Invasive Functional Assessment of Coronary Artery Disease in Patients with Severe Aortic Stenosis in the TAVI Era
Abstract
1. Introduction
2. Functional Assessment of the Coronary Arteries—Basic Principles and Parameters
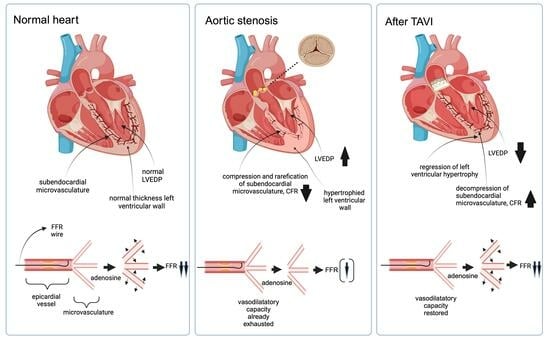
2.1. Myocardial and Coronary Hemodynamics in Aortic Valve Stenosis—Pathophysiological Background
2.2. Changes in Coronary Hemodynamics after TAVI
2.3. Functional Assessment of Epicardial Coronary Stenosis Using FFR
2.4. Functional Assessment of Epicardial Coronary Stenosis Using Non-Hyperemic Indices
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Larosa, C.; Rosa, I.; Biondi-Zoccai, G.; Nestola, P.L.; Di Cillo, O.; Bortone, A.S.; Giordano, A.; Favale, S. Degenerative Severe Aortic Stenosis and Concomitant Coronary Artery Disease: What Is Changing in the Era of the “Transcatheter Revolution”? Curr. Atheroscler. Rep. 2020, 22, 17. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Stortecky, S.; Meier, B.; Windecker, S.; Wenaweser, P. Severe aortic stenosis and coronary artery disease. EuroIntervention 2013, 9, S63–S68. [Google Scholar] [CrossRef]
- Kvidal, P.; Bergstrom, R.; Horte, L.G.; Stahle, E. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 2000, 35, 747–756. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Tarantini, G.; Tang, G.; Nai Fovino, L.; Blackman, D.; Van Mieghem, N.M.; Kim, W.K.; Karam, N.; Carrilho-Ferreira, P.; Fournier, S.; Pregowski, J.; et al. Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2023, 19, 37–52. [Google Scholar] [CrossRef]
- Rheude, T.; Costa, G.; Ribichini, F.L.; Pilgrim, T.; Amat Santos, I.J.; De Backer, O.; Kim, W.K.; Ribeiro, H.B.; Saia, F.; Bunc, M.; et al. Comparison of different percutaneous revascularisation timing strategies in patients undergoing transcatheter aortic valve implantation. EuroIntervention 2023. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- De Bruyne, B.; Fearon, W.F.; Pijls, N.H.; Barbato, E.; Tonino, P.; Piroth, Z.; Jagic, N.; Mobius-Winckler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N. Engl. J. Med. 2014, 371, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; De Bruyne, B.; Peels, K.; Van Der Voort, P.H.; Bonnier, H.J.; Bartunek, J.K.J.J.; Koolen, J.J. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 1996, 334, 1703–1708. [Google Scholar] [CrossRef]
- Stegehuis, V.E.; Wijntjens, G.W.; Piek, J.J.; van de Hoef, T.P. Fractional Flow Reserve or Coronary Flow Reserve for the Assessment of Myocardial Perfusion: Implications of FFR as an Imperfect Reference Standard for Myocardial Ischemia. Curr. Cardiol. Rep. 2018, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Escaned, J.; Malik, I.S.; Mikhail, G.W.; Foale, R.A.; Mila, R.; Tarkin, J.; Petraco, R.; Broyd, C.; Jabbour, R.; et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: Results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol. 2012, 59, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Maehara, A.; Genereux, P.; Asrress, K.N.; Berry, C.; De Bruyne, B.; Davies, J.E.; Escaned, J.; Fearon, W.F.; Gould, K.L.; et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: The RESOLVE study. J. Am. Coll. Cardiol. 2014, 63, 1253–1261. [Google Scholar] [CrossRef]
- Gotberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Ohagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Sen, S.; Dehbi, H.M.; Al-Lamee, R.; Petraco, R.; Nijjer, S.S.; Bhindi, R.; Lehman, S.J.; Walters, D.; Sapontis, J.; et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N. Engl. J. Med. 2017, 376, 1824–1834. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Park, J.; Hwang, D.; Rhee, T.M.; Kim, J.; Park, J.; Kim, H.Y.; Jung, H.W.; Cho, Y.K.; et al. Physiological and Clinical Assessment of Resting Physiological Indexes. Circulation 2019, 139, 889–900. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.H.; Hwang, D.; Park, J.; Fan, Y.; Na, S.H.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Koo, B.K. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J. Am. Coll. Cardiol. 2016, 67, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Barbato, E.; Aarnoudse, W.; Aengevaeren, W.R.; Werner, G.; Klauss, V.; Bojara, W.; Herzfeld, I.; Oldroyd, K.G.; Pijls, N.H.; De Bruyne, B.; et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur. Heart J. 2004, 25, 219–223. [Google Scholar] [CrossRef]
- Doucette, J.W.; Corl, P.D.; Payne, H.M.; Flynn, A.E.; Goto, M.; Nassi, M.; Segal, J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 1992, 85, 1899–1911. [Google Scholar] [CrossRef]
- Majmudar, M.D.; Murthy, V.L.; Shah, R.V.; Kolli, S.; Mousavi, N.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Sitek, A.; et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 900–909. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Hachamovitch, R.; Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015, 131, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Melikian, N.V.S.; Fearon, W.F.; Cuisset, T.; MacCarthy, P.A.; Davidavicius, G.; Aarnoudse, W.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; Wijns, W.; et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. Eurointervention 2010, 5, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Long, M.; Hu, X.; Huang, Z.; Hu, C.; Gao, X.; Du, Z. Thermodilution-derived coronary microvascular resistance and flow reserve in patients with cardiac syndrome X. Circ. Cardiovasc. Interv. 2014, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Solberg, O.G.; Ragnarsson, A.; Kvarsnes, A.; Endresen, K.; Kongsgard, E.; Aakhus, S.; Gullestad, L.; Stavem, K.; Aaberge, L. Reference interval for the index of coronary microvascular resistance. EuroIntervention 2014, 9, 1069–1075. [Google Scholar] [CrossRef]
- Gould, K.L.; Carabello, B.A. Why angina in aortic stenosis with normal coronary arteriograms? Circulation 2003, 107, 3121–3123. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef]
- Carabello, B.A. Introduction to aortic stenosis. Circ. Res. 2013, 113, 179–185. [Google Scholar] [CrossRef]
- Ross, J., Jr.; Braunwald, E. Aortic stenosis. Circulation 1968, 38 (Suppl. S1), 61–67. [Google Scholar] [CrossRef]
- Selzer, A. Changing aspects of the natural history of valvular aortic stenosis. N. Engl. J. Med. 1987, 317, 91–98. [Google Scholar] [CrossRef]
- Garcia, D.; Camici, P.G.; Durand, L.G.; Rajappan, K.; Gaillard, E.; Rimoldi, O.E.; Pibarot, P. Impairment of coronary flow reserve in aortic stenosis. J. Appl. Physiol. 2009, 106, 113–121. [Google Scholar] [CrossRef]
- Marcus, M.L.; Doty, D.B.; Hiratzka, L.F.; Wright, C.B.; Eastham, C.L. Decreased coronary reserve: A mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N. Engl. J. Med. 1982, 307, 1362–1366. [Google Scholar] [CrossRef]
- Nemes, A.; Balazs, E.; Csanady, M.; Forster, T. Long-term prognostic role of coronary flow velocity reserve in patients with aortic valve stenosis-insights from the SZEGED Study. Clin. Physiol. Funct. Imaging 2009, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Aleksandric, S.; Banovic, M.; Beleslin, B. Challenges in Diagnosis and Functional Assessment of Coronary Artery Disease in Patients With Severe Aortic Stenosis. Front. Cardiovasc. Med. 2022, 9, 849032. [Google Scholar] [CrossRef]
- Nemes, A.; Forster, T.; Csanady, M. Decreased aortic distensibility and coronary flow velocity reserve in patients with significant aortic valve stenosis with normal epicardial coronary arteries. J. Heart Valve Dis. 2004, 13, 567–573. [Google Scholar]
- Banovic, M.D.; Vujisic-Tesic, B.D.; Kujacic, V.G.; Callahan, M.J.; Nedeljkovic, I.P.; Trifunovic, D.D.; Aleksandric, S.B.; Petrovic, M.Z.; Obradovic, S.D.; Ostojic, M.C. Coronary flow reserve in patients with aortic stenosis and nonobstructed coronary arteries. Acta Cardiol. 2011, 66, 743–749. [Google Scholar] [CrossRef]
- Wiegerinck, E.M.; van de Hoef, T.P.; Rolandi, M.C.; Yong, Z.; van Kesteren, F.; Koch, K.T.; Vis, M.M.; de Mol, B.A.; Piek, J.J.; Baan, J., Jr. Impact of Aortic Valve Stenosis on Coronary Hemodynamics and the Instantaneous Effect of Transcatheter Aortic Valve Implantation. Circ. Cardiovasc. Interv. 2015, 8, e002443. [Google Scholar] [CrossRef] [PubMed]
- Camuglia, A.C.; Syed, J.; Garg, P.; Kiaii, B.; Chu, M.W.; Jones, P.M.; Bainbridge, D.; Teefy, P.J. Invasively assessed coronary flow dynamics improve following relief of aortic stenosis with transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2014, 63, 1808–1809. [Google Scholar] [CrossRef][Green Version]
- Stoller, M.; Gloekler, S.; Zbinden, R.; Tueller, D.; Eberli, F.; Windecker, S.; Wenaweser, P.; Seiler, C. Left ventricular afterload reduction by transcatheter aortic valve implantation in severe aortic stenosis and its prompt effects on comprehensive coronary haemodynamics. EuroIntervention 2018, 14, 166–173. [Google Scholar] [CrossRef]
- Wada, T.; Shiono, Y.; Honda, K.; Higashioka, D.; Taruya, A.; Takahata, M.; Fujita, S.; Ota, S.; Satogami, K.; Ozaki, Y.; et al. Serial changes of coronary flow reserve over one year after transcatheter aortic valve implantation in patients with severe aortic stenosis. Int. J. Cardiol. Heart Vasc. 2022, 42, 101090. [Google Scholar] [CrossRef]
- Vendrik, J.; Ahmad, Y.; Eftekhari, A.; Howard, J.P.; Wijntjens, G.W.M.; Stegehuis, V.E.; Cook, C.; Terkelsen, C.J.; Christiansen, E.H.; Koch, K.T.; et al. Long-Term Effects of Transcatheter Aortic Valve Implantation on Coronary Hemodynamics in Patients With Concomitant Coronary Artery Disease and Severe Aortic Stenosis. J. Am. Heart Assoc. 2020, 9, e015133. [Google Scholar] [CrossRef]
- Sabbah, M.; Olsen, N.T.; Holmvang, L.; Tilsted, H.H.; Pedersen, F.; Joshi, F.R.; Sorensen, R.; Jabbari, R.; Arslani, K.; Sondergaard, L.; et al. Long-term changes in coronary physiology after aortic valve replacement. EuroIntervention 2023, 18, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’ t Veer, M.; Klauss, V.; Manoharan, G.; Engstrom, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Mobius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Paulus, W.J.; Pijls, N.H. Rationale and application of coronary transstenotic pressure gradient measurements. Catheter. Cardiovasc. Diagn. 1994, 33, 250–261. [Google Scholar] [CrossRef]
- Ahmad, Y.; Vendrik, J.; Eftekhari, A.; Howard, J.P.; Cook, C.; Rajkumar, C.; Malik, I.; Mikhail, G.; Ruparelia, N.; Hadjiloizou, N.; et al. Determining the Predominant Lesion in Patients With Severe Aortic Stenosis and Coronary Stenoses: A Multicenter Study Using Intracoronary Pressure and Flow. Circ. Cardiovasc. Interv. 2019, 12, e008263. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, S.M.; Park, S.J.; Jeong, D.S.; Woo, M.A.; Jung, S.H.; Lee, S.C.; Park, S.W.; Choe, Y.H.; Park, P.W.; et al. Coronary Microvascular Dysfunction as a Mechanism of Angina in Severe AS: Prospective Adenosine-Stress CMR Study. J. Am. Coll. Cardiol. 2016, 67, 1412–1422. [Google Scholar] [CrossRef]
- Ahmad, Y.; Gotberg, M.; Cook, C.; Howard, J.P.; Malik, I.; Mikhail, G.; Frame, A.; Petraco, R.; Rajkumar, C.; Demir, O.; et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc. Interv. 2018, 11, 2019–2031. [Google Scholar] [CrossRef]
- Pesarini, G.; Scarsini, R.; Zivelonghi, C.; Piccoli, A.; Gambaro, A.; Gottin, L.; Rossi, A.; Ferrero, V.; Vassanelli, C.; Ribichini, F. Functional Assessment of Coronary Artery Disease in Patients Undergoing Transcatheter Aortic Valve Implantation: Influence of Pressure Overload on the Evaluation of Lesions Severity. Circ. Cardiovasc. Interv. 2016, 9, e004088. [Google Scholar] [CrossRef]
- Stundl, A.; Shamekhi, J.; Bernhardt, S.; Starke, M.; Al-Kassou, B.; Weber, M.; Sedaghat, A.; Treede, H.; Grube, E.; Nickenig, G.; et al. Fractional flow reserve in patients with coronary artery disease undergoing TAVI: A prospective analysis. Clin. Res. Cardiol. 2020, 109, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; Scarsini, R.; Venturi, G.; Pesarini, G.; Pighi, M.; Gratta, A.; Gottin, L.; Barbierato, M.; Caprioglio, F.; Piccoli, A.; et al. Physiological Versus Angiographic Guidance for Myocardial Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Hear. Assoc. 2019, 8, e012618. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Pesarini, G.; Zivelonghi, C.; Piccoli, A.; Ferrero, V.; Lunardi, M.; Barbierato, M.; Caprioglio, F.; Vassanelli, C.; Ribichini, F. Coronary physiology in patients with severe aortic stenosis: Comparison between fractional flow reserve and instantaneous wave-free ratio. Int. J. Cardiol. 2017, 243, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, F.; Shishido, K.; Ochiai, T.; Moriyama, N.; Yamazaki, K.; Sugitani, A.; Tani, T.; Tobita, K.; Mizuno, S.; Tanaka, Y.; et al. Instantaneous Wave-Free Ratio for the Assessment of Intermediate Coronary Artery Stenosis in Patients With Severe Aortic Valve Stenosis: Comparison With Myocardial Perfusion Scintigraphy. JACC Cardiovasc. Interv. 2018, 11, 2032–2040. [Google Scholar] [CrossRef]
- Scarsini, R.; Cantone, R.; Venturi, G.; De Maria, G.L.; Variola, A.; Braggio, P.; Lunardi, M.; Pesarini, G.; Ferdeghini, M.; Piccoli, A.; et al. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int. J. Cardiol. 2019, 292, 162–165. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Rzeszutko, L.; Dudek, D.; Legutko, J. Hyperemic versus non-hyperemic indexes for coronary physiology assessment in patients with severe aortic stenosis. Adv. Med. Sci. 2021, 66, 366–371. [Google Scholar] [CrossRef]
| References | Year | Study Design | n | Results | p-Values |
|---|---|---|---|---|---|
| Vendrik et al. [42] | 2020 | Prospective, observational; FFR and iFR measurements before TAVI, immediately after TAVI and at six-month FU | 13 | FFR values decreased from 0.85 (0.76–0.88) pre-TAVI to 0.79 (0.74–0.83) post-TAVI and 0.71 (0.65–0.77) at six-month FU; iFR values did not change: 0.82 (0.80–0.90) pre-TAVI, 0.83 (0.77–0.88) post-TAVI, and 0.83 (0.73–0.89) at six months | FFR before vs. after TAVI: p < 0.001; iFR: p = 0.735 |
| Ahmad al. [47] | 2019 | Prospective, observational; assessment of microvascular function, FFR and iFR in both AS and no AS pts with concomitant CAD before and immediately after TAVI; in no AS pts before and after PCI | 55 (AS pts) | FFR values decreased from 0.86 (±0.08) pre-TAVI to 0.83 (±0.09) post-TAVI; iFR values did not change: 0.87 (±0.10) pre-TAVI and 0.87 (±0.09) post-TAVI | FFR before vs. after TAVI: p < 0.001 iFR: p = 0.80 |
| Ahmad et al. [49] | 2018 | Prospective, observational; intracoronary flow-, FFR and iFR measurements immediately before and after TAVI | 28 | FFR values changed from 0.87 ± 0.08 pre-TAVI to 0.85 ± 0.09 post-TAVI; iFR values did not change: 0.88 ± 0.09 pre-TAVR vs. 0.88 ± 0.09 post-TAVR | FFR before vs. after TAVI: p < 0.001; iFR: p = 0.73 |
| Pesarini et al. [50] | 2016 | Prospective, observational; FFR measurements before and after TAVI during the same procedure | 54 | No relevant FFR change before and after TAVI: 0.89 ± 0.10 vs. 0.89 ± 0.13; however, FFR values in >50% stenosis worsened after TAVI 0.84 ± 0.12 vs. 0.82 ± 0.16, whereas FFR in mild stenosis (<50%) improved slightly: 0.90 ± 0.07 vs. 0.91 ± 0.09 | Overall FFR change before and after TAVI: p = 0.73; FFR values in >50% stenosis before vs. after TAVI: p = 0.02; FFR values in <50% stenosis: p = 0.69 |
| Stoller et al. [40] | 2018 | Prospective, observational; FFR and CFR measurements before and after TAVI | 40 | Improvement of FFR values after TAVI compared with FFR before TAVI: 0.90 ± 0.08 vs. 0.93 ± 0.08; CFR values did not change significantly: 1.9 ± 0.09 vs. 2.0 ± 1.0 | FFR before vs. after TAVI: p = 0.0021; CFR before vs. after TAVI: p = 0.72 |
| Lunardi et al. [52] | 2019 | Retrospective; comparison of FFR- vs. angiography guidance in patients with AS and concomitant CAD planned for TAVI in terms of MACCE at two-year FU | 216 | MACCE-free survival was better in the FFR-guided group versus angio-guided group: 92.6% vs. 82.0%; hazard ratio 0.4; 95% CI 0.2–1.0 | p = 0.035 |
| Stundl et al. [51] | 2020 | Prospective observational; FFR measurements in coronary artery stenosis >50% in AS pts before and six to eight weeks after TAVI | 13 | No relevant difference in FFR values before and after TAVI: 0.77 ± 0.04 vs. 0.76 ± 0.08 | p = 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weferling, M.; Kim, W.-K. Invasive Functional Assessment of Coronary Artery Disease in Patients with Severe Aortic Stenosis in the TAVI Era. J. Clin. Med. 2023, 12, 5414. https://doi.org/10.3390/jcm12165414
Weferling M, Kim W-K. Invasive Functional Assessment of Coronary Artery Disease in Patients with Severe Aortic Stenosis in the TAVI Era. Journal of Clinical Medicine. 2023; 12(16):5414. https://doi.org/10.3390/jcm12165414
Chicago/Turabian StyleWeferling, Maren, and Won-Keun Kim. 2023. "Invasive Functional Assessment of Coronary Artery Disease in Patients with Severe Aortic Stenosis in the TAVI Era" Journal of Clinical Medicine 12, no. 16: 5414. https://doi.org/10.3390/jcm12165414
APA StyleWeferling, M., & Kim, W.-K. (2023). Invasive Functional Assessment of Coronary Artery Disease in Patients with Severe Aortic Stenosis in the TAVI Era. Journal of Clinical Medicine, 12(16), 5414. https://doi.org/10.3390/jcm12165414