The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography
Abstract
:1. Introduction
1.1. Retinal Blood Flow Measurement in Patients with Carotid Artery Stenosis
1.2. Role of CoW and Cerebral Collateral Blood Flow in Patients with Carotid Stenosis
2. Materials and Methods
2.1. Patients’ Enrollment and Surgical Treatment
2.2. Brain CT and CTA Examinations and Evaluation
2.3. OCTA Examination
2.4. Statistical Analysis
3. Results
3.1. Enrollment
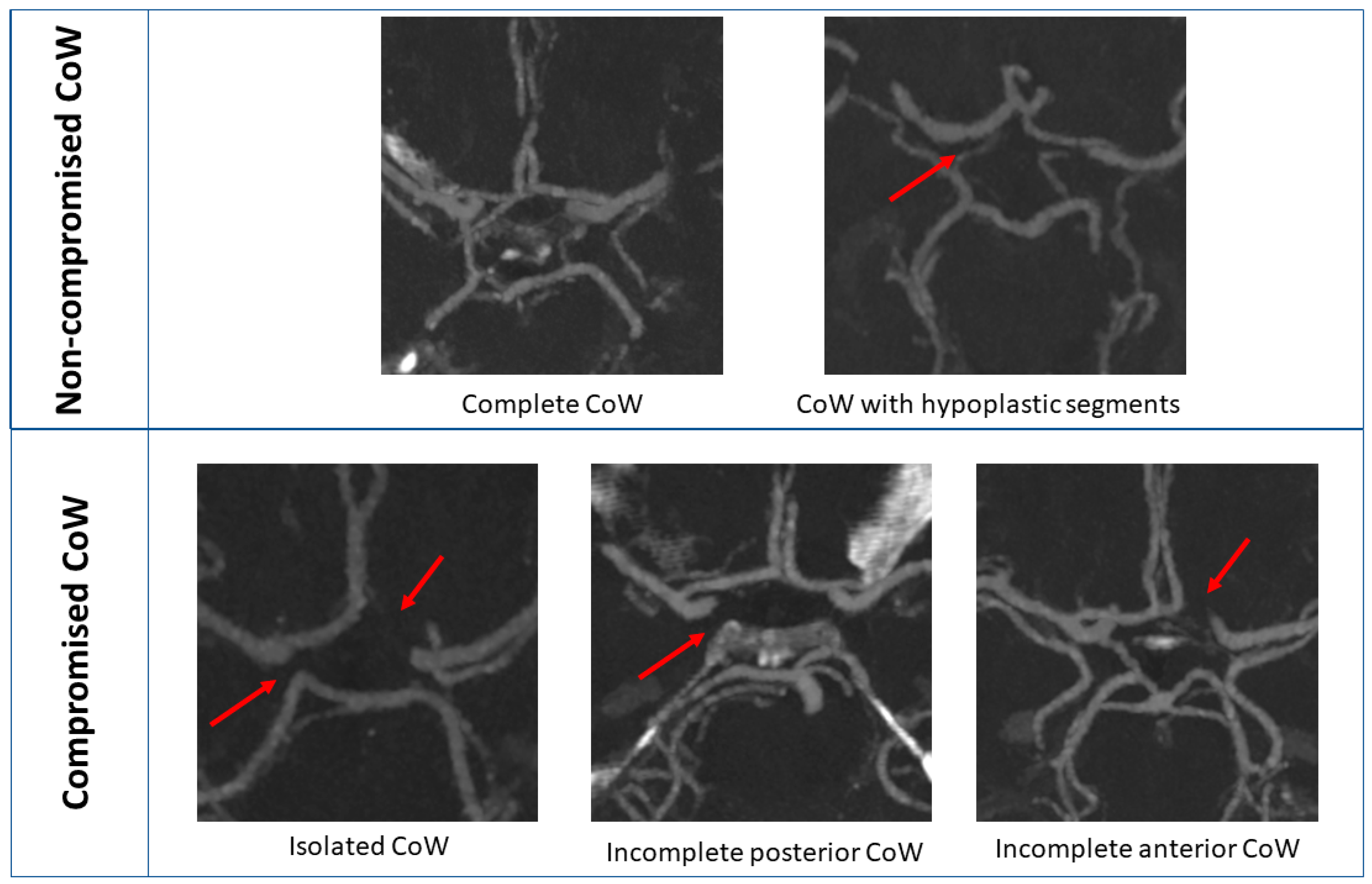
3.2. CoW Analysis Based on CT Angiography
3.3. OCT Measurements
4. Discussion
4.1. CoW Categorization
4.2. Role of Collateral Flow
4.3. OCT Image Quality
4.4. The Role of Ophthalmic Artery in Collateral Flow
4.5. Effect of CoW Morphology on Retinal Blood Flow
4.6. Positive Clinical Effect of Increased Retinal and Cerebral Blood Flow
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| All Patients | 70–89% ICA Stenosis | 90–99% ICA Stenosis | p-Value | |
|---|---|---|---|---|
| Number of patients n (%) | 56 | 44 | 12 | |
| Sex female n (%) | 17 (30.4%) | 12 (27.3%) | 5 (41.7%) | 0.48 |
| Contralateral ICA significant stenosis/occlusion n (%) | 10 (17.9%) | 9 (20.5%) | 1 (8.3%) | 0.67 |
| Symptomatic ICA stenosis n (%) | 4 (7.1%) | 3 (6.7%) | 1 (8.3%) | 1.00 |
| Comorbidities | ||||
| Smoking n (%) | 16 (28.6%) | 10 (22.7%) | 6 (50%) | 0.08 |
| Hypertension n (%) | 52 (92.9%) | 41 (93.1%) | 11 (91.7%) | 1.00 |
| Diabetes mellitus n (%) | 21 (37.5%) | 16 (36.4%) | 5 (41.7%) | 0.75 |
| Ischemic heart disease n (%) | 13 (23.2%) | 12 (27.3%) | 1 (8.3%) | 0.26 |
| COPD n (%) | 5 (8.9%) | 4 (9.1%) | 1 (8.3%) | 1.00 |
| Medical therapy | ||||
| Aspirin n (%) | 33 (58.9%) | 29 (65.9%) | 4 (33.3%) | 0.05 |
| Clopidogrel n (%) | 21(37.5%) | 15 (34.1%) | 6 (50%) | 0.34 |
| Statin n (%) | 33 (58.9%) | 25 (56.8%) | 8 (66.7%) | 0.74 |
| Circle of Willis morphology subgroups | ||||
| Non-compromised | 13 (23.2%) | 11 (25%) | 2 (16.7%) | 0.71 |
| Compromised | 43 (76.8%) | 33 (75%) | 10 (83.3%) | |
References
- AbuRahma, A.F.; Avgerinos, E.M.; Chang, R.W.; Darling, R.C., 3rd; Zhou, W.; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2021, 18, S0741–S5214. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A. Editor’s Choice-European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Eliasziw, M.; Gutnikov, S.A.; Fox, A.J.; Mayberg, M.R.; Mayberg, M.R.; Warlow, C.P.; Barnett, H.J.M. Carotid Endarterectomy Trialists’ Collaboration. Analysis of pooled data from the randomized controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003, 361, 107e16. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Gutnikov, S.A.; Warlow, C.P. European Carotid Surgery Trialist’s Collaboration. Sex differences in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischaemic attack and non-disabling stroke. Stroke 2004, 35, 2855e61. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Noubiap, J.J.; Wilman, A.H.; Saqqur, M.; Jickling, G.C.; Shuaib, A. Prevalence of High-risk Plaques and Risk of Stroke in Patients with Asymptomatic Carotid Stenosis: A Meta-analysis. JAMA Neurol. 2020, 77, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- István, L.; Czakó, C.; Benyó, F.; Élő, Á.; Kovács, I.; Mihály, Z.; Sótonyi, P.; Varga, A.; Nagy, Z.Z.; Kovács, I. The effect of systemic factors on retinal blood flow in patients with carotid stenosis: An optical coherence tomography angiography study. Geroscience 2022, 44, 389–401. [Google Scholar] [CrossRef]
- Reynolds, P.S.; Greenberg, J.P.; Lien, L.-M.; Meads, D.C.; Myers, L.G.; Tegeler, C.H. Ophthalmic artery flow direction on color flow duplex imaging is highly specific for severe carotid stenosis. J. Neuroimaging 2002, 12, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Coscas, F.; Sellam, A.; Glacet-Bernard, A.; Jung, C.; Goudot, M.; Miere, A.; Souied, E.H. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2016, 57, 211–223. [Google Scholar] [CrossRef]
- Shiihara, H.; Sakamoto, T.; Yamashita, T.; Kakiuchi, N.; Otsuka, H.; Terasaki, H.; Sonoda, S. Reproducibility and differences in area of foveal avascular zone measured by three different optical coherence tomographic angiography instruments. Sci. Rep. 2017, 7, 9853. [Google Scholar] [CrossRef]
- Guo, J.; She, X.; Liu, X.; Sun, X. Repeatability and Reproducibility of Foveal Avascular Zone Area Measurements Using AngioPlex Spectral Domain Optical Coherence Tomography Angiography in Healthy Subjects. Ophthalmologica 2017, 237, 21–28. [Google Scholar] [CrossRef]
- Lei, J.; Durbin, M.K.; Shi, Y.; Uji, A.; Balasubramanian, S.; Baghdasaryan, E.; Al-Sheikh, M.; Sadda, S.R. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA Ophthalmol. 2017, 135, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Tepelus, T.C.; Nazikyan, T.; Sadda, S.R. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 449–452. [Google Scholar] [CrossRef]
- Venugopal, J.P.; Rao, H.L.; Weinreb, R.N.; Pradhan, Z.S.; Dasari, S.; Riyazuddin, M.; Puttiah, N.K.; Rao, D.A.S.; Devi, S.; Mansouri, K.; et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br. J. Ophthalmol. 2018, 102, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Fenner, B.J.; Tan, G.S.W.; Tan, A.C.S.; Yeo, I.Y.S.; Wong, T.Y.; Cheung, G.C.M. Identification of imaging features that determine quality and repeatability of retinal capillary plexus density measurements in OCT angiography. Br. J. Ophthalmol. 2018, 102, 509–514. [Google Scholar] [CrossRef]
- Odabaş, Ö.; Demirel, S.; Özmert, E.; Batioğlu, F. Repeatability of automated vessel density and superficial and deep foveal avascular zone area measurements using optical coherence tomography angiography: Diurnal Findings. Retina 2018, 38, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Van Melkebeke, L.; Barbosa-Breda, J.; Huygens, M.; Stalmans, I. Optical Coherence Tomography Angiography in Glaucoma: A Review. Ophthalmic Res. 2018, 60, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ang, M.; Najjar, R.P.; Sng, C.; Cheung, C.Y.; Rukmini, A.V.; Schmetterer, L.; Milea, D. Optical coherence tomography angiography in acute non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2017, 101, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, K.M.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Repeatability of vessel density measurements using optical coherence tomography angiography in retinal diseases. Br. J. Ophthalmol. 2019, 103, 704–710. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease with Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242–1248. [Google Scholar] [CrossRef]
- Lahme, L.; Marchiori, E.; Panuccio, G.; Nelis, P.; Schubert, F.; Mihailovic, N.; Torsello, G.; Eter, N.; Alnawaiseh, M. Changes in retinal flow density measured by optical coherence tomography angiography in patients with carotid artery stenosis after carotid endarterectomy. Sci. Rep. 2018, 8, 17161. [Google Scholar] [CrossRef]
- Lee, C.W.; Cheng, H.C.; Chang, F.C.; Wang, A.G. Optical Coherence Tomography Angiography Evaluation of Retinal Microvasculature before and after Carotid Angioplasty and Stenting. Sci. Rep. 2019, 9, 14755. [Google Scholar] [CrossRef] [PubMed]
- Alpers, B.J.; Berry, R.G. Circle of Willis in cerebral vascular disorders. The anatomical structure. Arch. Neurol. 1963, 8, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Battacharji, S.K.; Hutchinson, E.C.; McCall, A.J. The Circle of Willis—The incidence of developmental abnormalities in normal and infarcted brains. Brain 1967, 90, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Di Leo, G.; Banga, P.V.; Csobay-Novák, C.; Kolossváry, M.; Maurovich-Horvat, P.; Hüttl, K. Multidetector CT angiography of the Circle of Willis: Association of its variants with carotid artery disease and brain ischemia. Eur. Radiol. 2019, 29, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Banga, P.V.; Varga, A.; Csobay-Novák, C.; Kolossváry, M.; Szántó, E.; Oderich, G.S.; Entz, L.; Sótonyi, P. Incomplete circle of Willis is associated with a higher incidence of neurologic events during carotid eversion endarterectomy without shunting. J. Vasc. Surg. 2018, 68, 1764–1771. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 7, 255–323. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3, 1–150. [Google Scholar]
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef]
- North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef]
- Niculescu, R.; Russu, E.; Arbănași, E.M.; Kaller, R.; Cotoi, O.S. Carotid Plaque Features and Inflammatory Biomarkers as Predictors of Restenosis and Mortality Following Carotid Endarterectomy. Int. J. Environ. Res. Public Health 2022, 19, 13934. [Google Scholar] [CrossRef] [PubMed]
- De Caro, J.; Ciacciarelli, A.; Tessitore, A.; Buonomo, O.; Calzoni, A.; Francalanza, I.; Dell’aera, C.; Cosenza, D.; Currò, C.T.; Granata, F.; et al. Variants of the circle of Willis in ischemic stroke patients. J. Neurol. 2021, 268, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Zaninovich, O.A.; Ramey, W.L.; Walter, C.M.; Dumont, T.M. Completion of the Circle of Willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg. 2017, 106, 953–963. [Google Scholar] [CrossRef]
- Hoksbergen, A.W.; Fülesdi, B.; Legemate, D.A.; Csiba, L. Collateral configuration of the circle of Willis: Transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke 2000, 31, 1346–1351. [Google Scholar] [CrossRef]
- Kluytmans, M.; Van Der Grond, J.; Van Everdingen, K.J.; Klijn, C.J.M.; Kappelle, L.J.; Viergever, M.A. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke 1999, 30, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Sundt, T.M., Jr.; Sharbrough, F.W.; Anderson, R.E.; Michenfelder, J.D. Cerebral blood flow measurements and electroencephalograms during carotid endarterectomy. J. Neurosurg. 1974, 41, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kaszczewski, P.; Elwertowski, M.; Leszczyński, J.; Ostrowski, T.; Gałązka, Z.; Kaszczewska, J. Intracranial Flow Volume Estimation in Patients with Internal Carotid Artery Occlusion. Diagnostics 2022, 12, 766. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Zarrinkoob, L.; Wåhlin, A.; Ambarki, K.; Birgander, R.; Eklund, A.; Malm, J. Blood Flow Lateralization and Collateral Compensatory Mechanisms in Patients with Carotid Artery Stenosis. J. Stroke 2019, 50, 1081–1088. [Google Scholar] [CrossRef]
- Badacz, R.; Przewłocki, T.; Karch, I.; Pieniążek, P.; Rosławiecka, A.; Mleczko, S.; Brzychczy, A.; Trystuła, M.; Żmudka, K.; Kabłak-Ziembicka, A. Low prevalence of collateral cerebral circulation in the circle of Willis in patients with severe carotid artery stenosis and recent ischemic stroke. Postep. Kardiol Interwencyjnej 2015, 11, 312–317. [Google Scholar] [CrossRef]
- Czakó, C.; István, L.; Ecsedy, M.; Récsán, Z.; Sándor, G.; Benyó, F.; Horváth, H.; Papp, A.; Resch, M.; Borbándy, Á.; et al. The effect of image quality on the reliability of OCT angiography measurements in patients with diabetes. Int. J. Retin. Vitr. 2019, 4, 46. [Google Scholar] [CrossRef]
- Yu, J.J.; Camino, A.; Liu, L.; Zhang, X.; Wang, J.; Gao, S.S.; Jia, Y.; Huang, D. Signal Strength Reduction Effects in OCT Angiography. Ophthalmol. Retin. 2019, 3, 835–842. [Google Scholar] [CrossRef]
- Yu, S.; Frueh, B.E.; Steinmair, D.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S.; Munk, M.R. Cataract significantly influences quantitative measurements on swept-source optical coherence tomography angiography imaging. PLoS ONE 2018, 13, e0204501. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Ghasemi, F.K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Holló, G. Influence of Posterior Subcapsular Cataract on Structural OCT and OCT Angiography Vessel Density Measurements in the Peripapillary Retina. J. Glaucoma 2019, 28, e61–e63. [Google Scholar] [CrossRef]
- Czakó, C.; István, L.; Benyó, F.; Élo, Á.; Erdei, G.; Horváth, H.; Nagy, Z.Z.; Kovács, I. The Impact of Deterministic Signal Loss on OCT Angiography Measurements. Transl. Vis. Sci. Technol. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Sakata, L.M.; Deleon-Ortega, J.; Sakata, V.; Girkin, C.A. Optical coherence tomography of the retina and optic nerve—A review. Clin. Exp. Ophthalmol. 2009, 37, 90–99. [Google Scholar] [CrossRef] [PubMed]
- István, L.; Czakó, C.; Élő, Á.; Mihály, Z.; Nagy, Z.Z.; Sótonyi, P.; Varga, A.; Ungvári, Z.; Csiszár, A.; Yabluchanskiy, A.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef]
- Costa, V.P.; Kuzniec, S.; Molnar, L.J.; Cerri, G.G.; Puech-Leão, P.; Carvalho, C.A. Collateral blood supply through the ophthalmic artery. A steal phenomenon analyzed by color Doppler imaging. Ophthalmology 1998, 105, 689–693. [Google Scholar] [CrossRef]
- Costa, V.P.; Kuzniec, S.; Molnar, L.J.; Cerri, G.G.; Puech-Leão, P.; Carvalho, C.A. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. An investigation by color Doppler imaging. Ophthalmology 1999, 106, 306–310. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schmidt-Kastner, R.; Hamasaki, D.D.; Yamamoto, H.; Parel, J.M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006, 82, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, H.; Yoon, K.Y.; Chang, Y.Y.; Song, H.B. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp. Eye Res. 2020, 201, 108275. [Google Scholar] [CrossRef] [PubMed]
- Orihashi, K.; Matsuura, Y.; Sueda, T.; Shikata, H.; Morita, S.; Hirai, S.; Sueshiro, M.; Okada, K. Flow velocity of central retinal artery and retrobulbar vessels during cardiovascular operations. J. Thorac. Cardiovasc. Surg. 1997, 114, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chazen, J.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Magyar-Stang, R.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Csipo, T.; Ungvari, Z.; Sótonyi, P.; Debreczeni, R.; et al. Assessment of Cerebral Autoregulatory Function and Inter-Hemispheric Blood Flow in Older Adults with Internal Carotid Artery Stenosis Using Transcranial Doppler Sonography-Based Measurement of Transient Hyperemic Response after Carotid Artery Compression. Geroscince 2023, JAAA-D-23-00333R1, accepted for publication. [Google Scholar]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Signed the informed consent | Withdrawal of the informed consent |
| Criteria of the vascular surgery part | |
| Significant carotid artery stenosis (≥70%) NASCET criteria in carotid CTA | Pacemaker implantation |
| Planned endarterectomy | Chronic kidney disease in Stage V |
| Age under 50 years | |
| Neurological event 15 days before operation | |
| Criteria of the carotid CT angiography | |
| Examination by Brilliance iCT 256 | Serious movement artifacts |
| Criteria of the OCTA examination | |
| Associated ocular disease (age-related macular degeneration, glaucoma, vitreomacular disease) | |
| Previous intraocular anti-VEGF injection | |
| Clinically significant media opacities Serious movement artifacts or scan quality below 5 | |
| All Patients | Non-Compromised CoW | Compromised CoW | p-Value | |
|---|---|---|---|---|
| Number of patients n (%) | 56 | 13 | 43 | |
| Sex female n (%) | 17 (30.4%) | 4 (9.3%) | 16 (37.2%) | 0.75 |
| Contralateral ICA significant stenosis/occlusion n (%) | 10 (17.9%) | 4 (9.3%) | 6 (13.9%) | 0.19 |
| Ipsilateral ICA stenosis 70–89% | 44 (78.6%) | 11 (84.6%) | 33 (76.4%) | 0.71 |
| Ipsilateral ICA stenosis 90–99% | 12 (21.4%) | 2 (15.4%) | 10 (23.25%) | |
| Symptomatic ICA stenosis n (%) | 4 (7.1%) | 1 (7.7%) | 3 (6.9%) | 1.00 |
| Comorbidities | ||||
| Smoking n (%) | 16 (28.6%) | 6 (46.1%) | 10 (23.3%) | 0.16 |
| Hypertension n (%) | 52 (92.9%) | 12 (92.3%) | 40 (93.0%) | 1.00 |
| Diabetes mellitus n (%) | 21 (37.5%) | 3 (23.1%) | 18 (41.9%) | 0.33 |
| Ischemic heart disease n (%) | 13 (23.2%) | 6 (46.1%) | 7 (16.3%) | 0.05 |
| COPD n (%) | 5 (8.9%) | 2 (15.4%) | 3 (6.9%) | 0.58 |
| Medical therapy | ||||
| Aspirin n (%) | 33 (58.9%) | 9 (69.2%) | 24 (55.8%) | 0.52 |
| Clopidogrel n (%) | 21(37.5%) | 7 (53.8%) | 14 (32.6%) | 0.20 |
| Statin n (%) | 33 (58.9%) | 7 (53.8%) | 26 (60.5%) | 0.75 |
| All (n = 56) | Normal Segment | Hypoplastic Segment | Non-Visualized Segment |
|---|---|---|---|
| AcomA (n) | 94.6% (53) | 1.7% (1) | 3.6% (2) |
| A1 (n × 2) | 91.9% (103) | 4.5% (5) | 3.6% (4) |
| PcomA (n × 2) | 44.6% (25) | 19.6% (11) | 35.7% (20) |
| P1 (n × 2) | 86.6% (97) | 8.0% (9) | 5.4% (6) |
| Anterior CoW semicircle | 64.3% (36) | 25.0% (14) | 10.7% (6) |
| Posterior CoW semicircle | 37.5% (21) | 28.6% (16) | 37.5% (21) |
| Predictors | ß | Confidence Interval | p-Value |
|---|---|---|---|
| Scan quality | 1.80 | 1.53–2.07 | <0.001 |
| Age * (years) | −0.12 | −0.07–−0.15 | <0.001 |
| CoW morphology * (non-compromised vs. compromised) | 0.87 | 0.26–1.50 | 0.005 |
| Carotid endarterectomy * | 0.71 | 0.18–1.25 | 0.01 |
| Laterality * (ipsi- vs. contralateral to the reconstruction) | 0.39 | −0.13–0.91 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihály, Z.; István, L.; Czakó, C.; Benyó, F.; Borzsák, S.; Varga, A.; Magyar-Stang, R.; Banga, P.V.; Élő, Á.; Debreczeni, R.; et al. The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography. J. Clin. Med. 2023, 12, 5335. https://doi.org/10.3390/jcm12165335
Mihály Z, István L, Czakó C, Benyó F, Borzsák S, Varga A, Magyar-Stang R, Banga PV, Élő Á, Debreczeni R, et al. The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography. Journal of Clinical Medicine. 2023; 12(16):5335. https://doi.org/10.3390/jcm12165335
Chicago/Turabian StyleMihály, Zsuzsanna, Lilla István, Cecilia Czakó, Fruzsina Benyó, Sarolta Borzsák, Andrea Varga, Rita Magyar-Stang, Péter Vince Banga, Ágnes Élő, Róbert Debreczeni, and et al. 2023. "The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography" Journal of Clinical Medicine 12, no. 16: 5335. https://doi.org/10.3390/jcm12165335
APA StyleMihály, Z., István, L., Czakó, C., Benyó, F., Borzsák, S., Varga, A., Magyar-Stang, R., Banga, P. V., Élő, Á., Debreczeni, R., Kovács, I., & Sótonyi, P. (2023). The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography. Journal of Clinical Medicine, 12(16), 5335. https://doi.org/10.3390/jcm12165335





