Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions
Abstract
:1. Introduction
2. Cardiovascular Imaging for Primary Prevention
2.1. Coronary Computed Tomography Angiography (CCTA)
2.2. Coronary Artery Calcium Score (CAC Score)
2.3. Other Imaging Techniques
3. Risk Assessment Using Cardiovascular Imaging for Secondary Prevention
4. New Perspectives for Secondary Prevention
5. Gaps in Evidence and Future Implications
5.1. Calcium Scoring and Beyond for Primary Prevention
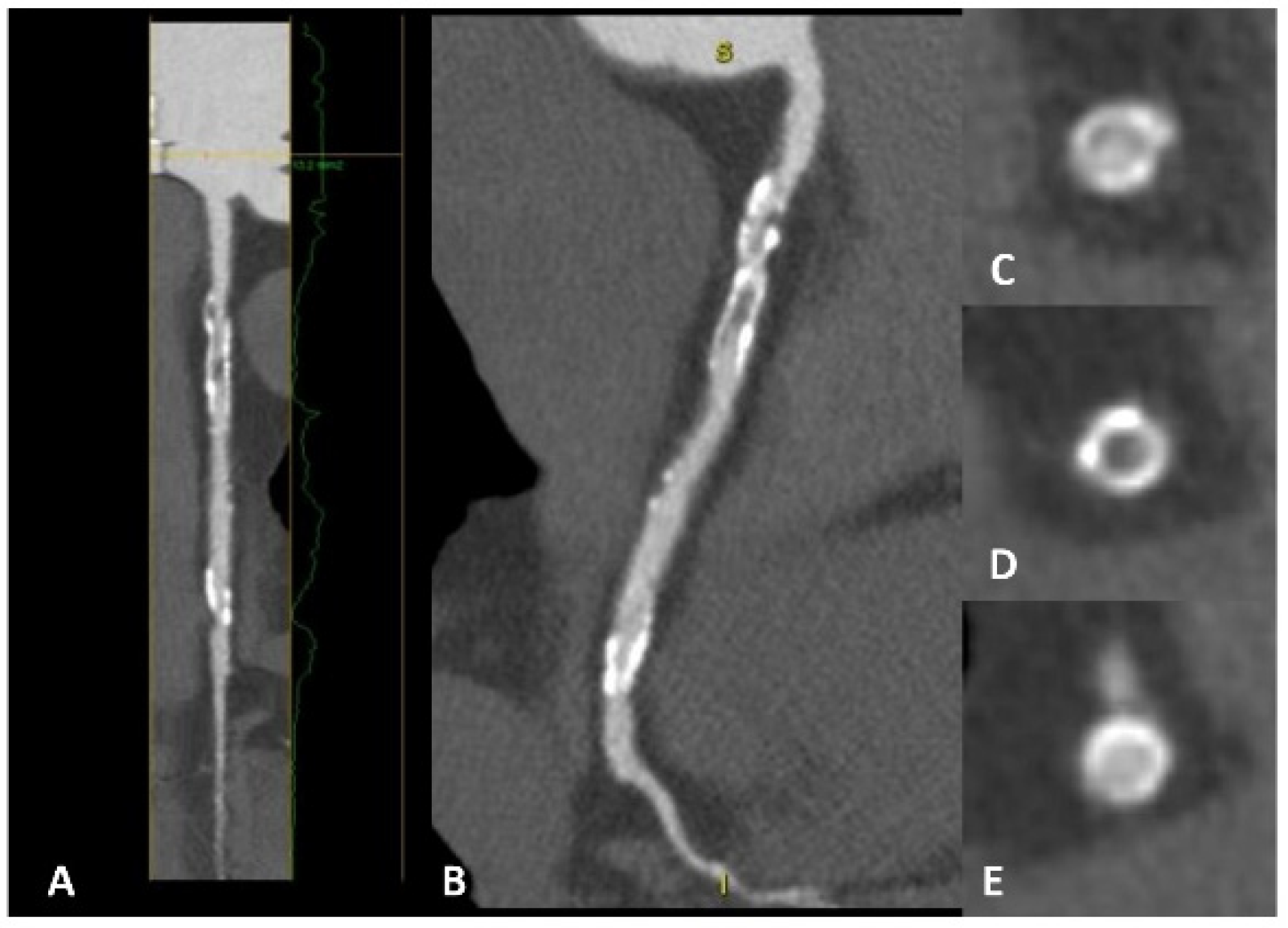
5.2. Advances in CAD Assessment Using CCTA
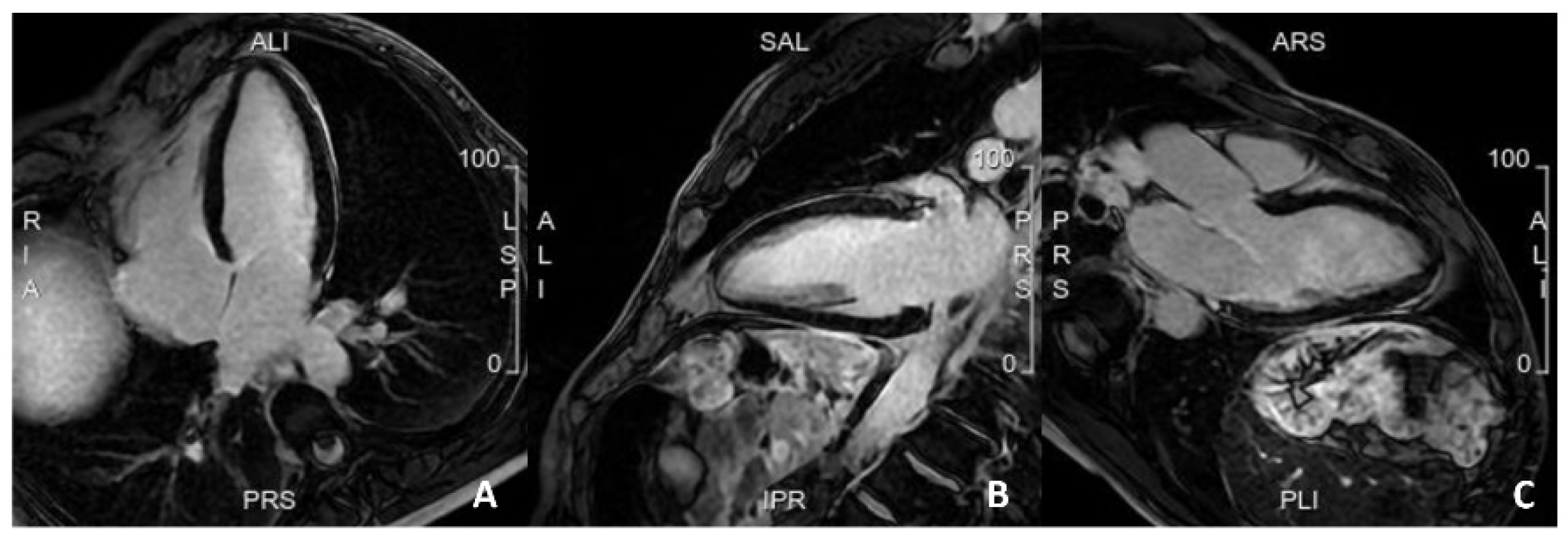
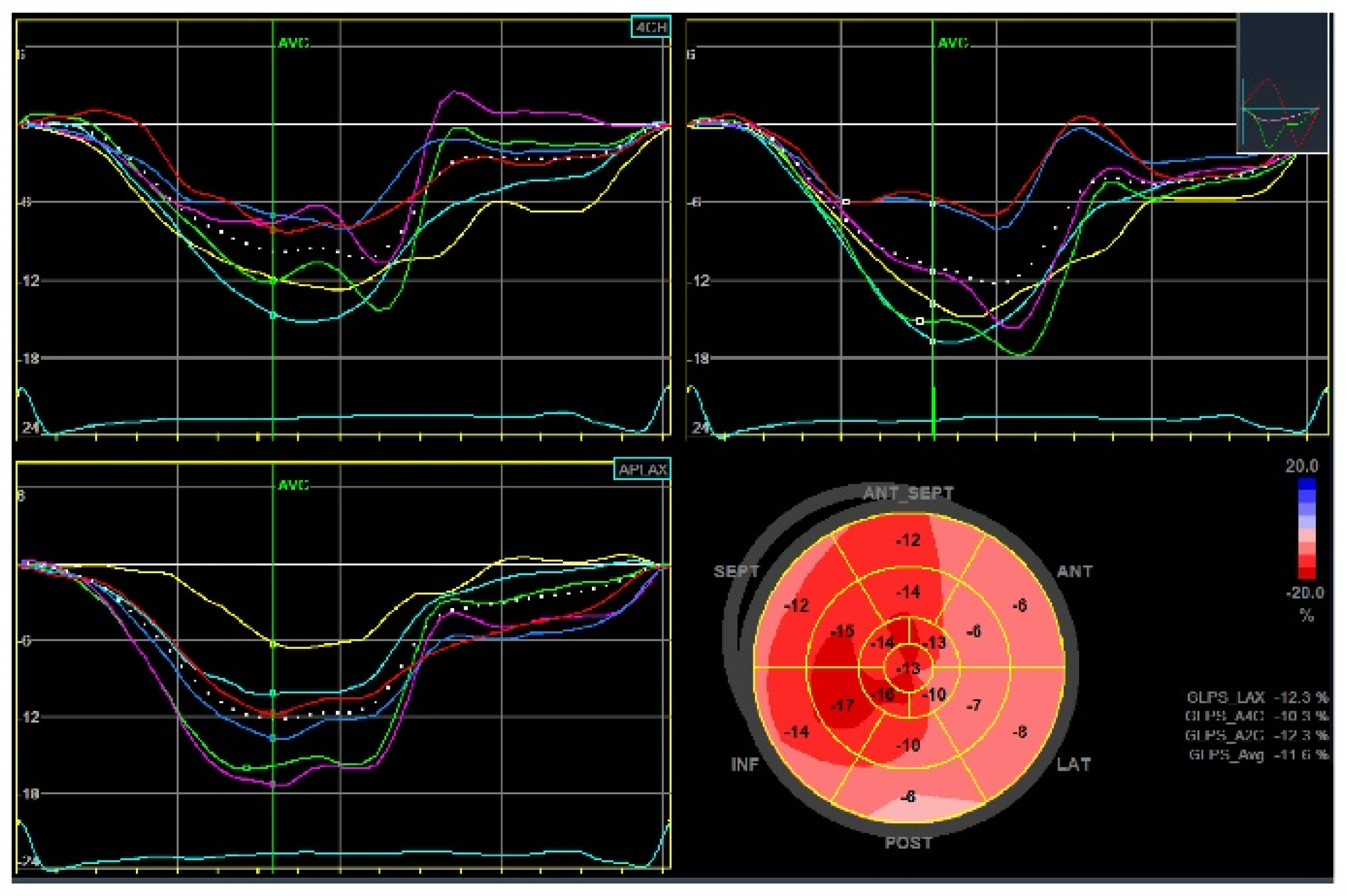
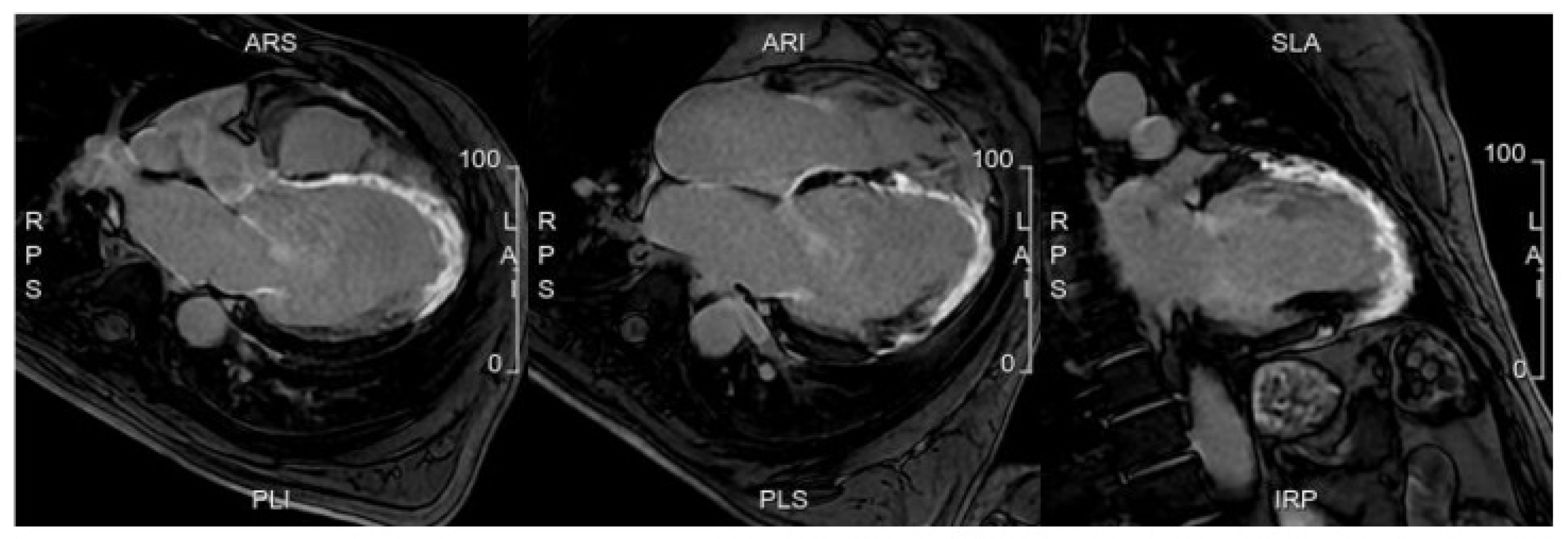
5.3. Advances in MRI for Risk Assessment
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Rabar, S.; Harker, M.; O’flynn, N.; Wierzbicki, A.S.; On behalf of the Guideline Development Group. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: Summary of updated NICE guidance. BMJ 2014, 349, g4356. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Rozanski, A.; Muhlestein, J.B.; Berman, D.S. Primary Prevention of CVD: The Role of Imaging Trials. JACC: Cardiovasc. Imaging 2017, 10, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- SCORE2-OP Working Group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Zeitouni, M.; Sulman, D.; Silvain, J.; Kerneis, M.; Guedeney, P.; Barthelemy, O.; Brugier, D.; Sabouret, P.; Procopi, N.; Collet, J.-P.; et al. Prevention and treatment of premature ischaemic heart disease with European Society of Cardiology Guidelines. Heart 2023, 109, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F. Non-Traditional Cardiovascular Risk Markers in the Era of Established Major Risk Factors and Multiple Guidelines. Curr. Vasc. Pharmacol. 2019, 17, 270–277. [Google Scholar] [CrossRef]
- Meah, M.N.; Dweck, M.R.; E Newby, D. Cardiovascular imaging to guide primary prevention. Heart 2020, 106, 1267–1275. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Lell, M.M.; Kachelrieß, M. Recent and Upcoming Technological Developments in Computed Tomography: High Speed, Low Dose, Deep Learning, Multienergy. Investig. Radiol. 2020, 55, 8–19. [Google Scholar] [CrossRef]
- Serruys, P.W.; Hara, H.; Garg, S.; Kawashima, H.; Nørgaard, B.L.; Dweck, M.R.; Bax, J.J.; Knuuti, J.; Nieman, K.; Leipsic, J.A.; et al. Coronary Computed Tomographic Angiography for Complete Assessment of Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 713–736. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals Without Known Coronary Artery Disease: Results From the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [CrossRef]
- Budoff, M.J.; Li, D.; Kazerooni, E.A.; Thomas, G.S.; Mieres, J.H.; Shaw, L.J. Diagnostic Accuracy of Noninvasive 64-row Computed Tomographic Coronary Angiography (CCTA) Compared with Myocardial Perfusion Imaging (MPI): The PICTURE Study, A Prospective Multicenter Trial. Acad. Radiol. 2016, 24, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Linde, J.J.; Kelbæk, H.; Hansen, T.F.; Sigvardsen, P.E.; Torp-Pedersen, C.; Bech, J.; Heitmann, M.; Nielsen, O.W.; Høfsten, D.; Kühl, J.T.; et al. Coronary CT Angiography in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 453–463. Available online: https://www.jacc.org/doi/abs/10.1016/j.jacc.2019.12.012 (accessed on 7 May 2023). [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Razavi, A.C.; Agatston, A.S.; Shaw, L.J.; De Cecco, C.N.; van Assen, M.; Sperling, L.S.; Bittencourt, M.S.; Daubert, M.A.; Nasir, K.; Blumenthal, R.S.; et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. JACC Cardiovasc. Imaging 2022, 15, 1648–1662. [Google Scholar] [CrossRef]
- Gepner, A.D.; Young, R.; Delaney, J.A.; Tattersall, M.C.; Blaha, M.J.; Post, W.S.; Gottesman, R.F.; Kronmal, R.; Budoff, M.J.; Burke, G.L.; et al. Comparison of Coronary Artery Calcium Presence, Carotid Plaque Presence, and Carotid Intima-Media Thickness for Cardiovascular Disease Prediction in the Multi-Ethnic Study of Atherosclerosis. Circ. Cardiovasc. Imaging 2015, 8, e002262. [Google Scholar] [CrossRef]
- Taylor, A.J.; Bindeman, J.; Feuerstein, I.; Cao, F.; Brazaitis, M.; O’malley, P.G. Coronary Calcium Independently Predicts Incident Premature Coronary Heart Disease Over Measured Cardiovascular Risk Factors: Mean Three-Year Outcomes in the Prospective Army Coronary Calcium (PACC) Project. J. Am. Coll. Cardiol. 2005, 46, 807–814. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.L.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Massaro, J.M.; D’Agostino Sr, R.B.; Kathiresan, S.; Fox, C.S.; O’Donnell, C.J. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J. Am. Heart Assoc. 2016, 5, e003144. [Google Scholar] [CrossRef]
- Grandhi, G.R.; Mirbolouk, M.; Dardari, Z.A.; Al-Mallah, M.H.; Rumberger, J.A.; Shaw, L.J.; Blankstein, R.; Miedema, M.D.; Berman, D.S.; Budoff, M.J.; et al. Interplay of Coronary Artery Calcium and Risk Factors for Predicting CVD/CHD Mortality: The CAC Consortium. JACC Cardiovasc. Imaging 2020, 13, 1175–1186. [Google Scholar] [CrossRef]
- Lin, J.S.; Evans, C.V.; Johnson, E.; Redmond, N.; Coppola, E.L.; Smith, N. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 281–297. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Miname, M.H.; Bittencourt, M.S.; Moraes, S.R.; Alves, R.I.; Silva, P.R.; Jannes, C.E.; Pereira, A.C.; Krieger, J.E.; Nasir, K.; Santos, R.D. Coronary Artery Calcium and Cardiovascular Events in Patients With Familial Hypercholesterolemia Receiving Standard Lipid-Lowering Therapy. JACC Cardiovasc. Imaging 2019, 12, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Navar, A.M. The Potential and Pitfalls of Coronary Artery Calcium Scoring. JAMA Cardiol. 2022, 7, 11–12. [Google Scholar] [CrossRef]
- Kutanzi, K.R.; Lumen, A.; Koturbash, I.; Miousse, I.R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public Health 2016, 13, 1057. [Google Scholar] [CrossRef]
- Naghavi, M.; Maron, D.J.; Kloner, R.A.; Berman, D.S.; Budoff, M.; Superko, H.R.; Shah, P. Coronary artery calcium testing: A call for universal coverage. Prev. Med. Rep. 2019, 15, 100879. [Google Scholar] [CrossRef] [PubMed]
- Den Ruijter, H.M.; Peters, S.A.E.; Anderson, T.J.; Britton, A.R.; Dekker, J.M.; Eijkemans, M.J.; Engström, G.; Evans, G.W.; De Graaf, J.; Grobbee, D.E.; et al. Common Carotid Intima-Media Thickness Measurements in Cardiovascular Risk Prediction. JAMA 2012, 308, 796–803. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Ho, Y.; Sin, S. Current status of carotid ultrasound in atherosclerosis. Quant. Imaging Med. Surg. 2016, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Yang, C.; Ballew, S.H.; Kalbaugh, C.A.; Meyer, M.L.; Tanaka, H.; Heiss, G.; Allison, M.; Salameh, M.; Coresh, J.; et al. Ankle-brachial index and subsequent risk of incident and recurrent cardiovascular events in older adults: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 2021, 336, 39–47. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; A Blom, N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ferrari, V.A.; Han, Y. Cardiovascular Magnetic Resonance Imaging and Heart Failure. Curr. Cardiol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Faganello, G.; Porcari, A.; Biondi, F.; Merlo, M.; De Luca, A.; Vitrella, G.; Belgrano, M.; Pagnan, L.; Di Lenarda, A.; Sinagra, G. Cardiac magnetic resonance in primary prevention of sudden cardiac death. J. Cardiovasc. Echography 2019, 29, 89–94. [Google Scholar] [CrossRef]
- E Petersen, S.; Khanji, M.Y.; Plein, S.; Lancellotti, P.; Bucciarelli-Ducci, C. European Association of Cardiovascular Imaging expert consensus paper: A comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1321–1331. [Google Scholar] [CrossRef]
- Keil, L.; Chevalier, C.; Kirchhof, P.; Blankenberg, S.; Lund, G.; Müllerleile, K.; Magnussen, C. CMR-Based Risk Stratification of Sudden Cardiac Death and Use of Implantable Cardioverter–Defibrillator in Non-Ischemic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 7115. [Google Scholar] [CrossRef] [PubMed]
- Kaasenbrood, L.; Boekholdt, S.M.; van der Graaf, Y.; Ray, K.K.; Peters, R.J.; Kastelein, J.J.; Amarenco, P.; LaRosa, J.C.; Cramer, M.J.; Westerink, J.; et al. Distribution of Estimated 10-Year Risk of Recurrent Vascular Events and Residual Risk in a Secondary Prevention Population. Circulation 2016, 134, 1419–1429. [Google Scholar] [CrossRef]
- Sirimarco, G.; Amarenco, P.; Labreuche, J.; Touboul, P.-J.; Alberts, M.; Goto, S.; Rother, J.; Mas, J.-L.; Bhatt, D.L.; Steg, P.G.; et al. Carotid Atherosclerosis and Risk of Subsequent Coronary Event in Outpatients With Atherothrombosis. Stroke 2013, 44, 373–379. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Eagle, K.A.; Ohman, E.M.; Hirsch, A.T.; Goto, S.; Mahoney, E.M.; Wilson, P.W.F.; Alberts, M.J.; D’agostino, R.; Liau, C.-S.; et al. Comparative Determinants of 4-Year Cardiovascular Event Rates in Stable Outpatients at Risk of or With Atherothrombosis. JAMA 2010, 304, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Shetye, A.; A Nazir, S.; Squire, I.B.; McCann, G.P. Global myocardial strain assessment by different imaging modalities to predict outcomes after ST-elevation myocardial infarction: A systematic review. World J. Cardiol. 2015, 7, 948–960. [Google Scholar] [CrossRef]
- Iwahashi, N.; Gohbara, M.; Kirigaya, J.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Kimura, Y.; Minamimoto, Y.; et al. Prognostic Significance of the Combination of Left Atrial Reservoir Strain and Global Longitudinal Strain Immediately After Onset of ST-Elevation Acute Myocardial Infarction. Circ. J. 2022, 86, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.; Sun, Y.; Shang, Z.; Wang, K.; Su, D.; Zhong, L.; Zhang, S.; Yang, Y. Prognostic Value of Speckle Tracking Echocardiography in Patients with ST-Elevation Myocardial Infarction Treated with Late Percutaneous Intervention. Echocardiography 2015, 32, 1384–1391. [Google Scholar] [CrossRef]
- Iwahashi, N.; Kirigaya, J.; Gohbara, M.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Kirigaya, H.; Minamimoto, Y.; et al. Mechanical dispersion combined with global longitudinal strain estimated by three dimensional speckle tracking in patients with ST elevation myocardial infarction. IJC Heart Vasc. 2022, 40, 101028. [Google Scholar] [CrossRef]
- Olsen, F.J.; Lindberg, S.; Pedersen, S.; Iversen, A.; Davidovski, F.S.; Galatius, S.; Fritz-Hansen, T.; Gislason, G.H.; Søgaard, P.; Møgelvang, R.; et al. Global longitudinal strain predicts cardiovascular events after coronary artery bypass grafting. Heart 2021, 107, 814–821. [Google Scholar] [CrossRef]
- Espersen, C.; Modin, D.; Hoffmann, S.; Hagemann, C.A.; Hagemann, R.A.; Olsen, F.J.; Fritz-Hansen, T.; Platz, E.; Møgelvang, R.; Biering-Sørensen, T. Layer-specific and whole wall global longitudinal strain predict major adverse cardiovascular events in patients with stable angina pectoris. Int. J. Cardiovasc. Imaging 2022, 38, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Peruzzi, M.; Conte, E.; Sciarra, L.; Frati, G.; Cavarretta, E.; Pingitore, A. An Overview of Sport Participation and Exercise Prescription in Mitral Valve Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Zile, M.R. Left ventricular function after surgical correction of chronic mitral regurgitation. Eur. Heart J. 1991, 12, 48–51. [Google Scholar] [CrossRef]
- Yu, M.; Li, W.; Lu, Z.; Wei, M.; Yan, J.; Zhang, J. Quantitative baseline CT plaque characterization of unrevascularized non-culprit intermediate coronary stenosis predicts lesion volume progression and long-term prognosis: A serial CT follow-up study. Int. J. Cardiol. 2018, 264, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Dedic, A.; Kurata, A.; Lubbers, M.; Meijboom, W.B.; Van Dalen, B.; Snelder, S.; Korbee, R.; Moelker, A.; Ouhlous, M.; Van Domburg, R.; et al. Prognostic implications of non-culprit plaques in acute coronary syndrome: Non-invasive assessment with coronary CT angiography. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Duguay, T.M.; Tesche, C.; Vliegenthart, R.; De Cecco, C.N.; Lin, H.; Albrecht, M.H.; Varga-Szemes, A.; De Santis, D.; Ebersberger, U.; Bayer, R.R.; et al. Coronary Computed Tomographic Angiography-Derived Fractional Flow Reserve Based on Machine Learning for Risk Stratification of Non-Culprit Coronary Narrowings in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2017, 120, 1260–1266. [Google Scholar] [CrossRef]
- Gu, H.; Gao, Y.; Hou, Z.; Schoepf, U.J.; Snyder, A.N.; Duguay, T.M.; Wang, X.; Lu, B. Prognostic value of coronary atherosclerosis progression evaluated by coronary CT angiography in patients with stable angina. Eur. Radiol. 2018, 28, 1066–1076. [Google Scholar] [CrossRef]
- Yoo, J.; Song, D.; Baek, J.-H.; Kim, K.; Kim, J.; Song, T.-J.; Lee, H.S.; Choi, D.; Kim, Y.D.; Nam, H.S.; et al. Poor long-term outcomes in stroke patients with asymptomatic coronary artery disease in heart CT. Atherosclerosis 2017, 265, 7–13. [Google Scholar] [CrossRef]
- Hur, J.; Lee, K.H.; Hong, S.R.; Suh, Y.J.; Hong, Y.J.; Lee, H.-J.; Kim, Y.J.; Chang, H.-J.; Choi, B.W. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis 2014, 238, 271–277. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, E.; Jeon, Y.; Yi, S.Y.; Bae, H.-J.; Jang, I.-K.; Lee, J.M.; Yoo, S.M.; White, C.S.; Chun, E.J. Prognostic Value of Coronary CT Angiography for Predicting Poor Cardiac Outcome in Stroke Patients without Known Cardiac Disease or Chest Pain: The Assessment of Coronary Artery Disease in Stroke Patients Study. Korean J. Radiol. 2020, 21, 1055–1064. [Google Scholar] [CrossRef]
- van ‘t Klooster, C.C.; van der Graaf, Y.; Nathoe, H.M.; Bots, M.L.; de Borst, G.J.; Visseren, F.L.J.; Leiner, T.; Asselbergs, F.W.; Geerlings, M.I.; Emmelot, M.H.; et al. Added value of cardiovascular calcifications for prediction of recurrent cardiovascular events and cardiovascular interventions in patients with established cardiovascular disease. Int. J. Cardiovasc. Imaging 2021, 37, 2051–2061. [Google Scholar] [CrossRef]
- Polkampally, P.; Jovin, I.; Bottinor, W. Adverse Reactions to Iodinated Contrast Media. Int. J. Angiol. 2013, 22, 149–154. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Earls, J.P.; Berman, E.L.; Urban, B.A.; Curry, C.A.; Lane, J.L.; Jennings, R.S.; McCulloch, C.C.; Hsieh, J.; Londt, J.H. Prospectively Gated Transverse Coronary CT Angiography versus Retrospectively Gated Helical Technique: Improved Image Quality and Reduced Radiation Dose. Radiology 2008, 246, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; Sarandrea, A.; Rigattieri, S.; Patrizi, R.; Cera, M.; Di Russo, C.; Perone, F.; Porretta, V.; Fedele, S.; Romano, S.; et al. Staff radiation dose during percutaneous coronary procedures: Role of adjunctive protective drapes. Cardiovasc. Revascularization Med. 2018, 19 Pt A, 755–758. [Google Scholar] [CrossRef]
- Azzalini, L.; Candilio, L.; McCullough, P.A.; Colombo, A. Current Risk of Contrast-Induced Acute Kidney Injury After Coronary Angiography and Intervention: A Reappraisal of the Literature. Can. J. Cardiol. 2017, 33, 1225–1228. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.L.; Raman, S.V.; Nayak, K.; Epstein, F.H.; Ferreira, P.; Axel, L.; Kraitchman, D.L. Myocardial first-pass perfusion cardiovascular magnetic resonance: History, theory, and current state of the art. J. Cardiovasc. Magn. Reson. 2008, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Gavara, J.; Rodriguez-Palomares, J.F.; Valente, F.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Bonanad, C.; Ferreira-Gonzalez, I.; del Blanco, B.G.; Rodriguez-Garcia, J.; Mutuberria, M.; et al. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1448–1457. [Google Scholar] [CrossRef]
- Reindl, M.; Tiller, C.; Holzknecht, M.; Lechner, I.; Beck, A.; Plappert, D.; Gorzala, M.; Pamminger, M.; Mayr, A.; Klug, G.; et al. Prognostic Implications of Global Longitudinal Strain by Feature-Tracking Cardiac Magnetic Resonance in ST-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2019, 12, e009404. [Google Scholar] [CrossRef]
- Zemrak, F.; Petersen, S.E. Late Gadolinium Enhancement CMR Predicts Adverse Cardiovascular Outcomes and Mortality in Patients With Coronary Artery Disease: Systematic Review and Meta-Analysis. Prog. Cardiovasc. Dis. 2011, 54, 215–229. [Google Scholar] [CrossRef]
- Reindl, M.; Holzknecht, M.; Tiller, C.; Lechner, I.; Schiestl, M.; Simma, F.; Pamminger, M.; Henninger, B.; Mayr, A.; Klug, G.; et al. Impact of infarct location and size on clinical outcome after ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Int. J. Cardiol. 2020, 301, 14–20. [Google Scholar] [CrossRef]
- van Kranenburg, M.; Magro, M.; Thiele, H.; de Waha, S.; Eitel, I.; Cochet, A.; Cottin, Y.; Atar, D.; Buser, P.; Wu, E.; et al. Prognostic Value of Microvascular Obstruction and Infarct Size, as Measured by CMR in STEMI Patients. JACC: Cardiovasc. Imaging 2014, 7, 930–939. [Google Scholar] [CrossRef]
- Galea, N.; Dacquino, G.M.; Ammendola, R.M.; Coco, S.; Agati, L.; De Luca, L.; Carbone, I.; Fedele, F.; Catalano, C.; Francone, M. Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur. Radiol. 2019, 29, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Ferré-Vallverdú, M.; Sánchez-Lacuesta, E.; Plaza-López, D.; Díez-Gil, J.L.; Sepúlveda-Sanchis, P.; Gil-Cayuela, C.; Maceira-Gonzalez, A.; Miró-Palau, V.; Montero-Argudo, A.; Martínez-Dolz, L.; et al. Prognostic value and clinical predictors of intramyocardial hemorrhage measured by CMR T2* sequences in STEMI. Int. J. Cardiovasc. Imaging 2021, 37, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Stiermaier, T.; Reindl, M.; Feistritzer, H.-J.; Fuernau, G.; Eitel, C.; Desch, S.; Klug, G.; Thiele, H.; Metzler, B.; et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 138–146. [Google Scholar] [CrossRef]
- Ishiyama, M.; Kurita, T.; Nakamura, S.; Omori, T.; Nakamori, S.; Ishida, M.; Fujimoto, N.; Kitagawa, K.; Sakuma, H.; Ito, M.; et al. Prognostic importance of acute phase extracellular volume evaluated by cardiac magnetic resonance imaging for patients with acute myocardial infarction. Int. J. Cardiovasc. Imaging 2021, 37, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, M.; Masi, A.; Burrage, M.K.; Kotronias, R.A.; Borlotti, A.; Scarsini, R.; Banerjee, A.; Terentes-Printzios, D.; Zhang, Q.; Hann, E.; et al. Acute Response in the Noninfarcted Myocardium Predicts Long-Term Major Adverse Cardiac Events After STEMI. JACC Cardiovasc. Imaging 2023, 16, 46–59. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Corban, M.T.; Yang, S.-W.; Lewis, B.R.; Bois, J.; Foley, T.; Lerman, L.O.; Herrmann, J.; Oh, J.K.; Lerman, A. Microvascular obstruction in non-infarct related coronary arteries is an independent predictor of major adverse cardiovascular events in patients with ST segment-elevation myocardial infarction. Int. J. Cardiol. 2018, 273, 22–28. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Fuster, V.; Muntendam, P.; Mehran, R.; Baber, U.; Sartori, S.; Falk, E. A Simple Disease-Guided Approach to Personalize ACC/AHA-Recommended Statin Allocation in Elderly People: The BioImage Study. J. Am. Coll. Cardiol. 2016, 68, 881–891. [Google Scholar] [CrossRef]
- Blaha, M.J.; Mortensen, M.B.; Kianoush, S.; Tota-Maharaj, R.; Cainzos-Achirica, M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar] [CrossRef]
- Blaha, M.J.; Matsushita, K. Coronary Artery Calcium: Need for More Clarity in Guidelines. JACC Cardiovasc. Imaging 2017, 10, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADS™ 2.0—2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2022, 16, 536–557. [Google Scholar] [CrossRef]
- Choi, A.D.; Marques, H.; Kumar, V.; Griffin, W.F.; Rahban, H.; Karlsberg, R.P.; Zeman, R.K.; Katz, R.J.; Earls, J.P. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 470–476. [Google Scholar] [CrossRef]
- Kueh, S.H.; Boroditsky, M.; Leipsic, J. Fractional flow reserve computed tomography in the evaluation of coronary artery disease. Cardiovasc. Diagn. Ther. 2017, 7, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Volpato, V.; Cau, R.; Chiesa, M.; Saba, L.; Guglielmo, M.; Senatieri, A.; Chierchia, G.; Pontone, G.; Dell’aversana, S.; et al. Application of AI in cardiovascular multimodality imaging. Heliyon 2022, 8, e10872. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Greco, F.; Salgado, R.; Van Hecke, W.; Del Buono, R.; Parizel, P.M.; Mallio, C.A. Epicardial and pericardial fat analysis on CT images and artificial intelligence: A literature review. Quant. Imaging Med. Surg. 2022, 12, 2075–2089. [Google Scholar] [CrossRef]
- Phan, F.; Boussouar, S.; Lucidarme, O.; Zarai, M.; Salem, J.-E.; Kachenoura, N.; Bouazizi, K.; Charpentier, E.; Niati, Y.; Bekkaoui, H.; et al. Cardiac adipose tissue volume and IL-6 level at admission are complementary predictors of severity and short-term mortality in COVID-19 diabetic patients. Cardiovasc. Diabetol. 2021, 20, 165. [Google Scholar] [CrossRef]
- Charpentier, E.; Redheuil, A.; Bourron, O.; Boussouar, S.; Lucidarme, O.; Zarai, M.; Kachenoura, N.; Bouazizi, K.; Salem, J.E.; Hekimian, G.; et al. Cardiac adipose tissue volume assessed by computed tomography is a specific and independent predictor of early mortality and critical illness in COVID-19 in type 2-diabetic patients. Cardiovasc. Diabetol. 2022, 21, 294. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Ghosn, M.G.; Khan, M.A.; Gramze, N.L.; Brunner, G.; Nabi, F.; Nambi, V.; Nagueh, S.F.; Nguyen, D.T.; Graviss, E.A.; et al. Myocardial Extracellular Volume Fraction Adds Prognostic Information Beyond Myocardial Replacement Fibrosis. Circ. Cardiovasc. Imaging 2019, 12, e009535. [Google Scholar] [CrossRef] [PubMed]
- Redheuil, A.; Blanchard, A.; Pereira, H.; Raissouni, Z.; Lorthioir, A.; Soulat, G.; Vargas-Poussou, R.; Amar, L.; Paul, J.-L.; Helley, D.; et al. Aldosterone-Related Myocardial Extracellular Matrix Expansion in Hypertension in Humans: A Proof-of-Concept Study by Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2020, 13, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Kachenoura, N.; Raissuni, Z.; Mousseaux, E.; Young, J.; Graves, M.J.; Jublanc, C.; Cluzel, P.; Chanson, P.; Kamenický, P.; et al. Effects of cortisol on the heart: Characterization of myocardial involvement in cushing’s disease by longitudinal cardiac MRI T1 mapping. J. Magn. Reson. Imaging 2017, 45, 147–156. [Google Scholar] [CrossRef]
- Redheuil, A.; Yu, W.-C.; Wu, C.O.; Mousseaux, E.; de Cesare, A.; Yan, R.; Kachenoura, N.; Bluemke, D.; Lima, J.A.C. Reduced Ascending Aortic Strain and Distensibility: Earliest manifestations of vascular aging in humans. Hypertension 2010, 55, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Redheuil, A.; Wu, C.O.; Kachenoura, N.; Ohyama, Y.; Yan, R.T.; Bertoni, A.G.; Hundley, G.W.; Duprez, D.A.; Jacobs, D.R.; Daniels, L.B.; et al. Proximal Aortic Distensibility Is an Independent Predictor of All-Cause Mortality and Incident CV Events: The MESA study. J. Am. Coll. Cardiol. 2014, 64, 2619–2629. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. https://doi.org/10.3390/jcm12175563
Perone F, Bernardi M, Redheuil A, Mafrica D, Conte E, Spadafora L, Ecarnot F, Tokgozoglu L, Santos-Gallego CG, Kaiser SE, et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. Journal of Clinical Medicine. 2023; 12(17):5563. https://doi.org/10.3390/jcm12175563
Chicago/Turabian StylePerone, Francesco, Marco Bernardi, Alban Redheuil, Dario Mafrica, Edoardo Conte, Luigi Spadafora, Fiona Ecarnot, Lale Tokgozoglu, Carlos G. Santos-Gallego, Sergio Emanuel Kaiser, and et al. 2023. "Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions" Journal of Clinical Medicine 12, no. 17: 5563. https://doi.org/10.3390/jcm12175563
APA StylePerone, F., Bernardi, M., Redheuil, A., Mafrica, D., Conte, E., Spadafora, L., Ecarnot, F., Tokgozoglu, L., Santos-Gallego, C. G., Kaiser, S. E., Fogacci, F., Sabouret, A., Bhatt, D. L., Paneni, F., Banach, M., Santos, R., Biondi Zoccai, G., Ray, K. K., & Sabouret, P. (2023). Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. Journal of Clinical Medicine, 12(17), 5563. https://doi.org/10.3390/jcm12175563










