tijssen, J.G.P.; Lagerqvist, B.; Wallentin, L. Long-Term Outcome of a Routine Versus Selective Invasive Strategy in Patients with Non–ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2010, 55, 2435–2445. [Google Scholar] [CrossRef]
- Mehta, S.R.; Granger, C.B.; Boden, W.E.; Steg, P.G.; Bassand, J.-P.; Faxon, D.P.; Afzal, R.; Chrolavicius, S.; Jolly, S.S.; Widimsky, P.; et al. Early versus Delayed Invasive Intervention in Acute Coronary Syndromes. N. Engl. J. Med. 2009, 360, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, K.F.; Kelbæk, H.; Hansen, P.R.; Torp-Pedersen, C.; Høfsten, D.; Kløvgaard, L.; Holmvang, L.; Helqvist, S.; Jørgensen, E.; Galatius, S.; et al. Early Versus Standard Care Invasive Examination and Treatment of Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Circulation 2018, 138, 2741–2750. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Verdoia, M.; Merlo, M.; Zilio, F.; Vatrano, M.; Bianco, F.; Mancone, M.; Zaffalon, D.; Bonci, A.; Boscutti, A.; et al. Pharmacological Therapy for the Prevention of Cardiovascular Events in Patients with Myocardial Infarction with Non-Obstructed Coronary Arteries (MINOCA): Insights from a Multicentre National Registry. Int. J. Cardiol. 2021, 327, 9–14. [Google Scholar] [CrossRef]
- Bossard, M.; Gao, P.; Boden, W.; Steg, G.; Tanguay, J.-F.; Joyner, C.; Granger, C.B.; Kastrati, A.; Faxon, D.; Budaj, A.; et al. Antiplatelet Therapy in Patients with Myocardial Infarction without Obstructive Coronary Artery Disease. Heart 2021, 107, 1739–1747. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients with Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef]
- De Filippo, O.; Russo, C.; Manai, R.; Borzillo, I.; Savoca, F.; Gallone, G.; Bruno, F.; Ahmad, M.; De Ferrari, G.M.; D’Ascenzo, F. Impact of secondary prevention medical therapies on outcomes of patients suffering from Myocardial Infarction with NonObstructive Coronary Artery disease (MINOCA): A meta-analysis. Int J Cardiol. 2022, 368, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Bashline, M.; Novelli, E.M.; Bliden, K.P.; Tantry, U.S.; Olafiranye, O.; Rahman, A.; Gurbel, P.A.; Pacella, J.J. Sex-related differences in clinical outcomes among patients with myocardial infarction with nonobstructive coronary artery disease: A systematic review and meta-analysis. Int J Cardiol. 2022, 369, 1–4. [Google Scholar] [CrossRef]
- Armillotta, M.; Amicone, S.; Bergamaschi, L.; Angeli, F.; Rinaldi, A.; Paolisso, P.; Stefanizzi, A.; Sansonetti, A.; Impellizzeri, A.; Bodega, F.; et al. Predictive Value of Killip Classification in MINOCA Patients. Eur. J. Intern. Med. 2023, in press. [Google Scholar] [CrossRef]
- Szolc, P.; Niewiara, Ł.; Kleczyński, P.; Bryniarski, K.; Ostrowska-Kaim, E.; Szkodoń, K.; Brzychczy, P.; Żmudka, K.; Legutko, J.; Guzik, B. Clinical Characteristics Predicting Worse Long-Term Outcomes in Patients with Myocardial Infarction and Non-Obstructive Coronary Arteries (MINOCA). J. Cardiovasc. Dev. Dis. 2022, 9, 286. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Rambaldi, P.; Gatta, G.; Foà, A.; Angeli, F.; Fabrizio, M.; Casella, G.; Barbieri, M.; Galiè, N.; et al. Impact of Admission Hyperglycemia on Heart Failure Events and Mortality in Patients with Takotsubo Syndrome at Long-Term Follow-up: Data From HIGH-GLUCOTAKO Investigators. Diabetes Care 2021, 44, 2158–2161. [Google Scholar] [CrossRef]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Taruya, A.; Tanaka, A.; Nishiguchi, T.; Ozaki, Y.; Kashiwagi, M.; Yamano, T.; Matsuo, Y.; Ino, Y.; Kitabata, H.; Takemoto, K.; et al. Lesion Characteristics and Prognosis of Acute Coronary Syndrome without Angiographically Significant Coronary Artery Stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhao, C.; Bao, X.; Liu, M.; He, L.; Xu, Y.; Meng, W.; Qin, Y.; Weng, Z.; Yi, B.; et al. Clinical Characteristics and Prognosis of MINOCA Caused by Atherosclerotic and Nonatherosclerotic Mechanisms Assessed by OCT. JACC Cardiovasc. Imaging 2023, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Abdu, F.A.; Liu, L.; Mohammed, A.-Q.; Yin, G.; Xu, B.; Zhang, W.; Xu, S.; Lv, X.; Fan, R.; Feng, C.; et al. Prognostic Impact of Coronary Microvascular Dysfunction in Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries. Eur. J. Intern. Med. 2021, 92, 79–85. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Foà, A.; Paolisso, P.; Renzulli, M.; Angeli, F.; Fabrizio, M.; Bartoli, L.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. Prognostic Role of Early Cardiac Magnetic Resonance in Myocardial Infarction with Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2023, in press. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640, Epub 2020. Erratum in: Circulation. 2023, 147, e624. [Google Scholar] [CrossRef]
- Usui, E.; Matsumura, M.; Smilowitz, N.R.; Mintz, G.S.; Saw, J.; Kwong, R.Y.; Hada, M.; Mahmud, E.; Giesler, C.; Shah, B.; et al. Coronary morphological features in women with non-ST-segment elevation MINOCA and MI-CAD as assessed by optical coherence tomography. Eur. Heart J. Open. 2022, 2, oeac058. [Google Scholar] [CrossRef]
- Pasupathy, S.; Beltrame, J.F. Refining the Role of CMR Imaging in MINOCA. JACC Cardiovasc Imaging. 2021, 14, 1784–1786. [Google Scholar] [CrossRef]
- Borzillo, I.; De Filippo, O.; Manai, R.; Bruno, F.; Ravetti, E.; Galanti, A.A.; Vergallo, R.; Porto, I.; De Ferrari, G.M.; D’Ascenzo, F. Role of Intracoronary Imaging in Myocardial Infarction with Non-Obstructive Coronary Disease (MINOCA): A Review. J. Clin. Med. 2023, 12, 2129. [Google Scholar] [CrossRef]
- Fluder-Wlodarczyk, J.; Milewski, M.; Roleder-Dylewska, M.; Haberka, M.; Ochala, A.; Wojakowski, W.; Gasior, P. Underlying Causes of Myocardial Infarction with Nonobstructive Coronary Arteries: Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging Pilot Study. J. Clin. Med. 2022, 11, 7495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).