Integration of a Smartphone HF-Dedicated App in the Remote Monitoring of Heart Failure Patients with Cardiac Implantable Electronic Devices: Patient Access, Acceptance, and Adherence to Use
Abstract
:1. Introduction
2. Methods
2.1. Survey Design and Collection
2.2. Patient HF-Dedicated App Description
2.3. Patient HF-Dedicated App Download and Training
2.4. Statistical Analysis
3. Results
3.1. Survey Results
3.2. Adherence in the Use of the HF-Dedicated App
4. Discussion
4.1. Smartphone/Tablet Technology Penetration
4.2. Health App User Profile
4.3. The Role of Caregivers
4.4. Patient Adherence to the Use of the App
4.5. Angels of HF Project’s Next Steps
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [PubMed]
- Russo, M.J.; Gelijns, A.C.; Stevenson, L.W.; Sampat, B.; Aaronson, K.D.; Renlund, D.G.; Ascheim, D.D.; Hong, K.N.; Oz, M.C.; Moskowitz, A.J.; et al. The cost of medical management in advanced heart failure during the final two years of life. J. Card. Fail. 2008, 14, 651–658. [Google Scholar] [CrossRef]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N. Engl. J. Med. 2009, 360, 1418–1428, Erratum in N. Engl. J. Med. 2011, 364, 1582. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; O’Connor, C.M. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar]
- Boriani, G.; Da Costa, A.; Ricci, R.P.; Quesada, A.; Favale, S.; Iacopino, S.; Romeo, F.; Risi, A.; Stefano, L.M.d.S.; Navarro, X.; et al. The monitoring resynchronization devices and cardiac patients (MORE-CARE) randomized controlled trial: Phase 1 results on dynamics of early intervention with remote monitoring. J. Med. Internet Res. 2013, 15, e167. [Google Scholar] [CrossRef]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef]
- Gula, L.J.; Wells, G.A.; Yee, R.; Koehler, J.; Sarkar, S.; Sharma, V.; Skanes, A.C.; Sapp, J.L.; Redfearn, D.P.; Manlucu, J.; et al. A novel algorithm to assess risk of heart failure exacerbation using ICD diagnostics: Validation from RAFT. Heart Rhythm. 2014, 11, 1626–1631. [Google Scholar] [CrossRef]
- Burri, H.; da Costa, A.; Quesada, A.; Ricci, R.P.; Favale, S.; Clementy, N.; Boscolo, G.; Villalobos, F.S.; Stefano, L.M.d.S.; Sharma, V.; et al. Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. Europace 2018, 20, e69–e77. [Google Scholar] [CrossRef]
- Sammut-Powell, C.; Taylor, J.K.; Motwani, M.; Leonard, C.M.; Martin, G.P.; Ahmed, F.Z. Remotely Monitored Cardiac Implantable Electronic Device Data Predict All-Cause and Cardiovascular Unplanned Hospitalization. J. Am. Heart Assoc. 2022, 11, e024526. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Sammut-Powell, C.; Kwok, C.S.; Tay, T.; Motwani, M.; Martin, G.P.; Taylor, J.K. Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2022, 24, 245–255. [Google Scholar] [CrossRef]
- Seto, E.; Leonard, K.J.; Cafazzo, J.A.; Barnsley, J.; Masino, C.; Ross, H.J. Mobile phone-based telemonitoring for heart failure management: A randomized controlled trial. J. Med. Internet Res. 2012, 14, e31. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Gerber, B.S.; Gans, C.P.; Kansal, M.M.; Kitsiou, S. Smartphone Ownership and Interest in Mobile Health Technologies for Self-care among Patients with Chronic Heart Failure: Cross-sectional Survey Study. JMIR Cardio 2022, 6, e31982. [Google Scholar] [CrossRef] [PubMed]
- Cajita, M.I.; Hodgson, N.A.; Budhathoki, C.; Han, H.R. Intention to Use mHealth in Older Adults with Heart Failure. J. Cardiovasc. Nurs. 2017, 32, E1–E7. [Google Scholar] [CrossRef]
- Sohn, A.; Speier, W.; Lan, E.; Aoki, K.; Fonarow, G.; Ong, M.; Arnold, C. Assessment of Heart Failure Patients’ Interest in Mobile Health Apps for Self-Care: Survey Study. JMIR Cardio 2019, 3, e14332. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Ding, J.; Plante, T.B.; Martin, S.S. Mobile Health Access and Use Among Individuals with or At Risk for Cardiovascular Disease: 2018 Health Information National Trends Survey (HINTS). J. Am. Heart Assoc. 2019, 8, e014390. [Google Scholar] [CrossRef]
- Boriani, G.; Maisano, A.; Bonini, N.; Albini, A.; Imberti, J.F.; Venturelli, A.; Menozzi, M.; Ziveri, V.; Morgante, V.; Camaioni, G.; et al. Digital literacy as a potential barrier to implementation of cardiology tele-visits after COVID-19 pandemic: The INFO-COVID survey. J. Am. Heart Assoc. 2019, 18, 739–747. [Google Scholar]
- Anugu, P.; Ansari, A.Y.; Min, Y.-I.; Benjamin, E.J.; Murabito, J.; Winters, K.; Turner, E.; Correa, A. Digital Connectedness in the Jackson Heart Study: Cross-sectional Study. J. Med. Internet Res. 2022, 24, e37501. [Google Scholar] [CrossRef]
- Kitsiou, S.; Vatani, H.; Paré, G.; Gerber, B.S.; Buchholz, S.W.; Kansal, M.M.; Leigh, J.; Creber, R.M.M. Effectiveness of Mobile Health Technology Interventions for Patients with Heart Failure: Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 1248–1259. [Google Scholar] [CrossRef]
- Vuorinen, A.L.; LeppÃnen, J.; Kaijanranta, H.; Kulju, M.; Heliö, T.; van Gils, M.; LÃhteenmÃki, J. Use of Home Telemonitoring to Support Multidisciplinary Care of Heart Failure Patients in Finland: Randomized Controlled Trial. J. Med. Internet Res. 2014, 16, e282. [Google Scholar] [CrossRef]
- Yanicelli, L.M.; Goy, C.B.; González, V.d.C.; Palacios, G.N.; Martínez, E.C.; Herrera, M.C. Non-invasive home telemonitoring system for heart failure patients: A randomized clinical trial. J. Telemed. Telecare 2021, 27, 553–561. [Google Scholar]
- Mobile Fact Sheet. Pew Research Center. 2021. Available online: https://www.pewresearch.org/internet/fact-sheet/mobile/ (accessed on 6 August 2021).
- The Mobile Economy Europe 2022 Report by GSMA. Available online: https://www.gsma.com/mobileeconomy/europe/ (accessed on 9 February 2023).
- Gual-Montolio, P.; Suso-Ribera, C.; García-Palacios, A.; Castilla, D.; Zaragoza, I.; Bretón-López, J. Enhancing Internet-based psychotherapy for adults with emotional disorders using ecological momentary assessments and interventions: Study protocol of a feasibility trial with “My EMI, Emotional Well-being” app. Internet Interv. 2023, 31, 100601. [Google Scholar]
- Shaw, J.; Acharya, C.; Albhaisi, S.; Fagan, A.; McGeorge, S.; White, M.B.; Lachar, J.; Olson, J.; Olofson, A.; Bergstrom, L.; et al. Subjective and objective burden on providers from a multicenter app-based study of patients with cirrhosis and caregivers. Hepatol. Commun. 2023, 7, e0030. [Google Scholar] [CrossRef]
- Bhuyan, S.S.; Lu, N.; Chandak, A.; Kim, H.; Wyant, D.; Bhatt, J.; Kedia, S.; Chang, C.F. Use of mobile health applications for health-seeking behavior among US adults. J. Med. Syst. 2016, 40, 153–153:8. [Google Scholar] [PubMed]
- Shuren, J.; Patel, B.; Gottlieb, S. FDA Regulation of Mobile Medical Apps. JAMA 2018, 320, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, A.-S.; Rogers, W.A.; Bouwhuis, D.G. Older adults’ motivated choice for technological innovation: Evidence for benefit-driven selectivity. Psychol. Aging 2006, 21, 190–195. [Google Scholar] [PubMed]
- Mitzner, T.L.; Boron, J.B.; Fausset, C.B.; Adams, A.E.; Charness, N.; Czaja, S.J.; Dijkstra, K.; Fisk, A.D.; Rogers, W.A.; Sharit, J. Older Adults Talk Technology: Technology Usage and Attitudes. Comput. Hum. Behav. 2010, 26, 1710–1721. [Google Scholar]
- Liu, S.; Li, J.; Wan, D.Y.; Li, R.; Qu, Z.; Hu, Y.; Liu, J. Effectiveness of eHealth Self-management Interventions in Patients with Heart Failure: Systematic Review and Meta-analysis. J. Med. Internet Res. 2022, 24, e38697. [Google Scholar] [CrossRef]
- Schreier, G.; Eckmann, H.; Hayn, D.; Kreiner, K.; Kastner, P.; Lovell, N. Web versus App—Compliance of patients in a telehealth diabetes management programme using two different technologies. J. Telemed. Telecare 2012, 18, 476–480. [Google Scholar]
- Thodi, M.; Bistola, V.; Lambrinou, E.; Keramida, K.; Nikolopoulos, P.; Parissis, J.; Farmakis, D.; Filippatos, G. A randomized trial of a nurse-led educational intervention in patients with heart failure and their caregivers: Impact on caregiver outcomes. Eur. J. Cardiovasc. Nurs. 2022, zvac118. [Google Scholar]
- Purcell, C.; Purvis, A.; Cleland, J.G.F.; Cowie, A.; Dalal, H.M.; Ibbotson, T.; Murphy, C.; Taylor, R.S. Home-based cardiac rehabilitation for people with heart failure and their caregivers: A mixed-methods analysis of the roll out an evidence-based programme in Scotland (SCOT:REACH-HF study). Eur. J. Cardiovasc. Nurs. 2023, zvad004. [Google Scholar]
- Shih, P.C.; Han, K.; Poole, E.S.; Rosson, M.B.; Carroll, J.M. Use and Adoption Challenges of Wearable Activity Trackers. In iConference 2015 Proceedings; iSchools: Berlin, Germany, 2015. [Google Scholar]
- Clements, L.; Frazier, S.K.; Lennie, T.A.; Chung, M.L.; Moser, D.K. Improvement in Heart Failure Self-Care and Patient Readmissions with Caregiver Education: A Randomized Controlled Trial. West. J. Nurs. Res. 2022, 45, 402–415. [Google Scholar]
- Sgreccia, D.; Mauro, E.; Vitolo, M.; Manicardi, M.; Valenti, A.C.; Imberti, J.F.; Ziacchi, M.; Borini, G. Implantable cardioverter defibrillators and devices for cardiac resynchronization therapy: What perspective for patients’ apps combined with remote monitoring? Expert. Rev. Med. Devices 2022, 19, 155–160. [Google Scholar] [PubMed]
- Boriani, G.; Burri, H.; Svennberg, E.; Imberti, J.F.; Merino, J.L.; Leclercq, C. Current status of reimbursement practices for remote monitoring of cardiac implantable electrical devices across Europe. Europace 2022, 24, 1875–1880. [Google Scholar] [PubMed]
- Boriani, G.; Svennberg, E.; Guerra, F.; Linz, D.; Casado-Arroyo, R.; Malaczynska-Rajpold, K.; Duncker, D.; Boveda, S.; Merino, J.L.; Leclercq, C. Reimbursement practices for use of digital devices in atrial fibrillation and other arrhythmias: A European Heart Rhythm Association survey. Europace 2022, 24, 1834–1843. [Google Scholar] [PubMed]
- Svennberg, E.; Tjong, F.; Goette, A.; Akoum, N.; Di Biase, L.; Bordachar, P.; Boriani, G.; Burri, H.; Conte, G.; Deharo, J.C.; et al. How to use digital devices to detect and manage arrhythmias: An EHRA practical guide. Europace 2022, 24, 979–1005. [Google Scholar]
- Bonhorst, D.; Guerreiro, S.; Fonseca, C.; Cardim, N.; Macedo, F.; Adragão, P. Real-life data on heart failure before and after implantation of resynchronization and/or defibrillation devices—The Síncrone study. Rev. Port. Cardiol. 2019, 38, 33–41. [Google Scholar]
- Raafs, A.G.; Linssen, G.C.; Brugts, J.J.; Erol-Yilmaz, A.; Plomp, J.; Smits, J.P.; Nagelsmit, M.J.; Oortman, R.M.; Hoes, A.W.; Brunner-LaRocca, H. Contemporary use of devices in chronic heart failure in the Netherlands. ESC Heart Fail. 2020, 7, 1771–1780. [Google Scholar]




| Variable | Summary Statistics | Total N = 495 | App N = 311 | No App N = 184 | p-Value |
|---|---|---|---|---|---|
| DEMOGRAPHICS | |||||
| Age at first implant (years) | Mean ± SD | 66.8 ± 13.1 | 64.9 ± 14.0 | 70.2 ± 10.5 | <0.001 |
| Sex (male) | % (n/Pts) | 78.6 (389/495) | 79.1 (246/311) | 77.7(143/184) | 0.802 |
| MEDICAL HISTORY | |||||
| NYHA 3/4 | % (n/Pts) | 26.2 (118/450) | 25.0 (71/284) | 28.3 (47/166) | 0.441 |
| VT/VF | % (n/Pts) | 26.0 (109/419) | 27.2 (76/279) | 23.6 (33/140) | 0.419 |
| AT/AF | % (n/Pts) | 33.9 (158/466) | 31.6 (93/294) | 37.8 (65/172) | 0.175 |
| Ischemic cardiopathy | % (n/Pts) | 48.5 (230/474) | 47.3 (142/300) | 50.6 (88/174) | 0.496 |
| First-grade AV block | % (n/Pts) | 4.0 (20/495) | 2.9% (9/311) | 6.0 (11/184) | 0.092 |
| Second-grade AV block | % (n/Pts) | 2.2 (11/495) | 1.6 (5/311) | 3.3 (6/184) | 0.228 |
| Third-grade AV block | % (n/Pts) | 5.5 (27/495) | 5.8 (18/311) | 4.9 (9/184) | 0.671 |
| RBBB | % (n/Pts) | 3.2 (16/495) | 1.0 (3/311) | 7.1 (13/184) | <0.001 |
| LBBB | % (n/Pts) | 41.4 (205/495) | 41.5% (129/311) | 41.3 (76/184) | 0.970 |
| Left hemiblock | % (n/Pts) | 1.8 (9/495) | 1.3 (4/311) | 2.7 (5/184) | 0.249 |
| SND | % (n/Pts) | 8.0 (26/327) | 8.7 (19/218) | 6.4 (7/109) | 0.470 |
| History of stroke/TIA | % (n/Pts) | 4.1 (17/413) | 4.0 (11/275) | 4.3 (6/138) | 0.867 |
| Hypertension | % (n/Pts) | 64.2 (278/433) | 64.5 (187/290) | 63.6 (91/143) | 0.863 |
| Diabetes | % (n/Pts) | 30.1 (126/419) | 30.0 (85/283) | 30.1 (41/136) | 0.981 |
| Chronic kidney disease | % (n/Pts) | 21.0 (85/404) | 18.8 (51/271) | 25.6 (34/133) | 0.118 |
| COPD | % (n/Pts) | 10.4 (42/404) | 11.1 (30/270) | 9.0 (12/134) | 0.504 |
| CHA₂DS₂-VASc ≥ 4 | % (n/Pts) | 41.9 (126/301) | 41.1 (85/207) | 43.6 (41/94) | 0.677 |
| BASELINE ASSESSMENT | |||||
| Intrinsic QRS (ms) | Mean ± SD | 138.3 ± 29.5 | 134.8 ± 29.4 | 144.6 ± 28.7 | 0.006 |
| LVEF (%) | Mean ± SD | 34.9 ± 10.9 | 34.6 ± 10.4 | 35.3 ± 11.7 | 0.937 |
| DEVICE TYPE | |||||
| CRT-D | % (n/Pts) | 52.7 (259/491) | 54.7 (169/309) | 49.5 (90/182) | 0.371 |
| CRT-P | % (n/Pts) | 7.7 (38/491) | 7.1 (22/309) | 8.8 (16/182) | |
| DC-ICD | % (n/Pts) | 17.3 (85/491) | 17.8 (55/309) | 16.5 (30/182) | |
| SC-ICD | % (n/Pts) | 21.4 (105/491) | 20.1 (62/309) | 23.6 (43/182) | |
| IPG | % (n/Pts) | 0.8 (4/491) | 0.3 (1/309) | 1.6 (3/182) | |
| BASELINE MEDICATIONS | |||||
| Beta-blocker | % (n/Pts) | 75.1 (293/390) | 77.4 (199/257) | 70.7 (94/133) | 0.143 |
| ACE inhibitor/ARBs | % (n/Pts) | 58.2 (228/392) | 56.4 (146/259) | 61.7 (82/133) | 0.315 |
| Diuretic | % (n/Pts) | 76.1 (309/406) | 76.8 (208/271) | 74.8 (101/135) | 0.666 |
| ARNi | % (n/Pts) | 23.0 (70/305) | 24.8 (53/214) | 18.7 (17/91) | 0.248 |
| Antiplatelet | % (n/Pts) | 47.0 (186/396) | 46.7 (122/261) | 47.4 (64/135) | 0.900 |
| OAC | % (n/Pts) | 45.8 (164/358) | 42.6 (103/242) | 52.6 (61/116) | 0.075 |
| N Subjects | Population | N Sites | Geographies | Type of Investigated Health App | Endpoints | Smartphone/Tablet Access | Able to Use Apps | Using Health Apps | Intention to Use Health App for HF | Adherence in Using app for HF | App Intervention Effect on Patient Outcomes | Other Results | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANGELS OF HF PROJECT | |||||||||||||
| Ziacchi M et al. | 495 | HF patients with cardiac implantable electronic devices:
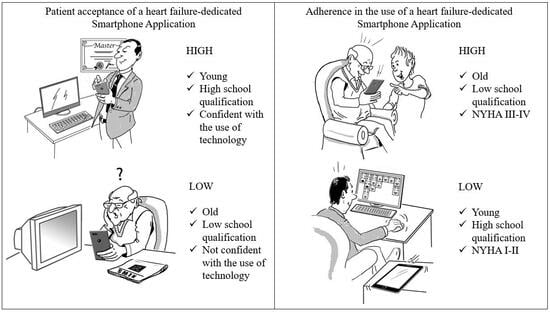
| 10 | Italy | App with remote HF data monitored by nurse/cardiologist + clinical feedback/intervention, if needed | Patient ownership/access to smartphone/tablet; Patients’ or caregivers’ acceptance to receive an HF-dedicated app; Patient adherence to using the HF-dedicated app during the first year of usage. | 60% on patient smartphone/ tablet; 24% on caregiver smartphone/tablet. | 69% | 21% | 85% | 49% (mean adherence at 1 year). | Not evaluated yet. | Younger patients with higher school qualifications are more likely to accept the technology, despite being less diligent in using it. The caregiver has a fundamental role in increasing the penetration of apps and in improving patient adherence. |
| PREVIOUS STUDIES | |||||||||||||
| Leigh JW et al. [13] | 100 | HF patients:
| 1 | US | HF self-care app | Smartphone ownership and patient attitudes toward using a health app for HF. | 68% | 60% | 22% | N.A. | N.A. | N.A. | Ethnic minorities had higher smartphone ownership rates compared with White patients with HF. Moderate significant association between smartphone ownership and age, education, and employment status. |
| Cajita MI et al. [14] | 129 | HF patients ≥65 y:
| 1 | US | HF self-care app | Factors that influence intention to use a health app for HF; current smartphone use; intention to use a health app if recommended by cardiologist. | 57.4% | N.A. | N.A. | 85% | N.A. | N.A. | Social influence and higher perceived ease of use and usefulness were both associated with higher intention to use a health app. Perceived financial cost and eHealth literacy were not significantly associated with intention to use mHealth. |
| Sohn A et al. [15] | 49 | HF patients between 50-80 y:
| 1 | US | HF self-care app | Interest in a smartphone app for HF; Determine factors that influence patient interest in app for HF. | 90% | N.A. | N.A. | 79% | N.A. | N.A. | Age correlated negatively with interest in activity tracking, HF symptoms management tips, and reminder features of the app for HF. |
| Shan R et al. [16] | 1903 | Patients with CV disease or CV risk factors:
| National Survey | US | All types | Prevalence of health app access and usage; Association between CV disease risk and health app uptake. | 73% | N.A. | 48% | N.A. | N.A. | N.A. | CVD risk was associated with sharing information from smartphone with a clinician. |
| Boriani et al. [17] | 1067 | Cardiology outpatients:
| 1 | Italy | App for teleconference | Evaluate digital literacy among cardiology outpatients to assess the possibilities to extend telemedicine/televisits during COVID pandemic. | 59% | 57% | N.A. | N.A. | N.A. | N.A. | The most used devices for internet access were smartphones, and WhatsApp represented the most used app. Internet users were younger compared to those who did not use the internet and had a higher educational level. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziacchi, M.; Molon, G.; Giudici, V.; Botto, G.L.; Viscusi, M.; Brasca, F.; Santoro, A.; Curcio, A.; Manzo, M.; Mauro, E.; et al. Integration of a Smartphone HF-Dedicated App in the Remote Monitoring of Heart Failure Patients with Cardiac Implantable Electronic Devices: Patient Access, Acceptance, and Adherence to Use. J. Clin. Med. 2023, 12, 5528. https://doi.org/10.3390/jcm12175528
Ziacchi M, Molon G, Giudici V, Botto GL, Viscusi M, Brasca F, Santoro A, Curcio A, Manzo M, Mauro E, et al. Integration of a Smartphone HF-Dedicated App in the Remote Monitoring of Heart Failure Patients with Cardiac Implantable Electronic Devices: Patient Access, Acceptance, and Adherence to Use. Journal of Clinical Medicine. 2023; 12(17):5528. https://doi.org/10.3390/jcm12175528
Chicago/Turabian StyleZiacchi, Matteo, Giulio Molon, Vittorio Giudici, Giovanni Luca Botto, Miguel Viscusi, Francesco Brasca, Amato Santoro, Antonio Curcio, Michele Manzo, Erminio Mauro, and et al. 2023. "Integration of a Smartphone HF-Dedicated App in the Remote Monitoring of Heart Failure Patients with Cardiac Implantable Electronic Devices: Patient Access, Acceptance, and Adherence to Use" Journal of Clinical Medicine 12, no. 17: 5528. https://doi.org/10.3390/jcm12175528
APA StyleZiacchi, M., Molon, G., Giudici, V., Botto, G. L., Viscusi, M., Brasca, F., Santoro, A., Curcio, A., Manzo, M., Mauro, E., Biffi, M., Costa, A., Dell’Aquila, A., Casale, M. C., & Boriani, G. (2023). Integration of a Smartphone HF-Dedicated App in the Remote Monitoring of Heart Failure Patients with Cardiac Implantable Electronic Devices: Patient Access, Acceptance, and Adherence to Use. Journal of Clinical Medicine, 12(17), 5528. https://doi.org/10.3390/jcm12175528