Upper Esophageal Sphincter Metrics across Eosinophilic Esophagitis, Gastroesophageal Reflux Disease and Functional Dysphagia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. High-Resolution Manometry and Protocol
2.3. High-Resolution Manometry Data Analysis
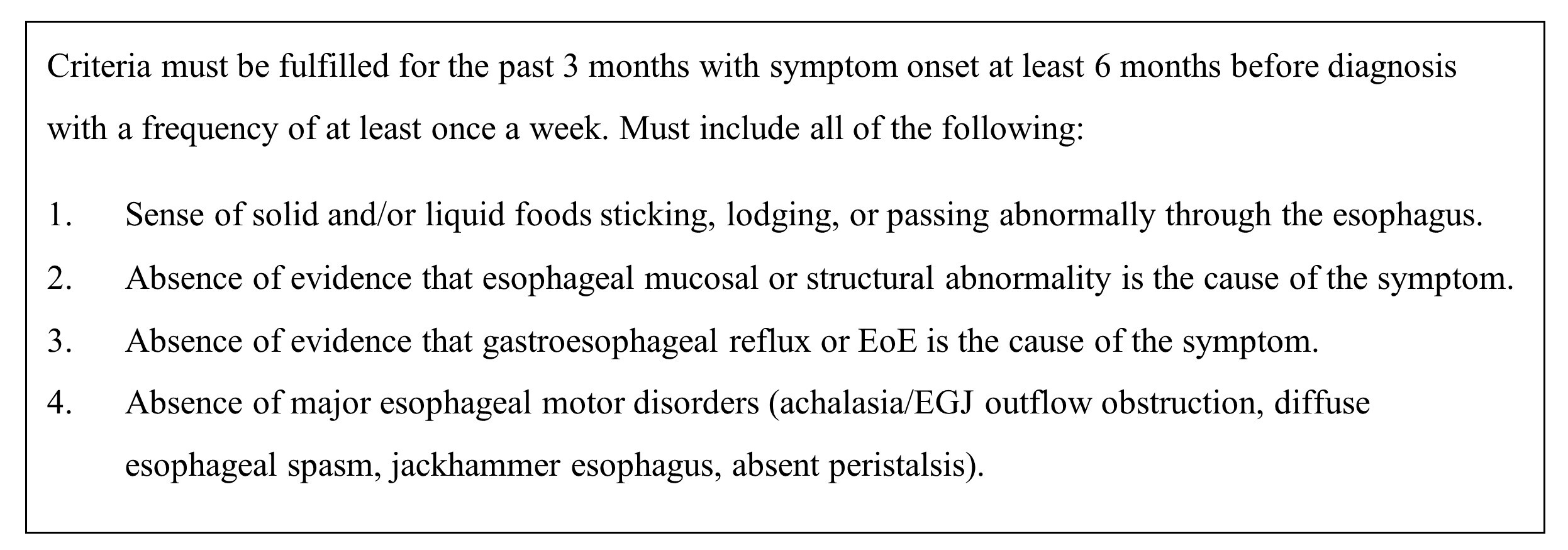
2.4. Standardized Symptoms Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
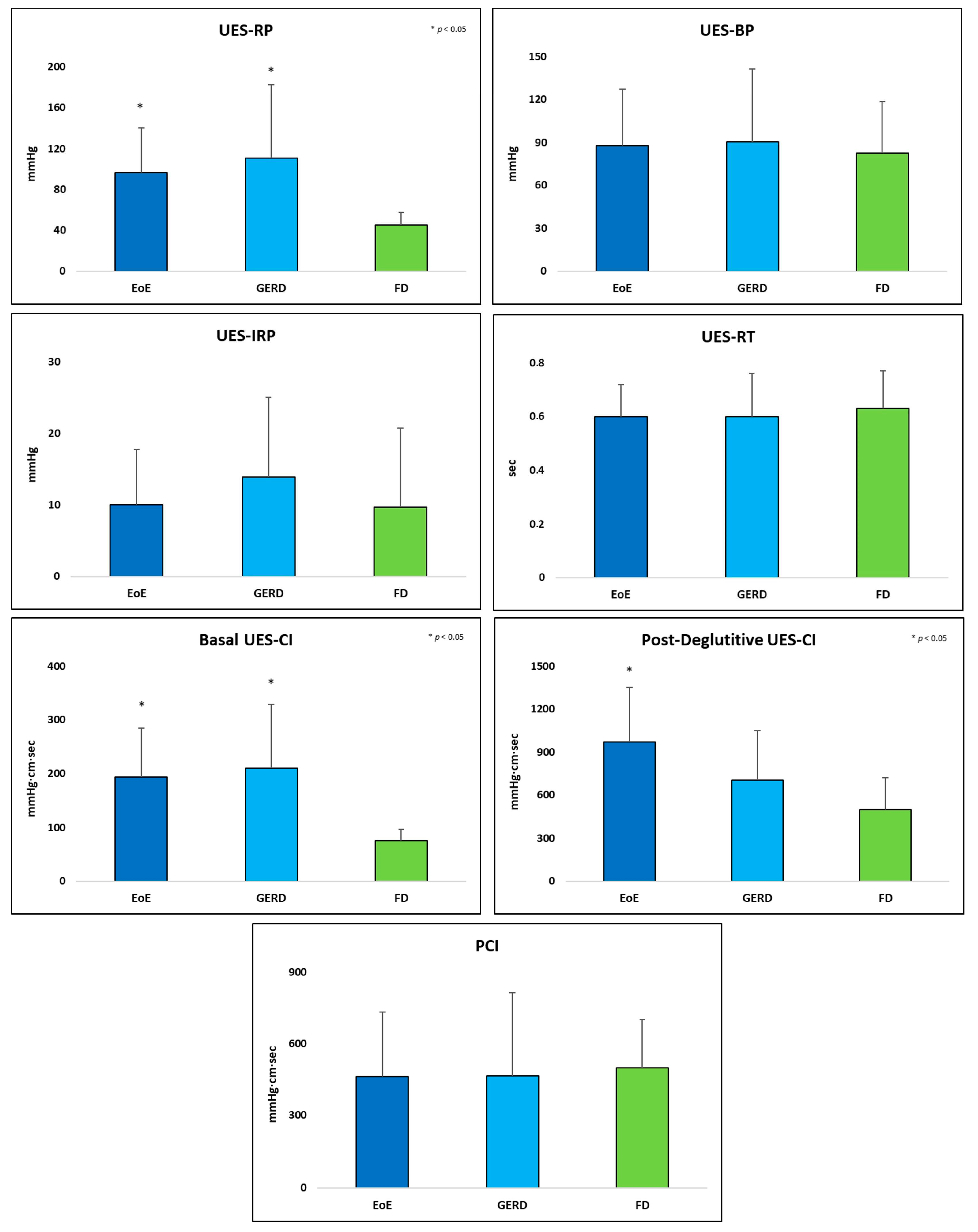
3.2. UES Metrics
3.3. Esophageal Body Contraction and EGJ Metrics
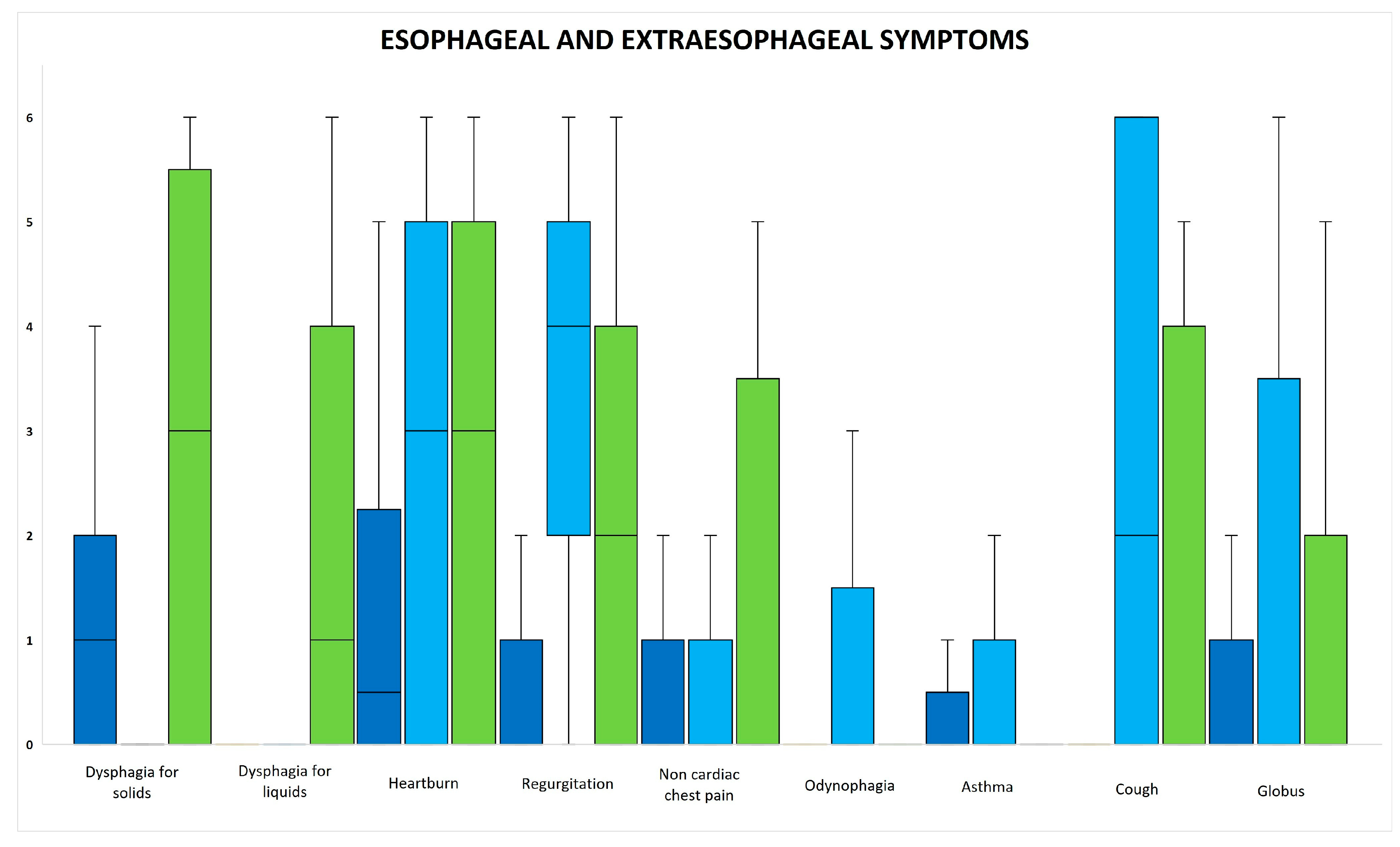
3.4. Esophageal and Extraesophageal Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivarao, D.V.; Goyal, R.K. Functional anatomy and physiology of the upper esophageal sphincter. Am. J. Med. 2000, 108 (Suppl. 4a), 27S–37S. [Google Scholar] [CrossRef]
- Lang, I.M.; Shaker, R. An overview of the upper esophageal sphincter. Curr. Gastroenterol. Rep. 2000, 2, 185–190. [Google Scholar] [CrossRef]
- Singh, S.; Hamdy, S. The upper oesophageal sphincter. Neurogastroenterol. Motil. 2005, 17 (Suppl. 1), 3–12. [Google Scholar] [CrossRef]
- Blais, P.; Patel, A.; Sayuk, G.S.; Gyawali, C.P. Upper esophageal sphincter (UES) metrics on high-resolution manometry (HRM) differentiate achalasia subtypes. Neurogastroenterol. Motil. 2017, 29, e13136. [Google Scholar] [CrossRef]
- Triantafyllou, T.; Theodoropoulos, C.; Mantides, A.; Chrysikos, D.; Smparounis, S.; Filis, K.; Zografos, G.; Theodorou, D. Can the upper esophageal sphincter contractile integral help classify achalasia? Ann. Gastroenterol. 2018, 31, 456–461. [Google Scholar] [CrossRef]
- Blais, P.; Bennett, M.C.; Gyawali, C.P. Upper esophageal sphincter metrics on high-resolution manometry differentiate etiologies of esophagogastric junction outflow obstruction. Neurogastroenterol. Motil. 2019, 31, e13558. [Google Scholar] [CrossRef]
- Nadaleto, B.F.; Herbella, F.A.; Pinna, B.R.; Patti, M.G. Upper esophageal sphincter motility in gastroesophageal reflux disease in the light of the high-resolution manometry. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef]
- de Bortoli, N.; Penagini, R.; Savarino, E.; Marchi, S. Eosinophilic esophagitis: Update in diagnosis and management. Position paper by the Italian Society of Gastroenterology and Gastrointestinal Endoscopy (SIGE). Dig. Liver Dis. 2017, 49, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Ghisa, M.; Barberio, B.; Marabotto, E.; de Bortoli, N.; Savarino, E. Systematic Review: Esophageal motility patterns in patients with eosinophilic esophagitis. Dig. Liver Dis. 2022, 54, 1143–1152. [Google Scholar] [CrossRef]
- Ghisa, M.; Laserra, G.; Marabotto, E.; Ziola, S.; Tolone, S.; de Bortoli, N.; Frazzoni, M.; Mauro, A.; Penagini, R.; Savarino, V.; et al. Achalasia and Obstructive Motor Disorders Are Not Uncommon in Patients With Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1554–1563. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2013, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Boyd, J.T.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.A.; et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef]
- Aziz, Q.; Fass, R.; Gyawali, C.P.; Miwa, H.; Pandolfino, J.E.; Zerbib, F. Functional Esophageal Disorders. Gastroenterology 2016, 150, 1368–1379. [Google Scholar] [CrossRef]
- Kahrilas, P.J.; Bredenoord, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.J.; Pandolfino, J.E. International High Resolution Manometry Working G: The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015, 27, 160–174. [Google Scholar] [CrossRef]
- Swallow Gateway High Resolution Manometry. Available online: https://swallowgateway.com/ (accessed on 15 February 2022).
- Omari, T.I.; Savilampi, J.; Kokkinn, K.; Schar, M.; Lamvik, K.; Doeltgen, S.; Cock, C. The Reliability of Pharyngeal High Resolution Manometry with Impedance for Derivation of Measures of Swallowing Function in Healthy Volunteers. Int. J. Otolaryngol. 2016, 2016, 2718482. [Google Scholar] [CrossRef][Green Version]
- Nollet, J.L.; Cajander, P.; Ferris, L.F.; Ramjith, J.; Omari, T.I.; Savilampi, J. Pharyngo-Esophageal Modulatory Swallow Responses to Bolus Volume and Viscosity Across Time. Laryngoscope 2022, 132, 1817–1824. [Google Scholar] [CrossRef]
- Amato, G.; Limongelli, P.; Pascariello, A.; Rossetti, G.; Del Genio, G.; Del Genio, A.; Iovino, P. Association between persistent symptoms and long-term quality of life after laparoscopic total fundoplication. Am. J. Surg. 2008, 196, 582–586. [Google Scholar] [CrossRef]
- Carpinelli, L.; Bucci, C.; Santonicola, A.; Zingone, F.; Ciacci, C.; Iovino, P. Anhedonia in irritable bowel syndrome and in inflammatory bowel diseases and its relationship with abdominal pain. Neurogastroenterol. Motil. 2019, 31, e13531. [Google Scholar] [CrossRef]
- Oliviero, G.; Ruggiero, L.; D’Antonio, E.; Gagliardi, M.; Nunziata, R.; Di Sarno, A.; Abbatiello, C.; Di Feo, E.; De Vivo, S.; Santonicola, A.; et al. Impact of COVID-19 lockdown on symptoms in patients with functional gastrointestinal disorders: Relationship with anxiety and perceived stress. Neurogastroenterol. Motil. 2021, 33, e14092. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L.; Iovino, P.; Ameno, C.; Palma, R.; Santonicola, A. Perception of the COVID-19 pandemic in patients with achalasia and its impact on gastrointestinal symptoms: A proof-of-concept study. Ann. Gastroenterol. 2022, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Passaretti, S.; Mazzoleni, G.; Vailati, C.; Testoni, P.A. Oropharyngeal acid reflux and motility abnormalities of the proximal esophagus. World J. Gastroenterol. 2016, 22, 8991–8998. [Google Scholar] [CrossRef]
- Jiao, H.; Mei, L.; Liang, C.; Dai, Y.; Fu, Z.; Wu, L.; Sanvanson, P.; Shaker, R. Upper esophageal sphincter augmentation reduces pharyngeal reflux in nasogastric tube-fed patients. Laryngoscope 2018, 128, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Sato, T.; Goto, T.; Koyama, M.; Yamauchi, A.; Mizukami, A.; Yamasoba, T. Effects of Aspiration Prevention Surgery on the Dynamics of the Pharynx and Upper Esophageal Sphincter. OTO Open 2021, 5, 2473974X211048505. [Google Scholar] [CrossRef]
- Rengarajan, A.; Gyawali, C.P. High-resolution Manometry can Characterize Esophagogastric Junction Morphology and Predict Esophageal Reflux Burden. J. Clin. Gastroenterol. 2020, 54, 22–27. [Google Scholar] [CrossRef]
- Blanchard, C.; Wang, N.; Stringer, K.F.; Mishra, A.; Fulkerson, P.C.; Abonia, J.P.; Jameson, S.C.; Kirby, C.; Konikoff, M.R.; Collins, M.H.; et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Investig. 2006, 116, 536–547. [Google Scholar] [CrossRef]
- Fox, V.L.; Nurko, S.; Teitelbaum, J.E.; Badizadegan, K.; Furuta, G.T. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest. Endosc. 2003, 57, 30–36. [Google Scholar] [CrossRef]
- Persad, R.; Huynh, H.Q.; Hao, L.; Ha, J.R.; Sergi, C.; Srivastava, R.; Persad, S. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-alpha-NFkappaB pathway. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 251–260. [Google Scholar] [CrossRef]
- Dellon, E.S.; Kim, H.P.; Sperry, S.L.; Rybnicek, D.A.; Woosley, J.T.; Shaheen, N.J. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest. Endosc. 2014, 79, 577–585.e4. [Google Scholar] [CrossRef]
- Kwiatek, M.A.; Hirano, I.; Kahrilas, P.J.; Rothe, J.; Luger, D.; Pandolfino, J.E. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011, 140, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Saffari, H.; Peterson, K.A.; Fang, J.C.; Teman, C.; Gleich, G.J.; Pease, L.F., 3rd. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J. Allergy Clin. Immunol. 2012, 130, 798–800. [Google Scholar] [CrossRef]
- Korsapati, H.; Babaei, A.; Bhargava, V.; Dohil, R.; Quin, A.; Mittal, R.K. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut 2009, 58, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Nonevski, I.; Ma, J.; Ouyang, Z.; West, G.; Protheroe, C.; De Petris, G.; Schirbel, A.; Lapinski, J.; Goldblum, J.; et al. T-helper 2 cytokines, transforming growth factor beta1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology 2014, 146, 1266–1277.e9. [Google Scholar] [CrossRef] [PubMed]
- Mavi, P.; Rajavelu, P.; Rayapudi, M.; Paul, R.J.; Mishra, A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1347–G1355. [Google Scholar] [CrossRef]
- Daniel, E.E.; Crankshaw, J.; Sarna, S. Prostaglandins and myogenic control of tension in lower esophageal sphincter in vitro. Prostaglandins 1979, 17, 629–639. [Google Scholar] [CrossRef]
- Gleich, G.J.; Adolphson, C.R.; Leiferman, K.M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 1993, 44, 85–101. [Google Scholar] [CrossRef]
- Spechler, S.J. Eosinophilic esophagitis: Novel concepts regarding pathogenesis and clinical manifestations. J. Gastroenterol. 2019, 54, 837–844. [Google Scholar] [CrossRef]
- Lu, P.W.; Chen, C.C.; Wu, J.F.; Lee, H.C.; Lee, Y.C.; Wang, H.P.; Wu, M.S.; Tseng, P.H. Clinical Characteristics and Associated Psychosocial Dysfunction in Patients With Functional Dysphagia: A Study Based on High-Resolution Impedance Manometry and Rome IV Criteria. Clin. Transl. Gastroenterol. 2022, 13, e00511. [Google Scholar] [CrossRef]
- Lenti, M.V.; Savarino, E.; Mauro, A.; Penagini, R.; Racca, F.; Ghisa, M.; Laserra, G.; Merli, S.; Arsie, E.; Longoni, V.; et al. Diagnostic delay and misdiagnosis in eosinophilic oesophagitis. Dig. Liver Dis. 2021, 53, 1632–1639. [Google Scholar] [CrossRef]
- Lei, W.Y.; Omari, T.; Liu, T.T.; Wong, M.W.; Hung, J.S.; Yi, C.H.; Liang, S.W.; Cock, C.; Chen, C.L. Esophageal Bolus Domain Pressure and Peristalsis Associated With Experimental Induction of Esophagogastric Junction Outflow Obstruction. J. Neurogastroenterol. Motil. 2022, 28, 62–68. [Google Scholar] [CrossRef]
- Omari, T.; Rommel, N.; Jan, T.; Szczesniak, M.; Wu, P.; Schar, M.; Doeltgen, S.; Cock, C. Transient hypopharyngeal intrabolus pressurization patterns: Clinically relevant or normal variant? Neurogastroenterol. Motil. 2022, 34, e14276. [Google Scholar] [CrossRef]
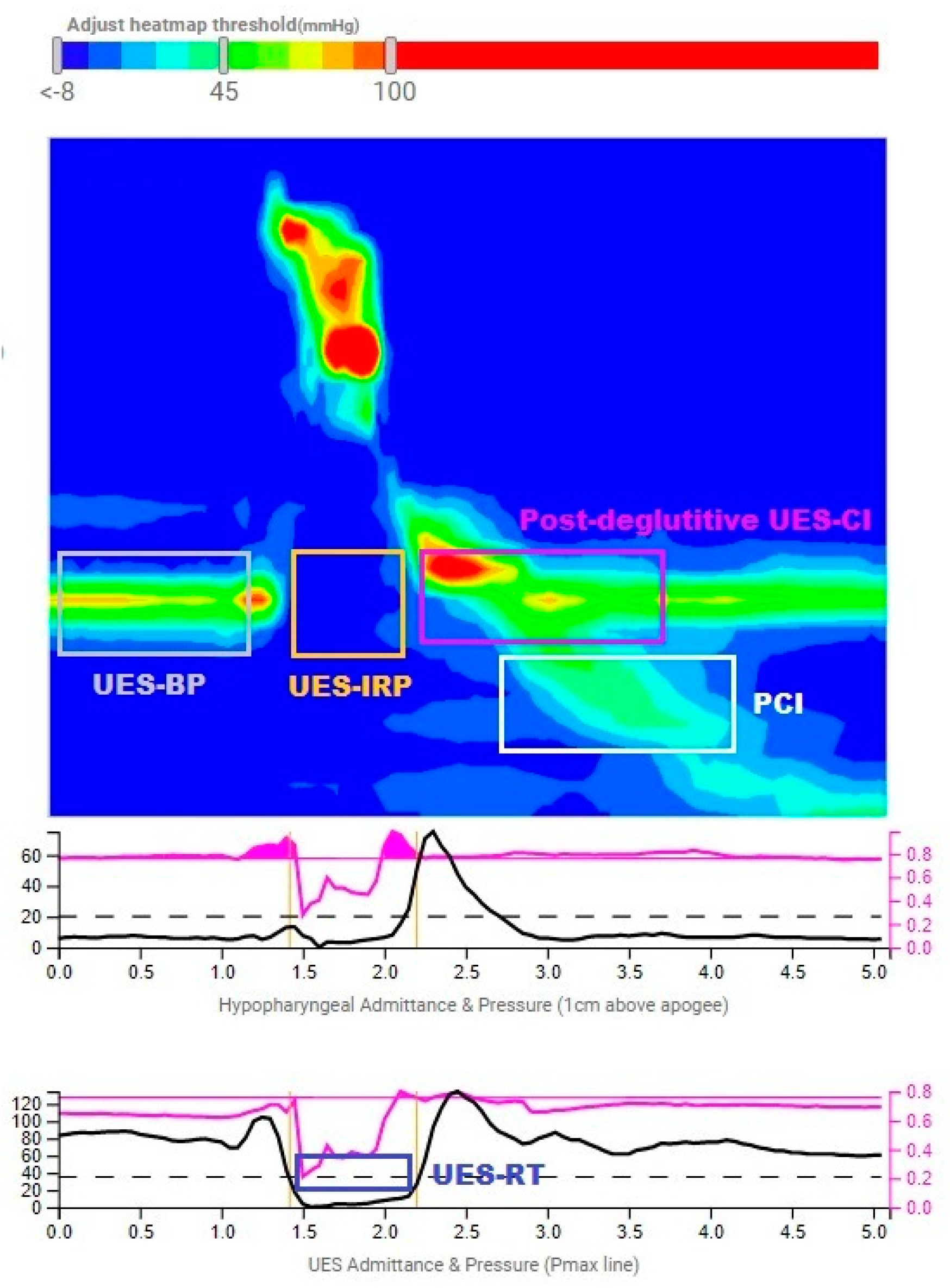
| Metric | Acronym (Units) | Definition |
|---|---|---|
| UES Resting Pressure | UES-RP (mmHg) | Mean of UES axial maximum pressures from the proximal limit of the UES to the distal point of the sphincter for three consecutive respiratory cycles [5] |
| UES Basal Pressure | UES-BP (mmHg) | Mean of UES axial maximum pressures preceding UES relaxation [19] |
| UES Integrated Relaxation Pressure | UES-IRP (mmHg) | The extent of UES relaxation defined as the median of the lowest pressures in a nonconsecutive window of 0.25 s [19] |
| UES Relaxation Time | UES-RT (s) | Duration of UES relaxation defined as the interval when pressure is <50% of baseline or <35 mmHg (the lowest) [19] |
| Basal UES Contractile Integral | Basal UES-CI (mmHg∙cm∙s) | The integral of pressures from the proximal limit of the UES to the distal point of the sphincter for three consecutive respiratory cycles [5] |
| Post-Deglutitive UES Contractile Integral | Post-Deglutitive UES-CI (mmHg∙cm∙s) | The integral of pressures of the UES post-swallow, indicating UES contractile vigor [19] |
| Proximal Contractile Integral | PCI (mmHg∙cm∙s) | The integral of pressures > 20 mmHg within the proximal esophagus region, indicating contractile vigor of the proximal esophagus [19] |
| EoE | GERD | FD | p-Value | |
|---|---|---|---|---|
| Number (%total) | 30 (39.0%) | 18 (23.4%) | 29 (37.7%) | - |
| Age (years, M ± SD) | 39.1 ± 13.2 | 52.1 ± 15.2 | 51.7 ± 14.6 | 0.005 |
| Gender (%M) | 27 (90%) | 9 (50.0%) | 12 (41.4%) | <0.001 |
| BMI (Kg/m2, M ± SD) | 24.7 ± 3.2 | 29.0 ± 5.0 | 24.7 ± 3.9 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggiero, L.; Iovino, P.; Gargano, D.; Caloro, A.; De Leo, L.; D’Antonio, A.; Caputo, A.; Santonicola, A. Upper Esophageal Sphincter Metrics across Eosinophilic Esophagitis, Gastroesophageal Reflux Disease and Functional Dysphagia: A Pilot Study. J. Clin. Med. 2023, 12, 5548. https://doi.org/10.3390/jcm12175548
Ruggiero L, Iovino P, Gargano D, Caloro A, De Leo L, D’Antonio A, Caputo A, Santonicola A. Upper Esophageal Sphincter Metrics across Eosinophilic Esophagitis, Gastroesophageal Reflux Disease and Functional Dysphagia: A Pilot Study. Journal of Clinical Medicine. 2023; 12(17):5548. https://doi.org/10.3390/jcm12175548
Chicago/Turabian StyleRuggiero, Luigi, Paola Iovino, Domenico Gargano, Angela Caloro, Luca De Leo, Antonio D’Antonio, Alessandro Caputo, and Antonella Santonicola. 2023. "Upper Esophageal Sphincter Metrics across Eosinophilic Esophagitis, Gastroesophageal Reflux Disease and Functional Dysphagia: A Pilot Study" Journal of Clinical Medicine 12, no. 17: 5548. https://doi.org/10.3390/jcm12175548
APA StyleRuggiero, L., Iovino, P., Gargano, D., Caloro, A., De Leo, L., D’Antonio, A., Caputo, A., & Santonicola, A. (2023). Upper Esophageal Sphincter Metrics across Eosinophilic Esophagitis, Gastroesophageal Reflux Disease and Functional Dysphagia: A Pilot Study. Journal of Clinical Medicine, 12(17), 5548. https://doi.org/10.3390/jcm12175548










