Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection
Abstract
1. Introduction
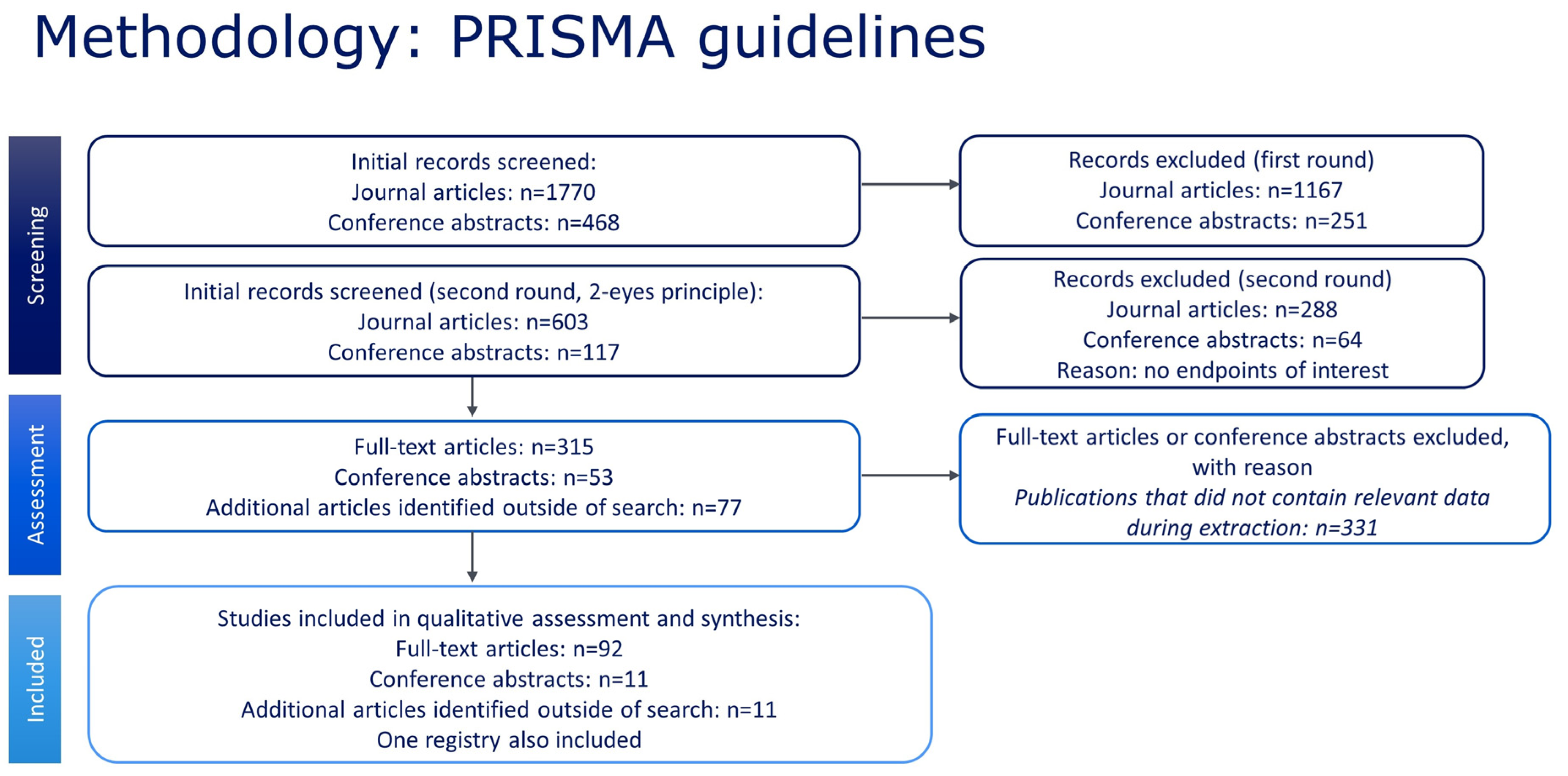
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria for Publications
2.3. Study Selection
2.4. Study Quality
2.5. Data Extraction, Reporting, and Synthesis
2.6. Quantitative Analyses of Prevalence and Birth Prevalence
2.7. Quantitative Analyses of Mortality
3. Results
3.1. Qualitative Data Analysis
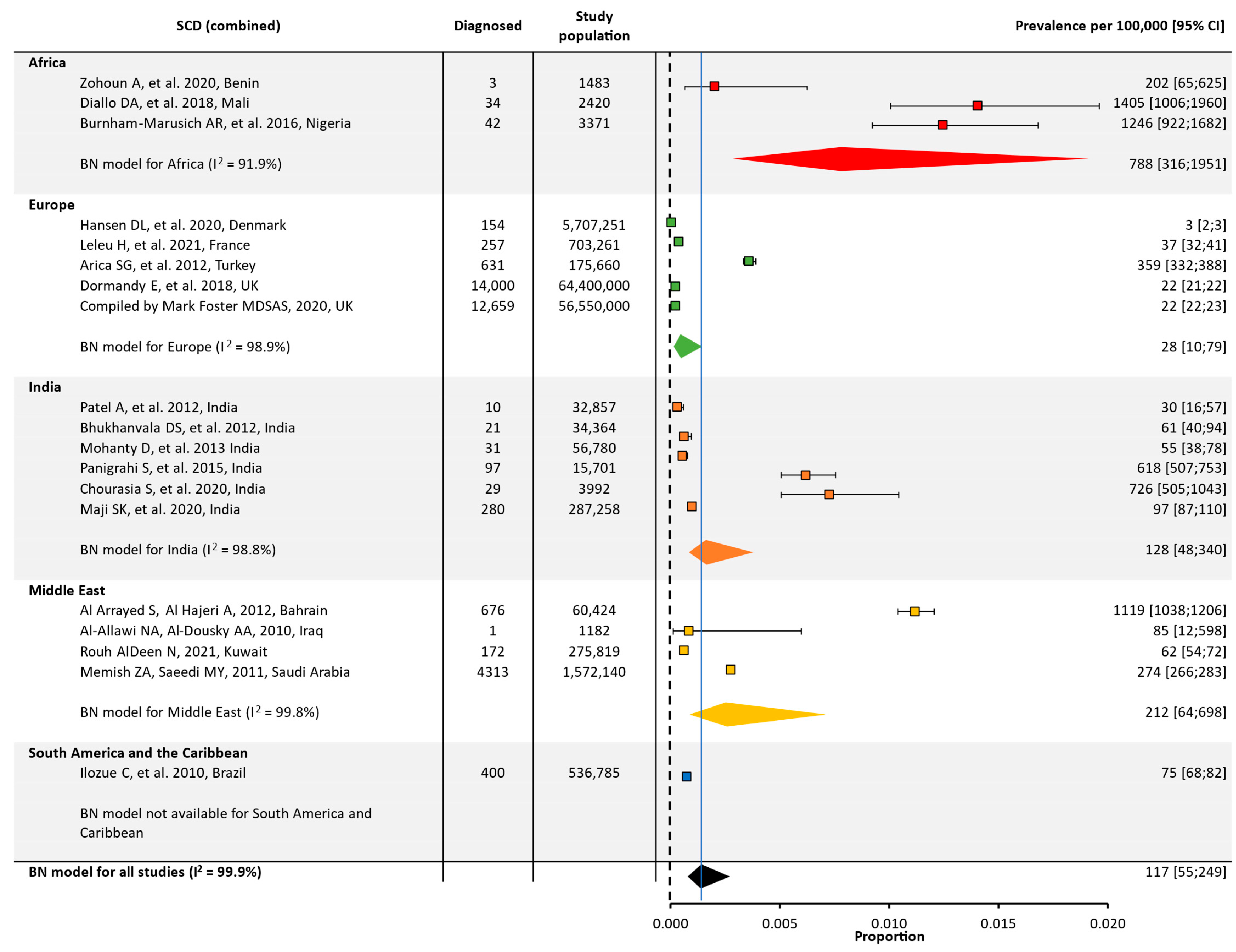
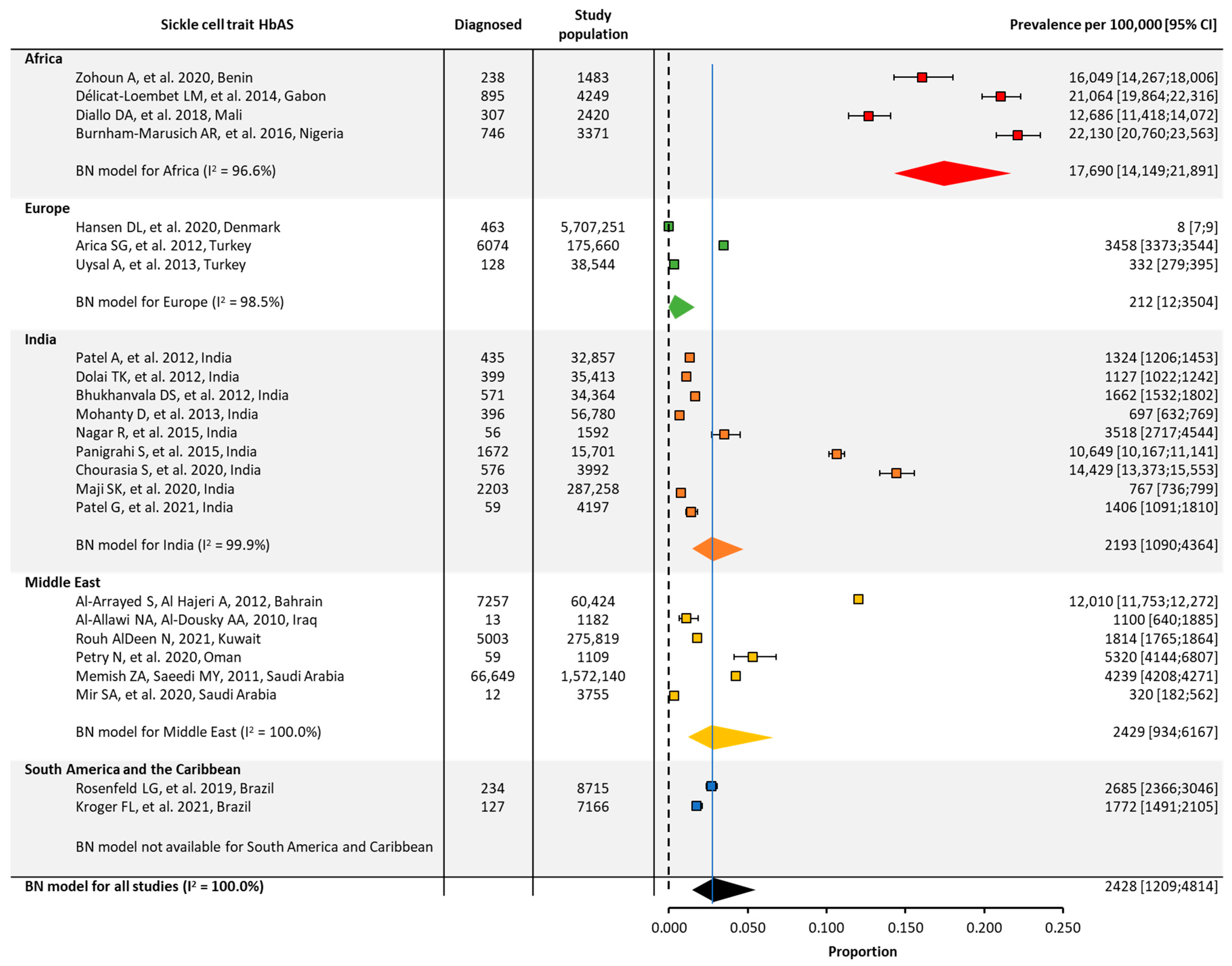
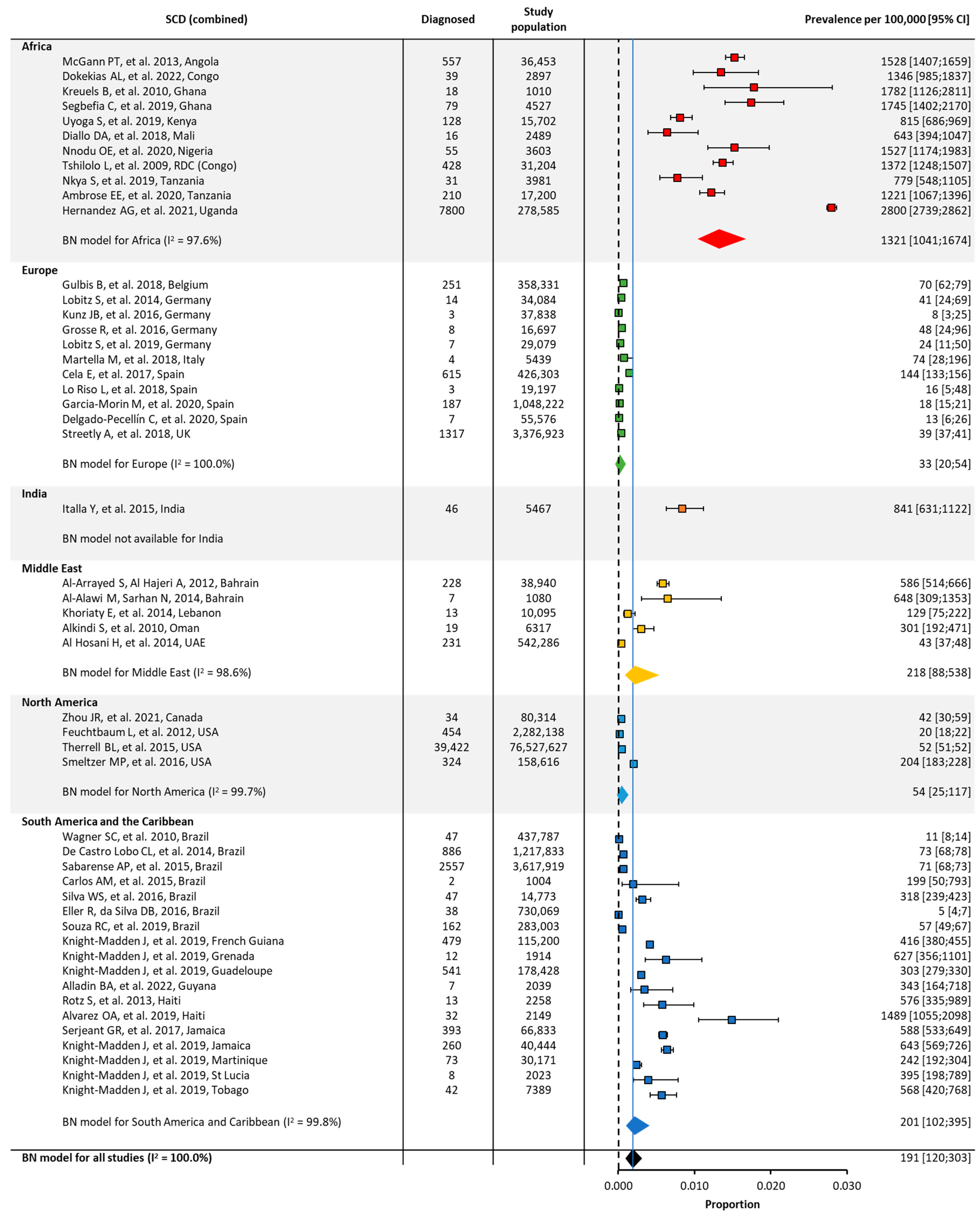
3.2. Quantitative Meta-Analysis of Prevalence and Birth Prevalence
3.3. Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Dis. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Kruse-Jarres, R. Management of sickle cell disease from childhood through adulthood. Blood Rev. 2013, 27, 279–287. [Google Scholar] [CrossRef]
- Ranque, B.; Kitenge, R.; Ndiaye, D.D.; Ba, M.D.; Adjoumani, L.; Traore, H.; Coulibaly, C.; Guindo, A.; Boidy, K.; Mbuyi, D.; et al. Estimating the risk of child mortality attributable to sickle cell anaemia in sub-Saharan Africa: A retrospective, multicentre, case-control study. Lancet Haematol. 2022, 9, e208–e216. [Google Scholar] [CrossRef] [PubMed]
- Lubeck, D.; Agodoa, I.; Bhakta, N.; Danese, M.; Pappu, K.; Howard, R.; Gleeson, M.; Halperin, M.; Lanzkron, S. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw. Open 2019, 2, e1915374. [Google Scholar] [CrossRef]
- Makani, J.; Ofori-Acquah, S.F.; Nnodu, O.; Wonkam, A.; Ohene-Frempong, K. Sickle cell disease: New opportunities and challenges in Africa. Sci. World J. 2013, 2013, 193252. [Google Scholar] [CrossRef]
- Smith, Y. Sickle-Cell Disease Classification. Available online: https://www.news-medical.net/health/Sickle-Cell-Disease-Classification.aspx (accessed on 21 August 2023).
- Notarangelo, L.D.; Agostini, A.; Casale, M.; Samperi, P.; Arcioni, F.; Gorello, P.; Perrotta, S.; Masera, N.; Barone, A.; Bertoni, E.; et al. HbS/b+ thalassemia: Really a mild disease? A National survey from the AIEOP Sickle Cell Disease Study Group with genotype-phenotype correlation. Eur. J. Haematol. 2020, 104, 214–222. [Google Scholar] [CrossRef]
- Torres, L.D.S.; Okumura, J.V.; Silva, D.G.J.; Bonini-Domingos, C.R. Hemoglobin D-Punjab: Origin, distribution and laboratory diagnosis. Rev. Bras. Hematol. Hemoter. 2015, 37, 120–126. [Google Scholar] [CrossRef]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef]
- Wastnedge, E.; Waters, D.; Patel, S.; Morrison, K.; Goh, M.Y.; Adeloye, D.; Rudan, I. The global burden of sickle cell disease in children under five years of age: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 021103. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions. 2011. Available online: https://training.cochrane.org/handbook (accessed on 21 August 2023).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Human Mortality Database. Max Planck Institute for Demographic Research (Germany), University of California, Berkeley (USA), and French Institute for Demographic Studies (France). Available online: www.mortality.org (accessed on 21 August 2023).
- Human Life-Table Database. 2022. Available online: https://www.lifetable.de/ (accessed on 21 August 2023).
- Burnham-Marusich, A.; Ezeanolue, C.; Obiefune, M.; Yang, W.; Osuji, A.; Ogidi, A.; Hunt, A.; Patel, D.; Ezeanolue, E.E. Prevalence Of Sickle Cell Trait And Reliability Of Self-Reported Status Among Expectant Parents In Nigeria: Implications For Targeted Newborn Screening. Public Health Genom. 2016, 19, 298–306. [Google Scholar] [CrossRef]
- Diallo, D.A.; Guindo, A.; Toure, B.A.; Sarro, Y.S.; Sima, M.; Tessougue, O.; Baraika, M.A.; Guindo, P.; Traore, M.; Diallo, M.; et al. Targeted newborn screening for sickle-cell anemia: Sickling test (Emmel test) boundaries in the prenatal assessment in West African area. Rev. Epidemiol. Sante Publique 2018, 66, 181–185. [Google Scholar] [CrossRef]
- Zohoun, A.; Baglo Agbodande, T.; Zohoun, L.; Anani, L. Prevalence of hemoglobin abnormalities in an apparently healthy population in Benin. Hematol. Transfus. Cell Ther. 2020, 42, 145–149. [Google Scholar] [CrossRef]
- Dormandy, E.; James, J.; Inusa, B.; Rees, D. How many people have sickle cell disease in the UK? J. Public Health 2018, 40, e291–e295. [Google Scholar] [CrossRef]
- Arıca, S.; Turhan, E.; Özer, C.; Arıca, V.; Şilfeler, D.; Şilfeler, I.; Altun, A. Evaluation of hemoglobinopathy screening results of a six year period in Turkey. Int. J. Collab. Res. Intern. Med. Public Health 2012, 4, 145–151. [Google Scholar]
- Leleu, H.; Arlet, J.B.; Habibi, A.; Etienne-Julan, M.; Khellaf, M.; Adjibi, Y.; Pirenne, F.; Pitel, M.; Granghaud, A.; Sinniah, C.; et al. Epidemiology and disease burden of sickle cell disease in France: A descriptive study based on a French nationwide claim database. PLoS ONE 2021, 16, e0253986. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.L.; Glenthoj, A.; Moller, S.; Biemond, B.J.; Andersen, K.; Gaist, D.; Petersen, J.; Frederiksen, H. Prevalence of Congenital Hemolytic Disorders in Denmark, 2000-2016. Clin. Epidemiol. 2020, 12, 485–495. [Google Scholar] [CrossRef] [PubMed]
- National Haemoglobinopathy Registry (Compiled by Mark Foster). Annual Data Report; 2019/2020. Available online: https://nhr.mdsas.com/wp-content/uploads/2021/01/2020_12_NHR_AnnualReport201920_Final.pdf (accessed on 21 August 2023).
- Chourasia, S.; Kumar, R.; Singh, M.; Vishwakarma, C.; Gupta, A.K.; Shanmugam, R. High Prevalence of Anemia and Inherited Hemoglobin Disorders in Tribal Populations of Madhya Pradesh State, India. Hemoglobin 2020, 44, 391–396. [Google Scholar] [CrossRef]
- Maji, S.K.; Dolai, T.K.; Pradhan, S.; Maity, A.; Mandal, S.; Mondal, T.; Manna, S.; Mandal, P.K. Implications of Population Screening for Thalassemias and Hemoglobinopathies in Rural Areas of West Bengal, India: Report of a 10-Year Study of 287,258 Cases. Hemoglobin 2020, 44, 432–437. [Google Scholar] [CrossRef]
- Panigrahi, S.; Patra, P.K.; Khodiar, P.K. The screening and morbidity pattern of sickle cell anemia in chhattisgarh. Indian J. Hematol. Blood Transfus. 2015, 31, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Colah, R.B.; Gorakshakar, A.C.; Patel, R.Z.; Master, D.C.; Mahanta, J.; Sharma, S.K.; Chaudhari, U.; Ghosh, M.; Das, S.; et al. Prevalence of beta-thalassemia and other haemoglobinopathies in six cities in India: A multicentre study. J. Community Genet. 2013, 4, 33–42. [Google Scholar] [CrossRef]
- Bhukhanvala, D.S.; Sorathiya, S.M.; Shah, A.P.; Patel, A.G.; Gupte, S.C. Prevalence and hematological profile of beta-thalassemia and sickle cell anemia in four communities of Surat city. Indian J. Hum. Genet. 2012, 18, 167–171. [Google Scholar] [PubMed]
- Patel, A.G.; Shah, A.P.; Sorathiya, S.M.; Gupte, S.C. Hemoglobinopathies in South Gujarat population and incidence of anemia in them. Indian J. Hum. Genet. 2012, 18, 294–298. [Google Scholar]
- Memish, Z.A.; Saeedi, M.Y. Six-year outcome of the national premarital screening and genetic counseling program for sickle cell disease and beta-thalassemia in Saudi Arabia. Ann. Saudi Med. 2011, 31, 229–235. [Google Scholar] [CrossRef]
- Rouh AlDeen, N.; Osman, A.; Alhabashi, M.; Al Khaldi, R.; Alawadi, H.; Alromh, M.; Alyafai, E.; Akbulut-Jeradi, N. The Prevalence of β-Thalassemia and Other Hemoglobinopathies in Kuwaiti Premarital Screening Program: An 11-Year Experience. J. Pers. Med. 2021, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Al-Allawi, N.; Al-Dousky, A. Frequency of haemoglobinopathies at premarital health screening in Dohuk, Iraq: Implications for a regional prevention programme. East Mediterr. Health J. 2010, 16, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Al Arrayed, S.; Al Hajeri, A. Newborn Screening Services in Bahrain between 1985 and 2010. Adv. Hematol. 2012, 2012, 903219. [Google Scholar] [CrossRef]
- Ilozue, C.; Cipolotti, R.; Melo, C.A.; Gurgel, R.Q.; Cuevas, L.E. Estimating the post-neonatal prevalence of sickle cell disease in a Brazilian population. Trop. Med. Int. Health 2010, 15, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Delicat-Loembet, L.M.; Elguero, E.; Arnathau, C.; Durand, P.; Ollomo, B.; Ossari, S.; Mezui-me-ndong, J.; Mbang Mboro, T.; Becquart, P.; Nkoghe, D.; et al. Prevalence of the sickle cell trait in Gabon: A nationwide study. Infect. Genet. Evol. 2014, 25, 52–56. [Google Scholar] [CrossRef]
- Uysal, A.; Genc, A.; Tasyurek, N.; Turkyilmaz, B. Prevalence of beta-thalassemia trait and abnormal hemoglobin in premarital screening in the province of Izmir, Turkey. Pediatr. Hematol. Oncol. 2013, 30, 46–50. [Google Scholar] [CrossRef]
- Dolai, T.K.; Dutta, S.; Bhattacharyya, M.; Ghosh, M.K. Prevalence of hemoglobinopathies in rural Bengal, India. Hemoglobin 2012, 36, 57–63. [Google Scholar] [CrossRef]
- Nagar, R.; Sinha, S.; Raman, R. Haemoglobinopathies in eastern Indian states: A demographic evaluation. J. Community Genet. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Patel, G.M.; Parmar, A.; Zalavadiya, D.; Talati, K. Tackling the Menace of Anemia and Hemoglobinopathies among Young Adults—Conceptualizing University-Level Screening. Indian J. Community Med. 2021, 46, 117–120. [Google Scholar]
- Mir, S.A.; Alshehri, B.M.; Alaidarous, M.; Banawas, S.S.; Dukhyil, A.; Alturki, M.K. Prevalence of Hemoglobinopathies (beta-Thalassemia and Sickle Cell Trait) in the Adult Population of Al Majma’ah, Saudi Arabia. Hemoglobin 2020, 44, 47–50. [Google Scholar] [CrossRef]
- Petry, N.; Al-Maamary, S.A.; Woodruff, B.A.; Alghannami, S.; Al-Shammakhi, S.M.; Al-Ghammari, I.K.; Tyler, V.; Rohner, F.; Wirth, J.P. National Prevalence of Micronutrient Deficiencies, Anaemia, Genetic Blood Disorders and Over- and Undernutrition in Omani Women of Reproductive Age and Preschool Children. Sultan Qaboos Univ. Med. J. 2020, 20, e151–e164. [Google Scholar] [CrossRef] [PubMed]
- Kroger, F.L.; Ernesto, I.C.; Silva, M.S.; Santos, O.F.D.; Medeiros, R.L.; Rodrigues, D.O.W. Hemoglobin S identification in blood donors: A cross section of prevalence. Hematol. Transfus. Cell Ther. 2022, 44, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.G.; Bacal, N.S.; Cuder, M.A.M.; Silva, A.G.D.; Machado, I.E.; Pereira, C.A.; Souza, M.F.M.; Malta, D.C. Prevalence of hemoglobinopathies in the Brazilian adult population: National Health Survey 2014–2015. Rev. Bras Epidemiol. 2019, 22, E190007-SUPL. [Google Scholar]
- Hernandez, A.G.; Kiyaga, C.; Howard, T.A.; Ssewanyana, I.; Ndeezi, G.; Aceng, J.R.; Ware, R.E. Trends in sickle cell trait and disease screening in the Republic of Uganda, 2014–2019. Trop. Med. Int. Health 2021, 26, 23–32. [Google Scholar] [CrossRef]
- Ambrose, E.E.; Smart, L.R.; Charles, M.; Hernandez, A.G.; Latham, T.; Hokororo, A.; Beyanga, M.; Howard, T.A.; Kamugisha, E.; McElhinney, K.E.; et al. Surveillance for sickle cell disease, United Republic of Tanzania. Bull. World Health Organ. 2020, 98, 859–868. [Google Scholar] [CrossRef]
- Nkya, S.; Mtei, L.; Soka, D.; Mdai, V.; Mwakale, P.; Mrosso, P.; Mchoropa, I.; Rwezaula, S.; Azayo, M.; Ulenga, N.; et al. Newborn screening for sickle cell disease: An innovative pilot program to improve child survival in Dar es Salaam, Tanzania. Int. Health 2019, 11, 589–595. [Google Scholar] [CrossRef]
- Tshilolo, L.; Aissi, L.M.; Lukusa, D.; Kinsiama, C.; Wembonyama, S.; Gulbis, B.; Vertongen, F. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: Experience from a pioneer project on 31 204 newborns. J. Clin. Pathol. 2009, 62, 35–38. [Google Scholar] [CrossRef]
- Nnodu, O.E.; Sopekan, A.; Nnebe-Agumadu, U.; Ohiaeri, C.; Adeniran, A.; Shedul, G.; Isa, H.A.; Owolabi, O.; Chianumba, R.I.; Tanko, Y.; et al. Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: A feasibility study. Lancet Haematol. 2020, 7, e534–e540. [Google Scholar] [CrossRef]
- Uyoga, S.; Macharia, A.W.; Mochamah, G.; Ndila, C.M.; Nyutu, G.; Makale, J.; Tendwa, M.; Nyatichi, E.; Ojal, J.; Otiende, M.; et al. The epidemiology of sickle cell disease in children recruited in infancy in Kilifi, Kenya: A prospective cohort study. Lancet Glob. Health 2019, 7, e1458–e1466. [Google Scholar] [CrossRef]
- Segbefia, C.; Goka, B.; Welbeck, J.; Amegan-Aho, K.; Dwuma-Badu, D.; Rao, S.; Salifu, N.; Oppong, S.; Odei, E.; Ohene-Frempong, K.; et al. Implementing newborn screening for sickle cell disease in Accra, Ghana: Results and challenges. Pediatr. Blood Cancer 2021, 68, e29068. [Google Scholar] [CrossRef]
- Kreuels, B.; Kreuzberg, C.; Kobbe, R.; Ayim-Akonor, M.; Apiah-Thompson, P.; Thompson, B.; Ehmen, C.; Adjei, S.; Langefeld, I.; Adjei, O.; et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood 2010, 115, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
- Dokekias, A.E.; Ocko Gokaba, L.T.; Louokdom, J.S.; Ocini, L.N.; Galiba Atipo Tsiba, F.O.; Ondzotto Ibatta, C.I.; Kouandzi, Q.N.; Tamekue, S.T.; Bango, J.C.; Nziengui Mboumba, J.V.; et al. Neonatal Screening for Sickle Cell Disease in Congo. Anemia 2022, 2022, 9970315. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.T.; Ferris, M.G.; Ramamurthy, U.; Santos, B.; de Oliveira, V.; Bernardino, L.; Ware, R.E. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am. J. Hematol. 2013, 88, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Streetly, A.; Sisodia, R.; Dick, M.; Latinovic, R.; Hounsell, K.; Dormandy, E. Evaluation of newborn sickle cell screening programme in England: 2010-2016. Arch. Dis. Child. 2018, 103, 648–653. [Google Scholar] [CrossRef]
- Delgado-Pecellin, C.; Alvarez Rios, I.; Bueno Delgado, M.D.A.; Jimenez Jambrina, M.M.; Quintana Gallego, M.E.; Ruiz Salas, P.; Marcos Luque, I.; Melguizo Madrid, E. Results of the neonatal screening on Western Andalusia after a decade of experience. Rev. Esp. Salud Publica 2020, 94, e202012174. [Google Scholar] [PubMed]
- Garcia-Morin, M.; Bardon-Cancho, E.J.; Belendez, C.; Zamarro, R.; Beliz-Mendiola, C.; Gonzalez-Rivera, M.; Vecilla, C.; Llorente-Otones, L.; Perez-Alonso, V.; Roman, S.S.; et al. Fifteen years of newborn sickle cell disease screening in Madrid, Spain: An emerging disease in a European country. Ann. Hematol. 2020, 99, 1465–1474. [Google Scholar] [CrossRef]
- Lo Riso, L.; Ortuño Cabrero, A.; Bauza, J.; Garcia-Recio, M.; Rodriguez, B.; Andrade, B.; Sanchez-Raga, J.; Mayol, A.; Pastor, M. Newborn screening for sickle cell disease: Experience in Balearic islands 2 years after of the implementation of the screening program. In Proceedings of the 23rd European Hematology Association Congress, Stockholm, Sweden, 14–17 June 2018. [Google Scholar]
- Cela, E.; Bellon, J.M.; de la Cruz, M.; Belendez, C.; Berrueco, R.; Ruiz, A.; Elorza, I.; Diaz de Heredia, C.; Cervera, A.; Valles, G.; et al. National registry of hemoglobinopathies in Spain (REPHem). Pediatr. Blood Cancer 2017, 64, e26322. [Google Scholar] [CrossRef]
- Martella, M.; Viola, G.; Azzena, S.; Schiavon, S.; Biondi, A.; Basso, G.; Corti, P.; Colombatti, R.; Masera, N.; Sainati, L. Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. Int. J. Neonatal. Screen 2019, 5, 2. [Google Scholar] [CrossRef]
- Grosse, R.; Lukacs, Z.; Cobos, P.N.; Oyen, F.; Ehmen, C.; Muntau, B.; Timmann, C.; Noack, B. The Prevalence of Sickle Cell Disease and Its Implication for Newborn Screening in Germany (Hamburg Metropolitan Area). Pediatr. Blood Cancer 2016, 63, 168–170. [Google Scholar] [CrossRef]
- Kunz, J.B.; Awad, S.; Happich, M.; Muckenthaler, L.; Lindner, M.; Gramer, G.; Okun, J.G.; Hoffmann, G.F.; Bruckner, T.; Muckenthaler, M.U.; et al. Significant prevalence of sickle cell disease in Southwest Germany: Results from a birth cohort study indicate the necessity for newborn screening. Ann. Hematol. 2016, 95, 397–402. [Google Scholar] [CrossRef]
- Lobitz, S.; Frommel, C.; Brose, A.; Klein, J.; Blankenstein, O. Incidence of sickle cell disease in an unselected cohort of neonates born in Berlin, Germany. Eur. J. Hum. Genet. 2014, 22, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Lobitz, S.; Telfer, P.; Cela, E.; Allaf, B.; Angastiniotis, M.; Backman Johansson, C.; Badens, C.; Bento, C.; Bouva, M.J.; Canatan, D.; et al. Newborn screening for sickle cell disease in Europe: Recommendations from a Pan-European Consensus Conference. Br. J. Haematol. 2018, 183, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Gulbis, B.; Le, P.Q.; Ketelslegers, O.; Dresse, M.F.; Adam, A.S.; Cotton, F.; Boemer, F.; Bours, V.; Minon, J.M.; Ferster, A. Neonatal Screening for Sickle Cell Disease in Belgium for More than 20 Years: An Experience for Comprehensive Care Improvement. Int. J. Neonatal. Screen 2018, 4, 37. [Google Scholar] [CrossRef]
- Italia, Y.; Krishnamurti, L.; Mehta, V.; Raicha, B.; Italia, K.; Mehta, P.; Ghosh, K.; Colah, R. Feasibility of a newborn screening and follow-up programme for sickle cell disease among South Gujarat (India) tribal populations. J. Med. Screen 2015, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Al Hosani, H.; Salah, M.; Osman, H.M.; Farag, H.M.; El-Assiouty, L.; Saade, D.; Hertecant, J. Expanding the comprehensive national neonatal screening programme in the United Arab Emirates from 1995 to 2011. East Mediterr. Health J. 2014, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Alkindi, S.; Al Zadjali, S.; Al Madhani, A.; Daar, S.; Al Haddabi, H.; Al Abri, Q.; Gravell, D.; Berbar, T.; Pravin, S.; Pathare, A.; et al. Forecasting hemoglobinopathy burden through neonatal screening in Omani neonates. Hemoglobin 2010, 34, 135–144. [Google Scholar] [CrossRef]
- Khoriaty, E.; Halaby, R.; Berro, M.; Sweid, A.; Abbas, H.A.; Inati, A. Incidence of sickle cell disease and other hemoglobin variants in 10,095 Lebanese neonates. PLoS ONE 2014, 9, e105109. [Google Scholar] [CrossRef]
- Al-Alawi, M.; Sarhan, N. Prevalence of anemia among nine-month-old infants attending primary care in Bahrain. J. Bahrain Med. Soc. 2014, 25, 29–32. [Google Scholar] [CrossRef]
- Smeltzer, M.P.; Nolan, V.G.; Yu, X.; Nottage, K.A.; Davis, B.A.; Yang, Y.; Wang, W.C.; Gurney, J.G.; Hankins, J.S. Birth Prevalence of Sickle Cell Trait and Sickle Cell Disease in Shelby County, TN. Pediatr. Blood Cancer 2016, 63, 1054–1059. [Google Scholar] [CrossRef]
- Therrell, B.L., Jr.; Lloyd-Puryear, M.A.; Eckman, J.R.; Mann, M.Y. Newborn screening for sickle cell diseases in the United States: A review of data spanning 2 decades. Semin. Perinatol. 2015, 39, 238–251. [Google Scholar] [CrossRef]
- Feuchtbaum, L.; Carter, J.; Dowray, S.; Currier, R.J.; Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 2012, 14, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.R.; Ridsdale, R.; MacNeil, L.; Lilley, M.; Hoang, S.; Christian, S.; Blumenschein, P.; Wolan, V.; Bruce, A.; Singh, G.; et al. The Alberta Newborn Screening Approach for Sickle Cell Disease: The Advantages of Molecular Testing. Int. J. Neonatal. Screen 2021, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Miranda Neto, P.A.D.; Santos, J.R.N.; Monteiro, S.G.; Goncalves, M.C.; Silva, F.B.; Holanda, R.A.; Santos, J.R.A. Sickle Cell Anaemia Prevalence Among Newborns in the Brazilian Amazon-Savanna Transition Region. Int. J. Environ. Res. Public Health 2019, 16, 1638. [Google Scholar] [CrossRef] [PubMed]
- Eller, R.; da Silva, D.B. Evaluation of a neonatal screening program for sickle-cell disease. J. Pediatr. 2016, 92, 409–413. [Google Scholar] [CrossRef][Green Version]
- Silva, W.S.; de Oliveira, R.F.; Ribeiro, S.B.; da Silva, I.B.; de Araujo, E.M.; Baptista, A.F. Screening for Structural Hemoglobin Variants in Bahia, Brazil. Int. J. Environ. Res. Public Health 2016, 13, 225. [Google Scholar] [CrossRef]
- Carlos, A.M.; Souza, R.A.; Souza, B.M.; Pereira Gde, A.; Tostes Junior, S.; Martins, P.R.; Moraes-Souza, H. Hemoglobinopathies in newborns in the southern region of the Triangulo Mineiro, Brazil. Cross-sectional study. Sao Paulo Med. J. 2015, 133, 439–444. [Google Scholar] [CrossRef]
- Sabarense, A.P.; Lima, G.O.; Silva, L.M.; Viana, M.B. Survival of children with sickle cell disease in the comprehensive newborn screening programme in Minas Gerais, Brazil. Paediatr. Int. Child Health 2015, 35, 329–332. [Google Scholar] [CrossRef]
- Lobo, C.L.; Ballas, S.K.; Domingos, A.C.; Moura, P.G.; do Nascimento, E.M.; Cardoso, G.P.; de Carvalho, S.M. Newborn screening program for hemoglobinopathies in Rio de Janeiro, Brazil. Pediatr. Blood Cancer 2014, 61, 34–39. [Google Scholar] [CrossRef]
- Wagner, S.C.; de Castro, S.M.; Gonzalez, T.P.; Santin, A.P.; Zaleski, C.F.; Azevedo, L.A.; Dreau, H.; Henderson, S.; Old, J.; Hutz, M.H. Neonatal screening for hemoglobinopathies: Results of a public health system in South Brazil. Genet. Test Mol. Biomark. 2010, 14, 565–569. [Google Scholar] [CrossRef]
- Serjeant, G.R.; Serjeant, B.E.; Mason, K.P.; Gardner, R.; Warren, L.; Gibson, F.; Coombs, M. Newborn screening for sickle cell disease in Jamaica: Logistics and experience with umbilical cord samples. J. Community Genet. 2017, 8, 17–22. [Google Scholar] [CrossRef]
- Alvarez, O.A.; Hustace, T.; Voltaire, M.; Mantero, A.; Liberus, U.; Saint Fleur, R. Newborn Screening for Sickle Cell Disease Using Point-of-Care Testing in Low-Income Setting. Pediatrics 2019, 144, e20184105. [Google Scholar] [CrossRef] [PubMed]
- Rotz, S.; Arty, G.; Dall’Amico, R.; De Zen, L.; Zanolli, F.; Bodas, P. Prevalence of sickle cell disease, hemoglobin S, and hemoglobin C among Haitian newborns. Am. J. Hematol. 2013, 88, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Alladin, B.A.; Mohamed-Rambaran, P.; Grey, V.; Hunter, A.; Chakraborty, P.; Henderson, M.; Milburn, J.; Tessier, L. Cross-sectional prospective feasibility study of newborn screening for sickle cell anaemia and congenital hypothyroidism in Guyana. BMJ Open 2022, 12, e046240. [Google Scholar] [CrossRef] [PubMed]
- Knight-Madden, J.; Lee, K.; Elana, G.; Elenga, N.; Marcheco-Teruel, B.; Keshi, N.; Etienne-Julan, M.; King, L.; Asnani, M.; Romana, M.; et al. Newborn Screening for Sickle Cell Disease in the Caribbean: An Update of the Present Situation and of the Disease Prevalence. Int. J. Neonatal. Screen 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, E.; Tarocco, A.; Marsella, M.; Bernardoni, R.; Carandina, G.; Melandri, C.; Guerra, G.; Patella, A.; Zucchelli, M.; Ferlini, A.; et al. Universal neonatal screening for sickle cell disease and other haemoglobinopathies in Ferrara, Italy. Blood Transfus. 2013, 11, 245–249. [Google Scholar]
- Gbadamosi-Akindele, M.; Aurit, S.; Johnson, A.; Nester, A. A State Level Retrospective Analysis of Newborn Screening for Hemoglobinopathies. Blood 2019, 134, 5806. [Google Scholar] [CrossRef]
- Ojodu, J.; Hulihan, M.M.; Pope, S.N.; Grant, A.M.; Centers for Disease Control and Prevention. Incidence of sickle cell trait—United States, 2010. MMWR Morb. Mortal Wkly Rep. 2014, 63, 1155–1158. [Google Scholar]
- INDEPTH Network. Population and Health in Developing Countries. Volume 1, Population, Health, and Survival at INDEPTH Sites 2002; International Development Research Centre: Ottawa, ON, Canada, 2002.
- Brousse, V.; Arnaud, C.; Lesprit, E.; Quinet, B.; Odievre, M.H.; Etienne-Julan, M.; Guillaumat, C.; Elana, G.; Belloy, M.; Garnier, N.; et al. Evaluation of outcomes and quality of care in children with sickle cell disease diagnosed by newborn screening: A real-world nation-wide study in France. J. Clin. Med. 2019, 8, 1594. [Google Scholar] [CrossRef]
- Bardón Cancho, E.J.; García-Morín, M.; Belendez, C.; Velasco, P.; Beneitez, D.; Ruiz-Llobet, A.; Berrueco, R.; Argiles, B.; Cervera, A.; Salinas, J.A.; et al. Update of the Spanish registry of haemoglobinopathies in children and adults. Med. Clin. 2020, 155, 95–103. [Google Scholar] [CrossRef]
- Rettenbacher, E.; Zaal, J.; Heijboer, H.; van der Plas, E.M.; Hof, M.; Biemond, B.J.; Fijnvandraat, K.; on behalf of the SCORE consortium. Mortality and Causes of Death From Sickle Cell Disease in The Netherlands, 1985–2017. J. Pediatr. Hematol. Oncol. 2021, 43, 258–265. [Google Scholar] [CrossRef]
- Ansari-Moghaddam, A.; Adineh, H.A.; Zareban, I.; Mohammadi, M.; Maghsoodlu, M. The survival rate of patients with beta-thalassemia major and intermedia and its trends in recent years in Iran. Epidemiol. Health 2018, 40, e2018048. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Taylor, R.; Naghavi, M.; Lopez, A.D. Mortality in the Islamic Republic of Iran, 1964–2004. Bull. World Health Organ. 2007, 85, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Paulukonis, S.T.; Harris, W.T.; Coates, T.D.; Neumayr, L.; Treadwell, M.; Vichinsky, E.; Feuchtbaum, L.B. Population based surveillance in sickle cell disease: Methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH). Pediatr. Blood Cancer 2014, 61, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Caggana, M.; Kennedy, J.; Zimmerman, R.; Oyeku, S.O.; Werner, E.M.; Grant, A.M.; Green, N.S.; Grosse, S.D. Mortality of New York children with sickle cell disease identified through newborn screening. Genet. Med. 2015, 17, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Paulukonis, S.T.; Eckman, J.R.; Snyder, A.B.; Hagar, W.; Feuchtbaum, L.B.; Zhou, M.; Grant, A.M.; Hulihan, M.M. Defining sickle cell disease mortality using a population-based surveillance system, 2004 through 2008. Public Health Rep. 2016, 131, 367–375. [Google Scholar] [CrossRef]
- Instituto Brasiliero de Geografia e Estatística (IBGE). Tábuas Abreviadas de Mortalidade por Sexo e Idade; Grandes Regiões e Unidades da Federaçao: Brasil, 2010. Available online: www.ibge.gov.br (accessed on 21 August 2023).
- Statistical Institute of Jamaica. STATIN: Abridged Life Table 2011. In Demographic Statistics 2014; Statistical Institute of Jamaica: Kingston, Jamaica, 2014; pp. 36–37. [Google Scholar]
- Muller, S.A.; Amoah, S.K.; Meese, S.; Spranger, J.; Mockenhaupt, F.P. High prevalence of anaemia among African migrants in Germany persists after exclusion of iron deficiency and erythrocyte polymorphisms. Trop. Med. Int. Health 2015, 20, 1180–1189. [Google Scholar] [CrossRef]
- Lobitz, S.; Klein, J.; Brose, A.; Blankenstein, O.; Frömmel, C. Newborn screening by tandem mass spectrometry confirms the high prevalence of sickle cell disease among German newborns. Ann. Hematol. 2019, 98, 47–53. [Google Scholar] [CrossRef]
- Kunz, J.B.; Lobitz, S.; Grosse, R.; Oevermann, L.; Hakimeh, D.; Jarisch, A.; Cario, H.; Beier, R.; Schenk, D.; Schneider, D.; et al. Sickle cell disease in Germany: Results from a national registry. Pediatr. Blood Cancer 2020, 67, e28130. [Google Scholar] [CrossRef]
- Gunjal, S.; Narlawar, U.; Humne, A.; Chaudhari, V. Prevalence of sickle cell disorder and anaemia in tribal school students from central India. Int. J. Collab. Res. Intern. Med. Public Health 2012, 4, 1321–1329. [Google Scholar]
- Oberoi, A.; Kanakia, S. Prevalence of thalassemia syndromes, hemoglobinopathies and mutation analysis in a tribal school in India. In Proceedings of the 11th World Hematology and Oncology Congress & 47th World Congress on Nursing Care, Rome, Italy, 24–25 July 2020. [Google Scholar]
- Seide, S.E.; Röver, C.; Friede, T. Likelihood-based random-effects meta-analysis with few studies: Empirical and simulation studies. BMC Med. Res. Methodol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Paintsil, V.; Amuzu, E.X.; Nyanor, I.; Asafo-Adjei, E.; Mohammed, A.R.; Yawnumah, S.A.; Oppong-Mensah, Y.G.; Nguah, S.B.; Obeng, P.; Dogbe, E.E.; et al. Establishing a Sickle Cell Disease Registry in Africa: Experience From the Sickle Pan-African Research Consortium, Kumasi-Ghana. Front. Genet. 2022, 13, 802355. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.; Adam, N.K.; Mohamed, B.A. Prevalence of sickle cell disease and sickle cell trait among children admitted to Al Fashir Teaching Hospital North Darfur State, Sudan. BMC Res. Notes 2019, 12, 659. [Google Scholar] [CrossRef]
- Adewoyin, A.; Busari, O.; Aworanti, O. Hemoglobin phenotypes in Nigeria: Data from a national reference laboratory. Am. J. Clin. Pathol. 2019, 152, S115–S116. [Google Scholar] [CrossRef]
- Al Arrayed, S. Prevalence of abnormal hemoglobins among students in Bahrain: A ten-year study. Bahrain Med. Bull. 2011, 33. [Google Scholar]
- Alayed, N.; Kezouh, A.; Oddy, L.; Abenhaim, H.A. Sickle cell disease and pregnancy outcomes: Population-based study on 8.8 million births. J. Perinat. Med. 2014, 42, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, E.S.; Farhat, G.N.; Assiri, A.M.; Memish, Z.; Ahmed, E.M.; Saeedi, M.Y.; Al-Dossary, M.F.; Bashawri, H. Distribution of hemoglobinopathy disorders in Saudi Arabia based on data from the premarital screening and genetic counseling program, 2011–2015. J. Epidemiol. Glob. Health 2018, 7 (Suppl. 1), S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Ameen, H.; Abidoye, A.; Alatishe-Muhammad, B.; Aderibigbe, S.; Uthman, M.; Bolarinwa, O.; Saludeen, A.; Musa, O.; Akande, T. Prevalence of heamoglobin genotype screening and awareness of SCD among undergraduate students of Unilorin. J. Med. Biomed. Res. 2016, 14–27. [Google Scholar]
- Brízido, H.; Silva, C.; Sousa, M.; Ribeiro, R.; Sousa, J.; Sousa, G. Hemoglobinopathies: A Five Year Revised Experience of Centro Medicina Laboratorial Germano de Sousa. Clin. Chem. Lab. Med. 2018, 56, eA20–eA21. [Google Scholar]
- Chunda-Liyoka, C.; Kumar, A.A.; Sambo, P.; Lubinda, F.; Nchimba, L.; Humpton, T.; Okuku, P.; Miyanda, C.; Im, J.; Maguire, K.; et al. Application of a public health strategy to large-scale point-of-care screening for sickle cell disease in rural sub-Saharan Africa. Blood Adv. 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Daak, A.A.; Elsamani, E.; Ali, E.H.; Mohamed, F.A.; Abdel-Rahman, M.E.; Elderdery, A.Y.; Talbot, O.; Kraft, P.; Ghebremeskel, K.; Elbashir, M.I.; et al. Sickle cell disease in western Sudan: Genetic epidemiology and predictors of knowledge attitude and practices. Trop. Med. Int. Health 2016, 21, 642–653. [Google Scholar] [CrossRef]
- De Assis, E.; Araújo, J.; de Rezende, M.; da Cunha Oliveira, C.; Francisco, P.; de Melo, M. Prevalence of variant hemoglobins and thalassemias in a maroon community in Sergipe, Brazil. Acta Sci. Health Sci. 2015, 37, 211–216. [Google Scholar] [CrossRef][Green Version]
- Desselas, E.; Thuret, I.; Kaguelidou, F.; Benkerrou, M.; de Montalembert, M.; Odievre, M.H.; Lesprit, E.; Rumpler, E.; Fontanet, A.; Pondarre, C.; et al. Mortality in children with sickle cell disease in mainland France from 2000 to 2015. Haematologica 2020, 105, e440–e443. [Google Scholar] [CrossRef] [PubMed]
- Eastburg, L.; Peckham, A.; Kawira, E.; Chirangi, B.; Adler, D.; Akungo, B.D.; Smart, L.R.; Ambrose, E.E. Extremely high birth prevalence of sickle cell disease in rural Tanzania. Pediatr. Blood Cancer 2020, 67, e28620. [Google Scholar] [CrossRef] [PubMed]
- El Ariss, A.B.; Younes, M.; Matar, J.; Berjaoui, Z. Prevalence of Sickle Cell Trait in the Southern Suburb of Beirut, Lebanon. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016015. [Google Scholar] [CrossRef]
- Elsayid, M.; Al-Shehri, M.J.; Alkulaibi, Y.A.; Alanazi, A.; Qureshi, S. Frequency distribution of sickle cell anemia, sickle cell trait and sickle/beta-thalassemia among anemic patients in Saudi Arabia. J. Nat. Sci. Biol. Med. 2015, 6, S85–S88. [Google Scholar]
- Gibbons, C.; Geoghegan, R.; Conroy, H.; Lippacott, S.; O’Brien, D.; Lynam, P.; Langabeer, L.; Cotter, M.; Smith, O.; McMahon, C. Sickle cell disease: Time for a targeted neonatal screening programme. Ir. Med. J. 2015, 108, 43–45. [Google Scholar]
- Gualandro, S.F.; Fonseca, G.H.; Yokomizo, I.K.; Gualandro, D.M.; Suganuma, L.M. Cohort study of adult patients with haemoglobin SC disease: Clinical characteristics and predictors of mortality. Br. J. Haematol. 2015, 171, 631–637. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Forsti, A.; Sundquist, J.; Sundquist, K. Thalassemia and sickle cell anemia in Swedish immigrants: Genetic diseases have become global. SAGE Open Med. 2015, 3, 2050312115613097. [Google Scholar] [CrossRef]
- Kjellander, C.; Hernlund, E.; Ivergård, M.; Svedbom, A.; Dibbern, T.; Stenling, A.; Sjöö, F.; Vertuani, S.; Glenthøj, A.; Cherif, H. Sickle Cell Disease in Sweden—Prevalence and Resource Use Estimated through Population-Based National Registers. Blood Rev. 2021, 138, 2040. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Updated Worldwide Epidemiology of Inherited Erythrocyte Disorders. Acta Haematol. 2020, 143, 196–203. [Google Scholar] [CrossRef]
- Madhubala, R.; Gupta, A.; Kumar, M.; Saini, S.; Purohit, A.; Didel, S.; Elhence, P.; Singh, K. Prevalence of Haemoglobinopathies in Western Rajasthan. Indian J. Hematol. Blood Transfus 2020, 36, S82. [Google Scholar]
- Marcheco-Teruel, B. Sickle Cell Anemia in Cuba: Prevention and Management, 1982–2018. MEDICC Rev. 2019, 21, 34–38. [Google Scholar] [PubMed]
- Moez, P.; Younan, D.N. High prevalence of haemoglobin S in the closed Egyptian community of Siwa Oasis. J. Clin. Pathol. 2016, 69, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, L.; Dascalu, I.; Marchand, K.; Khaira, A.; Yu, S.; Xu, R. Assessment of the Sickle Cell Disease Screening Program in the Indigenous Tharu Population in Nepal. J. Investig. Med. 2020, 68 (Suppl. 1), A178. [Google Scholar]
- Nalbandian, M.; Kaminsky, H.; Baghdasaryan, P.; Keleny, D.; Nalbandyan, K.; Jalonen, T. Epidemiology of Sickle Cell Disease in Grenada: A comparison with Haiti, Jamaica and the United States of America. West Indian Med. J. 2017, 66, 491–496. [Google Scholar]
- Okocha, C.; Onubogu, C.U.; Aneke, J.; Onah, C.; Ajuba, I.; Ibeh, N.; Egbuonu, I. Prevalence of sickle cell gene among apparently healthy under-two south-east Nigerian children: What is the role of parental premarital counselling and socio-demographic characteristics? A pilot study. Niger. J. Med. 2016, 25, 176–181. [Google Scholar]
- Oktay, G.; Acıpayam, C.; İlhan, G.; Karal, Y.; Sakallı, G.; Yılmazoglu, N.; Basun, S. The results of hemoglobinopathy screening in Hatay, the southern part of Turkey. J. Clin. Anal. Med. 2016, 7, 6–9. [Google Scholar]
- Okwi, A.L.; Byarugaba, W.; Ndugwa, C.M.; Parkes, A.; Ocaido, M.; Tumwine, J.K. An up-date on the prevalence of sickle cell trait in Eastern and Western Uganda. BMC Blood Disord. 2010, 10, 5. [Google Scholar] [CrossRef]
- Patel, J.; Patel, B.; Gamit, N.; Serjeant, G.R. Screening for the sickle cell gene in Gujarat, India: A village-based model. J. Community Genet. 2013, 4, 43–47. [Google Scholar] [CrossRef]
- Pinto, A.C.; Costa, F.F.; Gualandro, S.; Fonseca, P.; Bueno, C.; Cançado, R. Burden of sickle cell disease: A Brazilian societal perspective analysis. Blood 2020, 136, 10–11. [Google Scholar] [CrossRef]
- Pinto, V.M.; Graziadei, G.; Voi, V.; Quota, A.; Rigano, P.; Spadola, V.; Fidone, C.; Gianesin, B.; De Franceschi, L.; Forni, G.L. Survival rates and causes of death in elderly patients with sickle cell disease. HemaSphere 2019, 3, 322–323. [Google Scholar] [CrossRef]
- Purohit, P.; Dehury, S.; Patel, S.; Patel, D.K. Prevalence of deletional alpha thalassemia and sickle gene in a tribal dominated malaria endemic area of eastern India. ISRN Hematol. 2014, 2014, 745245. [Google Scholar] [PubMed]
- Santiago, R.P.; Oliveira, R.M.; Soares, L.F.; Figueiredo, C.V.B.; Silva, D.O.; Hurtado-Guerrero, A.F.; Fiuza, L.M.; Guarda, C.C.; Adorno, E.V.; Barbosa, C.G.; et al. Hemoglobin Variant Profiles among Brazilian Quilombola Communities. Hemoglobin 2017, 41, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hussain, R.; Fareed, M.; Afzal, M. Gene frequency of sickle cell trait among Muslim populations in a malarial belt of India, i.e., Manipur. Egypt. J. Med. Hum. Genet. 2012, 13, 323–330. [Google Scholar] [CrossRef]
- Shrestha, R.M.; Pandit, R.; Yadav, U.K.; Das, R.; Yadav, B.K.; Upreti, H.C. Distribution of Hemoglobinopathy in Nepalese Population. J. Nepal. Health Res. Counc. 2020, 18, 52–58. [Google Scholar] [CrossRef]
- Sidhu, H.; Donaldson, M.; Dayan, Z.; Dean, P.; Dhinsa, J.; Marchand, N.; Wang, A.; Zou, V.; Kapoor, V. Follow-up assessment of the sickle cell disease screening program in the indigenous Tharu population of Nepal. J. Investig. Med. 2019, 67, 167. [Google Scholar]
- Smart, L.; Ambrose, E.; Charles, M.; Hernandez, A.; Latham, T.; Hokororo, A.; Beyanga, M.; Kamugisha, E.; Tebuka, E.; Howard, T.; et al. Genetic Analysis in the Tanzania Sickle Surveillance Study (TS3): Modifiers of Sickle Cell Disease and Identification of Hemoglobin Variants. Blood 2019, 134, 988. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Ruth, L.J.; Earley, M.; Macharia, A.; Williams, T.N. The burden and consequences of inherited blood disorders among young children in western Kenya. Matern. Child Nutr. 2014, 10, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Telfer, P.; Agodoa, I.; Fox, K.M.; Burke, L.; Mant, T.; Jurek, M.; Tonda, M.; Lehrer-Graiwer, J. Impact of voxelotor (GBT440) on unconjugated bilirubin and jaundice in sickle cell disease. Hematol. Rep. 2018, 10, 7643. [Google Scholar] [CrossRef]
- Teli, A.B.; Deori, R.; Saikia, S.P.; Pathak, K.; Panyang, R.; Rajkakati, R. Beta-Thalassaemia and its Co-existence with Haemoglobin E and Haemoglobin S in Upper Assam Region of North Eastern India: A Hospital Based Study. J. Clin. Diagn. Res. 2016, 10, GC01–GC04. [Google Scholar]
- Theodoridou, S.; Prapas, N.; Balassopoulou, A.; Boutou, E.; Vyzantiadis, T.A.; Adamidou, D.; Delaki, E.E.; Yfanti, E.; Economou, M.; Teli, A.; et al. Efficacy of the National Thalassaemia and Sickle Cell Disease Prevention Programme in Northern Greece: 15-Year Experience, Practice and Policy Gaps for Natives and Migrants. Hemoglobin 2018, 42, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Umoh, A.V.; Abah, G.M.; Ekanem, T.I.; Essien, E.M. Haemoglobin genotypes: A prevalence study and implications for reproductive health in Uyo, Nigeria. Niger. J. Med. 2010, 19, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Vovor, A.; Feteke, L.; Kueviakoe, I.M.; Kpatarou, L.; Mawussi, K.; Magnang, H.; Segbena, A.Y. Blood typing profile of a school-aged population of a North Togo township. Hemoglobin 2014, 38, 316–318. [Google Scholar] [CrossRef] [PubMed]
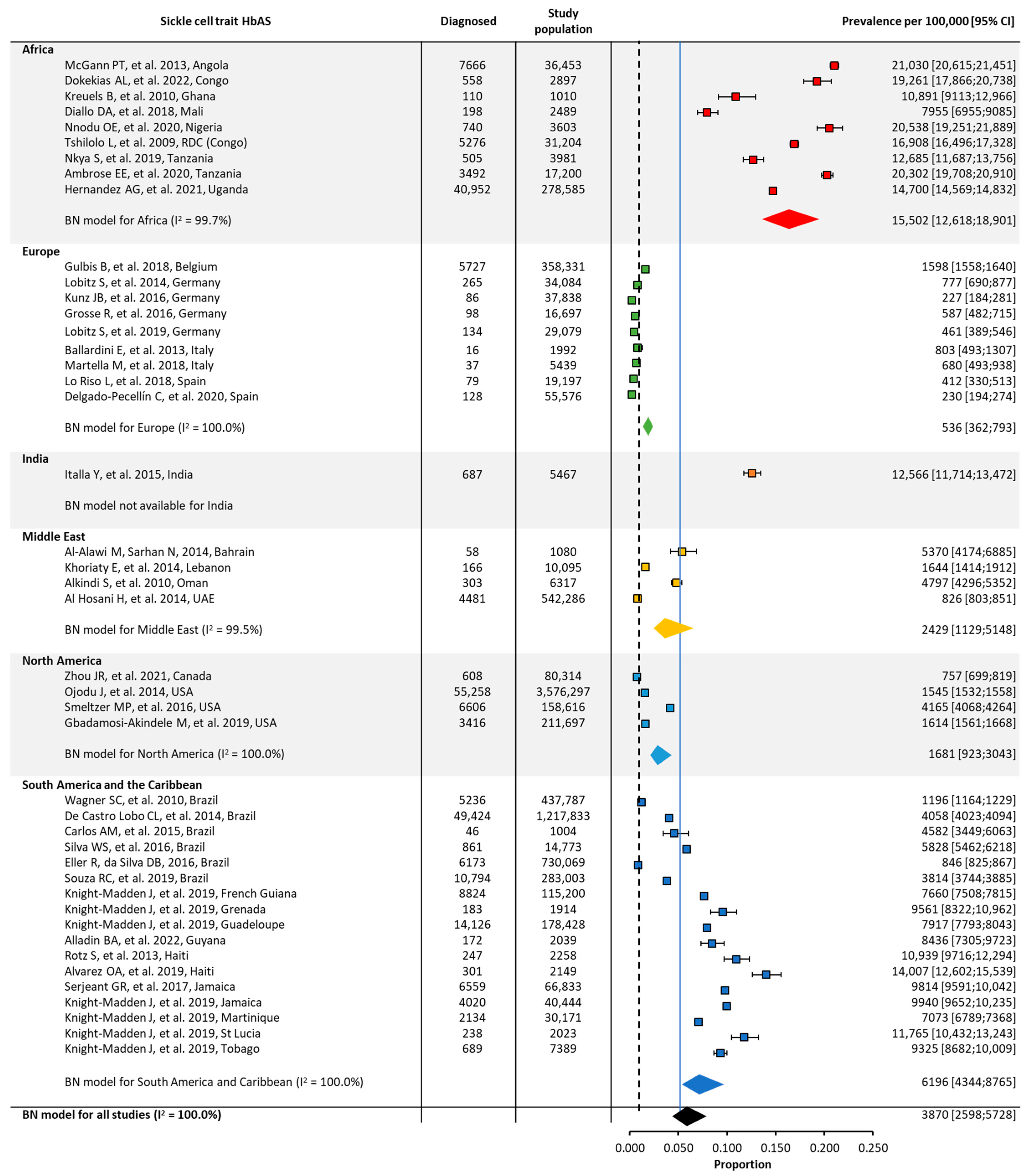

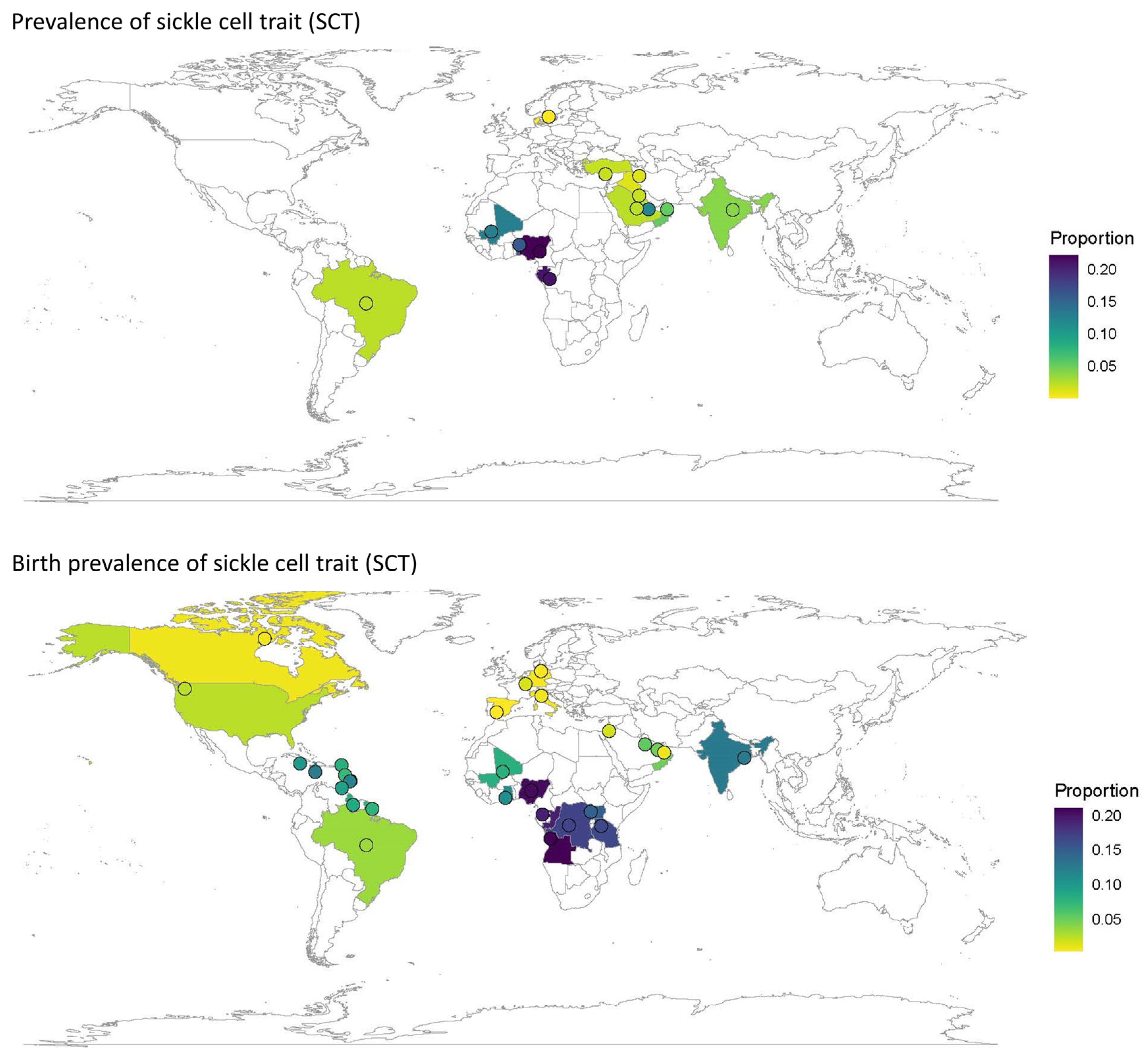

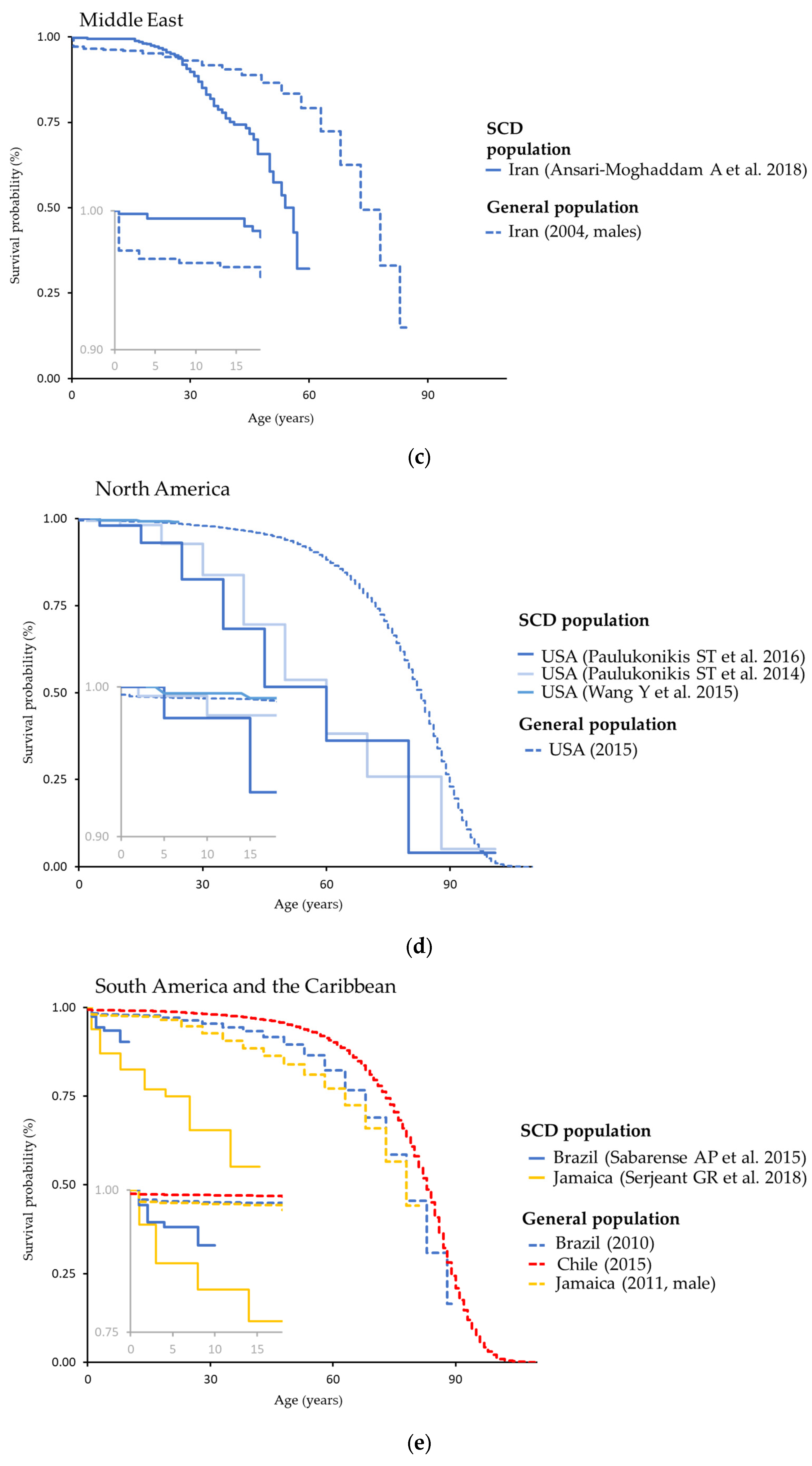
| Prevalence | ||||||||
| SCD | SCT | |||||||
| Region | No. of Studies | Total Studied Population | Prevalence per 100,000 [95% CI] | No. of Studies | Total Studied Population | Prevalence per 100,000 [95% CI] | ||
| Global | 19 | 130,420,748 | 117 [55;249] | 24 | 8,335,442 | 2428 [1209;4814] | ||
| Africa | 3 | 7274 | 788 [316;1951] | 4 | 11,523 | 17,690 [14,149;21,891] | ||
| Europe | 5 | 127,536,172 | 28 [10;79] | 3 | 5,921,455 | 212 [12;3503] | ||
| India | 6 | 430,952 | 128 [48;340] | 9 | 472,154 | 2193 [1090;4364] | ||
| Middle East | 4 | 1,909,565 | 212 [64;698] | 6 | 1,914,429 | 2429 [934;6167] | ||
| North America | – | – | NA | – | – | NA | ||
| South America/ the Caribbean | 1 | 536,785 | NA | 2 | 15,881 | NA | ||
| Birth Prevalence | ||||||||
| SCD | SCT | |||||||
| Region | No. of Studies | Total Studied Population | Prevalence per 100,000 [95% CI] | No. of Studies | Total Studied Population | Prevalence per 100,000 [95% CI] | ||
| Global | 44 | 92,209,456 | 191 [120;303] | 44 | 8,661,141 | 3870 [2598;5728] | ||
| Africa | 11 | 397,651 | 1321 [1041;1674] | 9 | 377,422 | 15,502 [12,618;18,901] | ||
| Europe | 11 | 5,407,689 | 33 [20;54] | 9 | 558,233 | 535 [362;792] | ||
| India | 1 | 5467 | NA | 1 | 5467 | NA | ||
| Middle East | 5 | 598,718 | 218 [88;538] | 4 | 559,778 | 2429 [1129;5148] | ||
| North America | 4 | 79,048,695 | 54 [25;117] | 4 | 4,026,924 | 1681 [923;3043] | ||
| South America/ the Caribbean | 12 | 6,751,236 | 201 [102;395] | 11 | 3,133,317 | 6196 [4344;8765] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombatti, R.; Hegemann, I.; Medici, M.; Birkegård, C. Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection. J. Clin. Med. 2023, 12, 5538. https://doi.org/10.3390/jcm12175538
Colombatti R, Hegemann I, Medici M, Birkegård C. Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection. Journal of Clinical Medicine. 2023; 12(17):5538. https://doi.org/10.3390/jcm12175538
Chicago/Turabian StyleColombatti, Raffaella, Inga Hegemann, Morten Medici, and Camilla Birkegård. 2023. "Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection" Journal of Clinical Medicine 12, no. 17: 5538. https://doi.org/10.3390/jcm12175538
APA StyleColombatti, R., Hegemann, I., Medici, M., & Birkegård, C. (2023). Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection. Journal of Clinical Medicine, 12(17), 5538. https://doi.org/10.3390/jcm12175538






