Proliferative Vitreoretinopathy: A Reappraisal
Abstract
1. Introduction
2. Epidemiology and Risk Factors
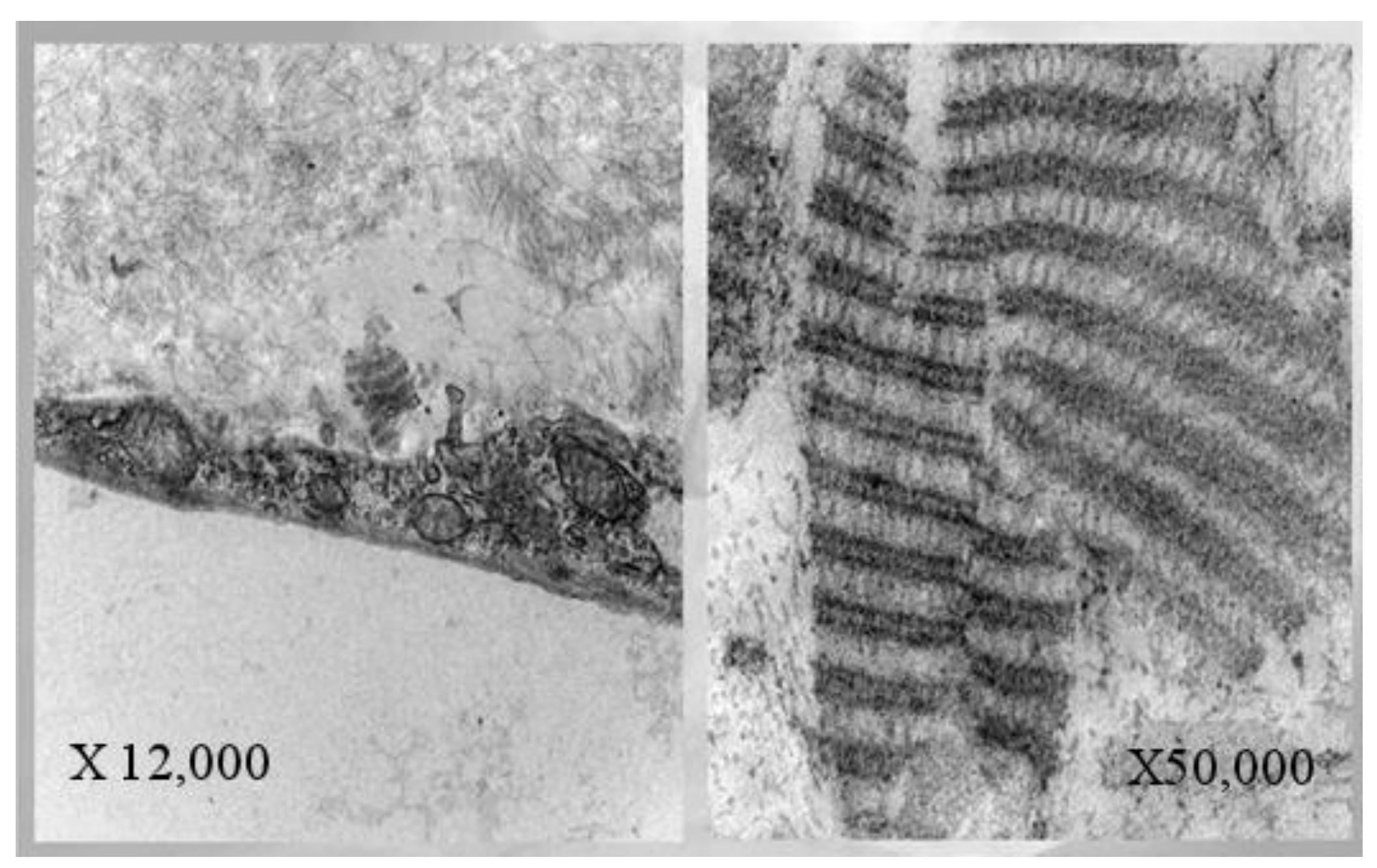
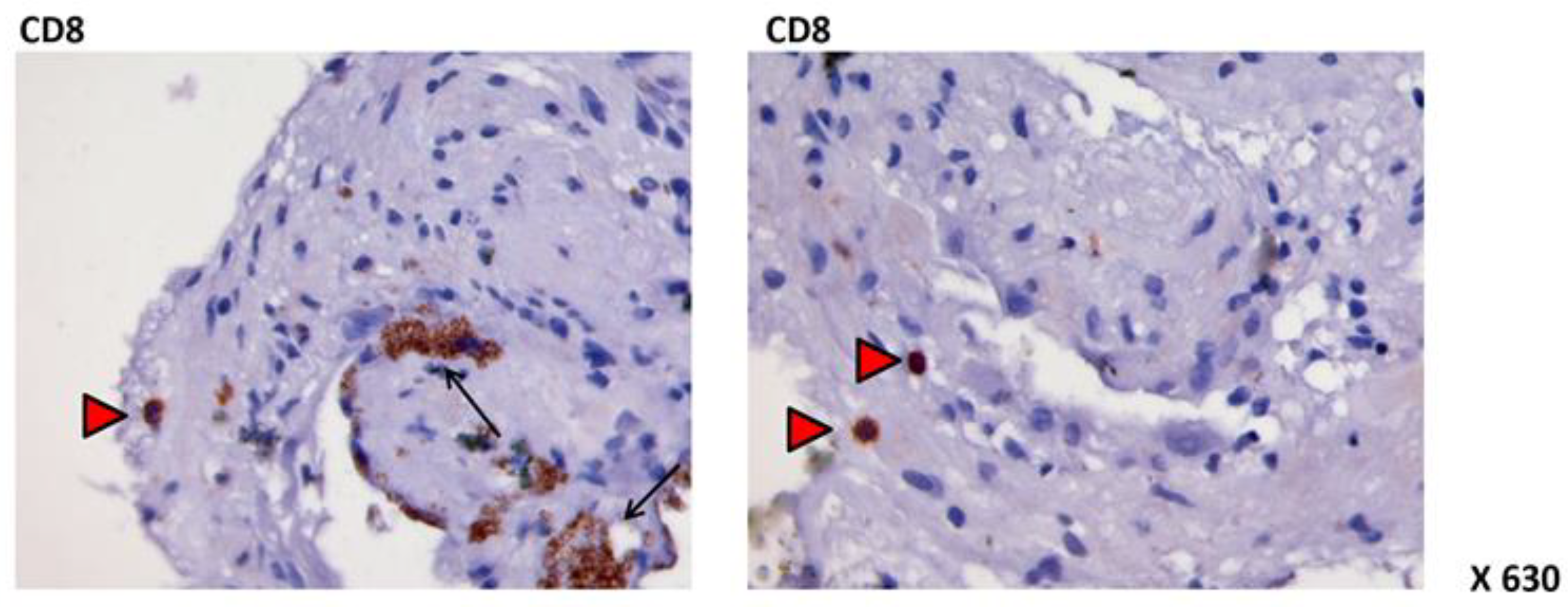
3. Physiopathology: “EPR Dysfunction”
4. PVR Staging
- Grade A or minimal PVR: vitreous haze, vitreous pigment clumps, and/or pigment clusters on inferior retina.
- Grade B or moderate PVR: wrinkling of inner retinal surface, retinal stiffness, vessels tortuosity, and/or rolled edges of the retinal breaks.
- Grade C or marked PVR: full thickness retinal folds in 1 (C-1), 2 (C-2), or 3 (C-3) quadrants.
- Grade D or massive PVR: fixed retinal folds in four quadrants with a wide (D-1), narrow (D-2), or closed funnel retinal detachment configuration, without visualization of the optic disc (D-3).
- Grade C-P: Full-thickness retinal folds and/or subretinal strands (Figure 3) posterior to the equator (1–12 clock hours involvement).
- Grade C-A: Full-thickness retinal folds and/or subretinal strands anterior to the equator (1–12 clock hours involvement), and anterior displacement.
5. Therapeutic Strategies
- Taxol and colchicine stabilize and inhibit the formation of microtubules. They could reduce RPE migration and proliferation [115].
- Glucosamine is an aminomonosaccharide that suppresses RPE proliferation in vitro, interfering with the TGF-β signaling pathway [116].
- Alkylphosphocholine is a protein kinase C inhibitor that was shown to be effective against RPE migration and proliferation in vitro [119].
- AG1295, a specific PDGFR inhibitor, attenuated PVR development without significant side effects in rabbits [120].
- N-acetylcysteine is an antioxidant agent that showed efficacy in protecting rabbits from PVR formation by blocking the activation of PDGFR-α [121].
- Palomid 529 is an inhibitor of the Akt/mTOR pathways that regulate cell signaling, and in an experimental model of RD in rabbits, it was effective in suppressing Muller cell proliferation, glial scar formation, and photoreceptors death [127].
- Melatonin inhibited the proliferation and migration induced by EGF in human ARPE-19 cells by suppressing the AKT/mTOR-mediated Sp1/c-Jun signaling pathways [130].
- IL-10 and antibodies to TGF-beta2 and PDGF have been hypothesized to be therapeutic options since they can inhibit RPE-mediated retinal contraction [131].
Surgical Treatment
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gloor, B.P.; Marmor, M.F. Controversy over the etiology and therapy of retinal detachment: The struggles of Jules Gonin. Surv. Ophthalmol. 2013, 58, 184–195. [Google Scholar] [CrossRef] [PubMed]
- The Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 1983, 90, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.W.; Song, J.H.; Roh, M.I. Retinal Detachment and Proliferative Vitreoretinopathy. Dev. Ophthalmol. 2016, 55, 154–162. [Google Scholar] [PubMed]
- Charteris, D.G.; Sethi, C.S.; Lewis, G.P.; Fisher, S.K. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye 2002, 16, 369–374. [Google Scholar] [CrossRef]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef]
- Pastor, J.C.; de la Rúa, E.R.; Martín, F. Proliferative vitreoretinopathy: Risk factors and pathobiology. Prog. Retin. Eye Res. 2002, 21, 127–144. [Google Scholar] [CrossRef]
- Sadaka, A.; Giuliari, G.P. Proliferative vitreoretinopathy: Current and emerging treatments. Clin. Ophthalmol. 2012, 6, 1325–1333. [Google Scholar]
- Girard, P.; Mimoun, G.; Karpouzas, I.; Montefiore, G. Clinical risk factors for proliferative vitreoretinopathy after retinal detachment surgery. Retina 1994, 14, 417–424. [Google Scholar] [CrossRef]
- Eliott, D.; Stryjewski, T.P.; Andreoli, M.T.; Andreoli, C.M. Smoking is a risk factor for proliferative vitreoretinopathy after traumatic retinal detachment. Retina 2017, 37, 1229–1235. [Google Scholar] [CrossRef]
- Xu, K.; Chin, E.K.; Bennett, S.R.; Williams, D.F.; Ryan, E.H.; Dev, S.; Mittra, R.A.; Quiram, P.A.; Davies, J.B.; Parke, D.W.I.; et al. Predictive Factors for Proliferative Vitreoretinopathy Formation after Uncomplicated Primary Retinal Detachment Repair. Retina 2019, 39, 1488–1495. [Google Scholar] [CrossRef]
- Rojas, J.; Fernandez, I.; Pastor, J.C.; Garcia-Gutierrez, M.T.; Sanabria, M.R.; Brion, M.; Coco, R.M.; Ruiz-Moreno, J.M.; García-Arumí, J.; Elizalde, J.; et al. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: The retina 4 project. Ophthalmology 2010, 117, 2417–2423.e2. [Google Scholar] [CrossRef] [PubMed]
- Lumi, X.; Jelen, M.M.; Jevšinek Skok, D.; Boštjančič, E.; Ravnik-Glavač, M.; Hawlina, M.; Glavač, D. Comparison of SNP Genotypes Related to Proliferative Vitreoretinopathy (PVR) across Slovenian and European Subpopulations. J. Ophthalmol. 2018, 2018, 8761625. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Idoate, S.; Rodríguez-Hernández, I.; Rojas, J.; Fernández, I.; García-Gutiérrez, M.T.; Ruiz-Moreno, J.M.; Rocha-Sousa, A.; Ramkissoon, Y.; Harsum, S.; MacLaren, R.E.; et al. The T309G MDM2 gene polymorphism is a novel risk factor for proliferative vitreoretinopathy. PLoS ONE 2013, 8, e82283. [Google Scholar] [CrossRef]
- Pastor-Idoate, S.; Rodriguez-Hernández, I.; Rojas, J.; Fernández, I.; García-Gutierrez, M.T.; Ruiz-Moreno, J.M.; Rocha-Sousa, A.; Ramkissoon, Y.; Harsum, S.; MacLaren, R.E.; et al. The p53 codon 72 polymorphism (rs1042522) is associated with proliferative vitreoretinopathy: The Retina 4 Project. Ophthalmology 2013, 120, 623–628. [Google Scholar] [CrossRef]
- Pastor-Idoate, S.; Rodríguez-Hernández, I.; Rojas, J.; Fernández, I.; García-Gutierrez, M.T.; Ruiz-Moreno, J.M.; Rocha-Sousa, A.; Ramkissoon, Y.D.; Harsum, S.; MacLaren, R.E.; et al. BAX and BCL-2 polymorphisms, as predictors of proliferative vitreoretinopathy development in patients suffering retinal detachment: The Retina 4 project. Acta Ophthalmol. 2015, 93, e541–e549. [Google Scholar] [CrossRef]
- Pastor, J.C. Proliferative vitreoretinopathy: An overview. Surv. Ophthalmol. 1998, 43, 3–18. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, O.W.; Seong, G.J.; Kim, S.H.; Ahn, J.E.; Kay, E.D. Epitheliomesenchymal transdifferentiation of cultured RPE cells. Ophthalmic Res. 2001, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Deora, A.A.; Philp, N.; Hu, J.; Bok, D.; Rodriguez-Boulan, E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc. Natl. Acad. Sci. USA 2005, 102, 16245–16250. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, S.; Karl, M.O.; Strauss, O. Ion channels in the RPE. Prog. Retin. Eye Res. 2007, 26, 263–301. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Krejcova, S.; Reichhart, N.; Wagner, A.; Strauss, O. Interaction of bestrophin-1 and Ca2+ channel β-subunits: Identification of new binding domains on the bestrophin-1 C-terminus. PLoS ONE 2011, 6, e19364. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; McLeod, D.S.; Hasegawa, T.; Kim, S.Y.; Merges, C.; Tong, P.; Lutty, G.A. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp. Eye Res. 2006, 82, 99–110. [Google Scholar] [CrossRef]
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Bonente, D.; Bianchi, L.; De Salvo, R.; Nicoletti, C.; De Benedetto, E.; Bacci, T.; Bini, L.; Inzalaco, G.; Franci, L.; Chiariello, M.; et al. Co-Expression of Podoplanin and CD44 in Proliferative Vitreoretinopathy Epiretinal Membranes. Int. J. Mol. Sci. 2023, 24, 9728. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Fredj-Reygrobellet, D.; Gordon, W.C.; Baudouin, F.; Peyman, G.; Lapalus, P.; Gastaud, P.; Bazan, N.G.; Fredj-Reygrobellet, D. Immunohistologic study of epiretinal membranes in proliferative vitreoretinopathy. Am. J. Ophthalmol. 1990, 110, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.; Heimann, K.; Wiedemann, P. Macrophages in proliferative vitreoretinopathyand proliferative diabetic reti nopathy: Differentiation of subpopulations. Br. J. Ophthalmol. 1993, 77, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Charteris, D.G.; Hiscott, P.; Grierson, I.; Lightman, S.L. Proliferative vitreoretinopathy. Lymphocytes in epiretinal membranes. Ophthalmology 1992, 99, 1364–1367. [Google Scholar] [CrossRef]
- Charteris, D.G. Proliferative vitreoretinopathy: Pathobiology, surgical management, and adjunctive treatment. Br. J. Ophthalmol. 1995, 79, 953–960. [Google Scholar] [CrossRef]
- Chaudhary, R.; Scott, R.A.H.; Wallace, G.; Berry, M.; Logan, A.; Blanch, R.J. Inflammatory and Fibrogenic Factors in Proliferative Vitreoretinopathy Development. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
- Lei, H.; Velez, G.; Hovland, P.; Hirose, T.; Gilbertson, D.; Kazlauskas, A. Growth factors outside the PDGF family drive experimental PVR. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3394–3403. [Google Scholar] [CrossRef]
- Ricker, L.J.; Kessels, A.G.; de Jager, W.; Hendrikse, F.; Kijlstra, A.; la Heij, E.C. Prediction of proliferative vitreoretinopathy after retinal detachment surgery: Potential of biomarker profiling. Am. J. Ophthalmol. 2012, 154, 347–354.e2. [Google Scholar] [CrossRef]
- Banerjee, P.J.; Quartilho, A.; Bunce, C.; Xing, W.; Zvobgo, T.M.; Harris, N.; Charteris, D.G. Slow-Release Dexamethasone in Proliferative Vitreoretinopathy: A Prospective, Randomized Controlled Clinical Trial. Ophthalmology 2017, 124, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.; Balciunaite, E.; Leong, F.L.; Tallquist, M.; Soriano, P.; Refojo, M.; Kazlauskas, A. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2683–2689. [Google Scholar]
- Cui, J.Z.; Chiu, A.; Maberley, D.; Ma, P.; Samad, A.; Matsubara, J.A. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye 2007, 21, 200–208. [Google Scholar] [CrossRef]
- Terasaki, H.; Kase, S.; Shirasawa, M.; Otsuka, H.; Hisatomi, T.; Sonoda, S.; Ishida, S.; Ishibashi, T.; Sakamoto, T. TNF-α decreases VEGF secretion in highly polarized RPE cells but increases it in non-polarized RPE cells related to crosstalk between JNK and NF-κB pathways. PLoS ONE 2013, 8, e69994. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.K.; Samuel, W.; Boyce, K.; Cherukuri, A.; Duncan, T.; Jaworski, C.; Nagineni, C.N.; Redmond, T.M. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol. Vis. 2016, 22, 1156–1168. [Google Scholar] [PubMed]
- Shirasawa, M.; Sonoda, S.; Terasaki, H.; Arimura, N.; Otsuka, H.; Yamashita, T.; Uchino, E.; Hisatomi, T.; Ishibashi, T.; Sakamoto, T. TNF-α disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Exp. Eye Res. 2013, 110, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.R.; He, S.; Jin, M.L.; Barron, E.; Ryan, S.J. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye 2002, 16, 422–428. [Google Scholar] [CrossRef]
- Dieudonné, S.C.; La Heij, E.C.; Diederen, R.M.; Kessels, A.G.; Liem, A.T.; Kijlstra, A.; Hendrikse, F. Balance of vascular endothelial growth factor and pigment epithelial growth factor prior to development of proliferative vitreoretinopathy. Ophthalmic Res. 2007, 39, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Limb, G.A.; Alam, A.; Earley, O.; Green, W.; Chignell, A.H.; Dumonde, D.C. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr. Eye Res. 1994, 13, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Shi, Y.; Zhang, X.; Yang, K.; Yu, C. [TNF-alpha, IL-8 and IL-6 in the early inflammatory stage of experimental PVR model induced by macrophages]. Zhonghua Yan Ke Za Zhi 1999, 35, 140–143. [Google Scholar] [PubMed]
- Asaria, R.H.; Kon, C.H.; Bunce, C.; Sethi, C.S.; Limb, G.A.; Khaw, P.T.; Aylward, G.W.; Charteris, D.G. Silicone oil concentrates fibrogenic growth factors in the retro-oil fluid. Br. J. Ophthalmol. 2004, 88, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- La Heij, E.C.; van de Waarenburg, M.P.; Blaauwgeers, H.G.; Kessels, A.G.; Liem, A.T.; Theunissen, C.; Steinbusch, H.; Hendrikse, F. Basic fibroblast growth factor, glutamine synthetase, and interleukin-6 in vitreous fluid from eyes with retinal detachment complicated by proliferative vitreoretinopathy. Am. J. Ophthalmol. 2002, 134, 367–375. [Google Scholar] [CrossRef]
- Mukherjee, S.; Guidry, C. The insulin-like growth factor system modulates retinal pigment epithelial cell tractional force generation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1892–1899. [Google Scholar] [CrossRef]
- Morino, I.; Hiscott, P.S.; McKechnie, N.; Grierson, I. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br. J. Ophthalmol. 1990, 74, 393–399. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Jerdan, J.A.; Glaser, B.M.; Cardin, A.; Michels, R.G. Vitreous aspirates from patients with proliferative vitreoretinopathy stimulate retinal pigment epithelial cell migration. Arch. Ophthalmol. 1985, 103, 1403–1405. [Google Scholar] [CrossRef]
- Hiscott, P.; Waller, H.A.; Grierson, I.; Butler, M.G.; Scott, D. Local production of fibronectin by ectopic human retinal cells. Cell Tissue Res. 1992, 267, 185–192. [Google Scholar] [CrossRef]
- Kon, C.H.; Occleston, N.L.; Charteris, D.; Daniels, J.; Aylward, G.W.; Khaw, P.T. A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1524–1529. [Google Scholar]
- Dvashi, Z.; Goldberg, M.; Adir, O.; Shapira, M.; Pollack, A. TGF-β1 induced transdifferentiation of rpe cells is mediated by TAK1. PLoS ONE. 2015, 10, e0122229. [Google Scholar] [CrossRef]
- Proulx, S.; Guérin, S.L.; Salesse, C. Effect of quiescence on integrin alpha5beta1 expression in human retinal pigment epithelium. Mol. Vis. 2003, 9, 473–481. [Google Scholar] [PubMed]
- Miller, C.G.; Budoff, G.; Prenner, J.L.; Schwarzbauer, J.E. Fibronectin in retinal disease. Exp. Biol. Med. 2017, 242, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Marigliani, D.; Romeo, N.; Toti, P. Disease Pathways in Proliferative Vitreoretinopathy: An Ongoing Challenge. J. Cell. Physiol. 2014, 229, 1577–1583. [Google Scholar] [CrossRef]
- Khankan, R.; Oliver, N.; He, S.; Ryan, S.J.; Hinton, D.R. Regulation of fibronectin-EDA through CTGF domain-specific interactions with TGF-β2 and its receptor TGF-βRII. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5068–5078. [Google Scholar] [CrossRef]
- Sheridan, C.M.; Magee, R.M.; Hiscott, P.S.; Hagan, S.; Wong, D.H.; McGalliard, J.N.; Grierson, I. The role of matricellular proteins thrombospondin-1 and osteonectin during RPE cell migration in proliferative vitreoretinopathy. Curr. Eye Res. 2002, 25, 279–285. [Google Scholar] [CrossRef]
- Carpineto, P.; Aharrh-Gnama, A.; Ciciarelli, V.; Borrelli, E.; Petti, F.; Aloia, R.; Lamolinara, A.; Di Nicola, M.; Mastropasqua, L. Subretinal Fluid Levels of Signal-Transduction Proteins and Apoptosis Molecules in Macula-Off Retinal Detachment Undergoing Scleral Buckle Surgery. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, S.; Hata, Y.; Kita, T.; Arita, R.; Miura, M.; Nakao, S.; Mochizuki, Y.; Enaida, H.; Kagimoto, T.; Goto, Y.; et al. Potent inhibition of cicatricial contraction in proliferative vitreoretinal diseases by statins. Diabetes 2008, 57, 2784–2793. [Google Scholar] [CrossRef]
- Kon, C.H.; Occleston, N.L.; Aylward, G.W.; Khaw, P.T. Expression of vitreous cytokines in proliferative vitreoretinopathy: A prospective study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 705–712. [Google Scholar]
- Flanders, K.C. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, 47–64. [Google Scholar] [CrossRef]
- Saika, S.; Yamanaka, O.; Flanders, K.C.; Okada, Y.; Miyamoto, T.; Sumioka, T.; Shirai, K.; Kitano, A.; Miyazaki, K.-I.; Tanaka, S.-I.; et al. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Yamanaka, O.; Nishikawa-Ishida, I.; Kitano, A.; Flanders, K.C.; Okada, Y.; Ohnishi, Y.; Nakajima, Y.; Ikeda, K. Effect of Smad7 gene overexpression on transforming growth factor beta-induced retinal pigment fibrosis in a proliferative vitreoretinopathy mouse model. Arch. Ophthalmol. 2007, 125, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Connor, T.B., Jr.; Roberts, A.B.; Sporn, M.B.; Danielpour, D.; Dart, L.L.; Michels, R.G.; de Bustros, S.; Enger, C.; Kato, H.; Lansing, M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Investig. 1989, 83, 1661–1666. [Google Scholar] [CrossRef]
- Pennock, S.; Haddock, L.J.; Mukai, S.; Kazlauskas, A. Vascular endothelial growth factor acts primarily via platelet-derived growth factor receptor α to promote proliferative vitreoretinopathy. Am. J. Pathol. 2014, 184, 3052–3068. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Rhéaume, M.A.; Velez, G.; Mukai, S.; Kazlauskas, A. Expression of PDGFRα is a determinant of the PVR potential of ARPE19 cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Velez, G.; Kazlauskas, A. Pathological signaling via platelet-derived growth factor receptor {alpha} involves chronic activation of Akt and suppression of p53. Mol. Cell Biol. 2011, 31, 1788–1799. [Google Scholar] [CrossRef]
- Wang, C.H.; Cao, G.F.; Jiang, Q.; Yao, J. TNF-α promotes human retinal pigment epithelial (RPE) cell migration by inducing matrix metallopeptidase 9 (MMP-9) expression through activation of Akt/mTORC1 signaling. Biochem. Biophys. Res. Commun. 2012, 425, 33–38. [Google Scholar] [CrossRef]
- Rojas, J.; Fernandez, I.; Pastor, J.C.; Maclaren, R.E.; Ramkissoon, Y.; Harsum, S.; Charteris, D.G.; Van Meurs, J.C.; Amarakoon, S.; Ruiz-Moreno, J.M.; et al. A genetic case-control study confirms the implication of SMAD7 and TNF locus in the development of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1665–1678. [Google Scholar] [CrossRef]
- Chang, C.J.; Lai, W.W.; Edward, D.P.; Tso, M.O. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch. Ophthalmol. 1995, 113, 880–886. [Google Scholar] [CrossRef]
- Lavrik, I.N.; Golks, A.; Krammer, P.H. Caspases: Pharmacological manipulation of cell death. J. Clin. Investig. 2005, 115, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.; Lewis, G.P.; Fisher, S.K.; Adler, R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 1995, 36, 990–996. [Google Scholar]
- Lo, A.C.; Woo, T.T.; Wong, R.L.; Wong, D. Apoptosis and other cell death mechanisms after retinal detachment: Implications for photoreceptor rescue. Ophthalmologica 2011, 226 (Suppl. 1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, K.; Banerjee, P.J.; Angbohang, A.; Charteris, D.G.; Khaw, P.T.; Limb, G.A. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia 2016, 64, 495–506. [Google Scholar] [CrossRef]
- Hui, Y.N.; Sorgente, N.; Ryan, S.J. Posterior vitreous separation and retinal detachment induced by macrophages. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 279–284. [Google Scholar] [CrossRef]
- Fischer, A.J.; Zelinka, C.; Milani-Nejad, N. Reactive retinal microglia, neuronal survival, and the formation of retinal folds and detachments. Glia 2015, 63, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.; Charteris, D.G. Glial cell changes of the human retina in proliferative vitreoretinopathy. Dev. Ophthalmol. 2009, 44, 37–45. [Google Scholar]
- Pastor, J.C.; Mendez, M.C.; de la Fuente, M.A.; Coco, R.M.; GarciaArumi, J.; Rodriguez de la Rua, E.; Fernández, N.; Saornil, M.A.; Gayoso, M.J. Intraretinal immunohistochemistry findings in proliferative vitreoretinopathy with retinal shortening. Ophthalmic Res. 2006, 38, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Walshe, R.; Esser, P.; Wiedemann, P.; Heimann, K. Proliferative retinal diseases: Myofibroblasts cause chronic vitreoretinal traction. Br. J. Ophthalmol. 1992, 76, 550–552. [Google Scholar] [CrossRef]
- Umazume, K.; Barak, Y.; McDonald, K.; Liu, L.; Kaplan, H.J.; Tamiya, S. Proliferative vitreoretinopathy in the Swine—A new model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4910–4916. [Google Scholar] [CrossRef]
- Glaser, B.M.; Cardin, A.; Biscoe, B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology 1987, 94, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Carpineto, P.; Di Filippo, E.S.; Aharrh Gnama, A.; Bondi, D.; Iafigliola, C.; Licata, A.M.; Fulle, S. MicroRNA Expression in Subretinal Fluid in Eyes Affected by Rhegmatogenous Retinal Detachment. Int. J. Mol. Sci. 2023, 24, 3032. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Reibaldi, M.; Avitabile, T.; Bucolo, C.; Salomone, S.; Rejdak, R.; Nowomiejska, K.; Tripodi, S.; Posarelli, C.; Ragusa, M.; et al. MicroRNAs in the Vitreous Humor of Patients with Retinal Detachment and a Different Grading of Proliferative Vitreoretinopathy: A Pilot Study. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
- Lean, J.S.; Stern, W.H.; Irvine, A.R.; Azen, S.P. Classification of proliferative vitreoretinopathy used in the silicone study. The Silicone Study Group. Ophthalmology 1989, 96, 765–771. [Google Scholar] [CrossRef]
- Machemer, R.; Aaberg, T.M.; Freeman, H.M.; Irvine, A.R.; Lean, J.S.; Michels, R.M. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am. J. Ophthalmol. 1991, 112, 159–165. [Google Scholar] [CrossRef]
- Di Lauro, S.; Kadhim, M.R.; Charteris, D.G.; Pastor, J.C. Classifications for proliferative vitreoretinopathy (PVR): An analysis of their use in publications over the last 15 years. J. Ophthalmol. 2016, 2016, 7807596. [Google Scholar] [CrossRef] [PubMed]
- Moysidis, S.N.; Thanos, A.; Vavvas, D.G. Mechanisms of inflammation in proliferative vitreoretinopathy: From bench to bedside. Mediat. Inflamm. 2012, 2012, 815937. [Google Scholar] [CrossRef]
- Pastor, J.C.; Rodríguez, E.; Marcos, M.A.; Lopez, M.I. Combined pharmacologic therapy in a rabbit model of proliferative vitreoretinopathy (PVR). Ophthalmic Res. 2000, 32, 25–29. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Feghhi, M.; Tabatabaei, H.; Shoeibi, N.; Ramezani, A.; Mohebbi, M.R. Triamcinolone acetonide in silicone filled eyes as adjunctive treatment for proliferative vitreoretinopathy: A randomized clinical trial. Ophthalmology 2008, 115, 1938–1943. [Google Scholar] [CrossRef]
- Yamakiri, K.; Sakamoto, T.; Noda, Y.; Nakahara, M.; Ogino, N.; Kubota, T.; Yokoyama, M.; Furukawa, M.; Ishibashi, T. One-year results of a multicenter controlled clinical trial of triamcinolone in pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 959–966. [Google Scholar] [CrossRef][Green Version]
- Williams, R.G.; Chang, S.; Comaratta, M.R.; Simoni, G. Does the presence of heparin and dexamethasone in the vitrectomy infusate reduce reproliferation in proliferative vitreoretinopathy? Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 496–503. [Google Scholar] [CrossRef]
- Dehghan, M.H.; Ahmadieh, H.; Soheilian, M.; Azarmina, M.; Moradian, S.; Ramezani, A.R.; Tavallal, A.; Naghibozakerin, J. Effect of oral prednisolone on visual outcomes and complications after scleral buckling. Eur. J. Ophthalmol. 2010, 20, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Koerner, F.; Koener-Stiefbold, U.; Garweg, J.G. Systemic corticosteroids reduce the risk of cellophane membranes after retinal detachment surgery: A prospective randomized placebo-controlled double-blind clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.; Bunce, C.; Wong, D.; McGurn, D.; Charteris, D.G. Randomized controlled trial of combined 5-Fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachment sunder going primary vitrectomy. Ophthalmology 2007, 114, 698–704. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, S.; Wu, M.; Cui, H.; Wu, R.; Chen, S.; Xu, C.; Lu, X.; Feng, S. Self-assembling hydrogel loaded with 5-FU PLGA microspheres as a novel vitreous substitute for proliferative vitreoretinopathy. J. Biomed. Mater. Res. A 2020, 108, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, M.; Albayrak, S.; Pehlivanoglu, S.; Ozkaya, A.; Gocgil, N.A. 5-Fluorouracyl added infusion fluid in patients with recurrent rhegmatogeneous retinal detachment. Saudi J. Ophthalmol. 2019, 33, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Hui, Y.N.; Wiedemann, P. Role of apoptosis in the cytotoxic effect mediated by daunorubicin in cultured human retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2002, 18, 377–387. [Google Scholar] [CrossRef]
- Hui, Y.N.; Hu, D. Prevention of experimental proliferative vitreoretinopathy with daunomycin and triamcinolone based on the time course of the disease. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 601–605. [Google Scholar] [CrossRef]
- Wiedemann, P.; Hilgers, R.D.; Bauer, P.; Heimann, K.; Daunomycin Study Group. Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: Results of a multicenter clinical trial. Am. J. Ophthalmol. 1998, 126, 550559. [Google Scholar] [CrossRef]
- Hou, H.; Huffman, K.; Rios, S.; Freeman, W.R.; Sailor, M.J.; Cheng, L. A Novel Approach of Daunorubicin Application on Formation of Proliferative Retinopathy Using a Porous Silicon Controlled Delivery System: Pharmacodynamics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2755–2763. [Google Scholar] [CrossRef]
- Kuo, H.K.; Chen, Y.H.; Wu, P.C.; Wu, Y.C.; Huang, F.; Kuo, C.W.; Lo, L.-H.; Shiea, J. Attenuated glial reaction in experimental proliferative vitreoretinopathy treated with liposomal doxorubicin. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Mandava, N.; Blackburn, P.; Paul, D.B.; Wilson, M.W.; Read, S.B.; Alspaugh, E.; Tritz, R.; Barber, J.R.; Robbins, J.M.; Kruse, C. Ribozyme to proliferating cell nuclear antigen to treat proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3338–3348. [Google Scholar]
- Schiff, W.M.; Hwang, J.C.; Ober, M.D.; Olson, J.L.; Dhrami-Gavazi, E.; Barile, G.R.; Chang, S.; Mandava, N. Safety and efficacy assessment of chimeric ribozyme to proliferating cell nuclear antigen to prevent recurrence of proliferative vitreoretinopathy. Arch. Ophthalmol. 2007, 125, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Pennock, S.; Kim, D.; Mukai, S.; Kuhnle, M.; Chun, D.W.; Matsubara, J.; Cui, J.; Ma, P.; Maberley, D.; Samad, A.; et al. Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. Am. J. Pathol. 2013, 182, 1659–1670. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Hashemi, M.; Modarres, M.; Hadavand Khani, A. Intrasilicone oil injection of bevacizumab at the end of retinal reattachment surgery for severe proliferative vitreoretinopathy. Eye 2014, 28, 576–580. [Google Scholar] [CrossRef]
- Tousi, A.; Hasanpour, H.; Soheilian, M. Intravitreal injection of bevacizumab in primary vitrectomy to decrease the rate of retinal redetachment: A randomized pilot study. J. Ophthalmic Vis. Res. 2016, 11, 271–276. [Google Scholar]
- Chang, Y.-C.; Hu, D.-N.; Wu, W.-C. Effect of oral 13-cis-retinoic acid treatment on postoperative clinical outcome of eyes with proliferative vitreoretinopathy. Am. J. Ophthalmol. 2008, 146, 440–446. [Google Scholar] [CrossRef]
- Schaub, F.; Abdullatif, A.M.; Fauser, S. Proliferative Vitreoretinopathieprophylaxe: Mission (im)possible [Proliferative vitreoretinopathy prophylaxis: Mission (im)possible]. Ophthalmologe 2021, 118, 3–9. [Google Scholar] [CrossRef]
- Amarnani, D.; Machuca-Parra, A.I.; Wong, L.L.; Marko, C.K.; Stefater, J.A.; Stryjewski, T.P.; Eliott, D.; Arboleda-Velasquez, J.F.; Kim, L.A. Effect of methotrexate on an in vitro patient-derived model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3940–3949. [Google Scholar] [CrossRef]
- Sadaka, A.; Sisk, R.A.; Osher, J.M.; Toygar, O.; Duncan, M.K.; Riemann, C.D. Intravitreal methotrexate infusion for proliferative vitreoretinopathy. Clin. Ophthalmol. 2016, 10, 1811–1817. [Google Scholar] [CrossRef]
- Benner, J.D.; Dao, D.; Butler, J.W.; Hamill, K.I. Intravitreal methotrexate for the treatment of proliferative vitreoretinopathy. BMJ Open Ophthalmol. 2019, 4, e000293. [Google Scholar] [CrossRef] [PubMed]
- Hardwig, P.W.; Pulido, J.S.; Bakri, S.J. The safety of intraocular methotrexate in silicone filled eyes. Retina 2008, 28, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- El Baha, S.; Leila, M.; Amr, A.; Lolah, M.M.A. Anatomical and Functional Outcomes of Vitrectomy with/without Intravitreal Methotrexate Infusion for Management of Proliferative Vitreoretinopathy Secondary to Rhegmatogenous Retinal Detachment. J. Ophthalmol. 2021, 2021, 3648134. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Toth, C.A.; Burnett, H.W.; Butler, J.W.; Levy, J.H.; Benner, J.D. Low-Dose Intravitreal Methotrexate for Proliferative Vitreoretinopathy. Ophthalmic Surg. Lasers Imaging Retina 2023, 54, 139–146. [Google Scholar] [CrossRef]
- Lemor, M.; de Bustros, S.; Glaser, B.M. Low-dose colchicine inhibits astrocyte, fibroblast, and retinal pigment epithelial cell migration and proliferation. Arch. Ophthalmol. 1986, 4, 1223–1225. [Google Scholar] [CrossRef]
- Liang, C.M.; Tai, M.C.; Chang, Y.H.; Chen, Y.H.; Chen, C.L.; Lu, D.W.; Chen, J.-T. Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy. Acta Ophthalmol. 2011, 89, e505–e514. [Google Scholar] [CrossRef]
- Machado, R.A.; Casella, A.M.; Malaguido, M.R.; Oguido, A.P. Experimental study of vitreoretinal proliferation inhibition by the use of hypericin. Arq. Bras. Oftalmol. 2009, 72, 650–654. [Google Scholar] [CrossRef][Green Version]
- Imai, K.; Loewenstein, A.; Koroma, B.; Grebe, R.; de Juan, E., Jr. Herbimycin A in the treatment of experimental proliferative vitreoretinopathy: Toxicity and efficacy study. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 440–447. [Google Scholar] [CrossRef]
- Eibl, K.H.; Lewis, G.P.; Betts, K.; Linberg, K.A.; Gandorfer, A.; Kampik, A.; Fisher, S.K. The effect of alkylphosphocholines on intraretinal proliferation initiated by experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1305–1311. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; Ikuno, Y.; Ohj, M.; Kusaka, S.; Jiang, R.; Cekiç, O.; Sawa, M.; Tano, Y. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn. J. Ophthalmol. 2003, 47, 158–165. [Google Scholar] [CrossRef]
- Lei, H.; Velez, G.; Cui, J.; Samad, A.; Maberley, D.; Matsubara, J.; Kazlauskas, A. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am. J. Pathol. 2010, 177, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Duan, Y.; Huang, X.; Qian, C.X.; Chee, Y.; Mukai, S.; Cui, J.; Samad, A.; Matsubara, J.A.; Kazlauskas, A.; et al. Prevention of Proliferative Vitreoretinopathy by Suppression of Phosphatidylinositol 5-Phosphate 4-Kinases. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3935–3943, Erratum in Investig. Ophthalmol. Vis. Sci. 2016, 57, 4213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alex, A.F.; Spitznas, M.; Tittel, A.P.; Kurts, C.; Eter, N. Inhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitro. Curr. Eye Res. 2010, 35, 1021–1033. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, Y.H.; Tai, M.C.; Liang, C.M.; Lu, D.W.; Chen, J.T. Resveratrol inhibits transforming growth factor-β2-induced epithelial-to-mesenchymal transition in human retinal pigment epithelial cells by suppressing the Smad pathway. Drug Des. Dev. Ther. 2017, 11, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Schuettauf, F.; Monnier, P.P.; Bartz-Schmidt, K.U.; Henke-Fahle, S. Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Investig. Ophthalmol. Vis. Sci. 2009, 50, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Halász, É.; Townes-Anderson, E.; Zarbin, M.A. Improving outcomes in retinal detachment: The potential role of rho-kinase inhibitors. Curr. Opin. Ophthalmol. 2020, 31, 192–198. [Google Scholar] [CrossRef]
- Lewis, G.P.; Chapin, E.A.; Byun, J.; Luna, G.; Sherris, D.; Fisher, S.K. Muller cell reactivity and photoreceptor cell death are reduced after experimental retinal detachment using an inhibitor of the Akt/mTOR pathway. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4429–4435. [Google Scholar] [CrossRef]
- Tseng, S.C. HC-HA/PTX3 Purified from Amniotic Membrane as Novel Regenerative Matrix: Insight into Relationship Between Inflammation and Regeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFh1-8. [Google Scholar] [CrossRef]
- He, H.; Kuriyan, A.E.; Su, C.W.; Mahabole, M.; Zhang, Y.; Zhu, Y.T.; Flynn, H.W.; Parel, J.M.; Tseng, S.C. Inhibition of Proliferation and Epithelial Mesenchymal Transition in Retinal Pigment Epithelial Cells by Heavy Chain-Hyaluronan/Pentraxin 3. Sci. Rep. 2017, 7, 43736. [Google Scholar] [CrossRef]
- Yang, S.F.; Chen, Y.S.; Chien, H.W.; Wang, K.; Lin, C.L.; Chiou, H.L.; Lee, C.; Chen, P.; Hsieh, Y. Melatonin attenuates epidermal growth factor-induced cathepsin S expression in ARPE-19 cells: Implications for proliferative vitreoretinopathy. J. Pineal Res. 2020, 68, e12615. [Google Scholar] [CrossRef]
- Carrington, L.; McLeod, D.; Boulton, M. IL-10 and antibodies to TGF-beta2 and PDGF inhibit RPE-mediated retinal contraction. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1210–1216. [Google Scholar]
- Coffee, R.E.; Jiang, L.; Rahman, S.A. Proliferative vitreoretinopathy: Advances in surgical management. Int. Ophthalmol. Clin. 2014, 54, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi-Shima, C.; Sato, T.; Bando, H.; Ikeda, T.; Emi, K. Anatomic and functional outcomes of 25- gauge vitrectomy for repair of eyes with rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy. Clin. Ophthalmol. 2013, 7, 2043–2049. [Google Scholar] [PubMed]
- Minarcik, J.R.; von Fricken, M.A. Virtual retinectomy: Indocyanine green-assisted internal limiting membrane peeling as a surgical adjunct in repair of recurrent rhegmatogenous retinal detachment due to PVR. Clin. Ophthalmol. 2012, 6, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.C.; Blinder, K.J.; Smith, B.T.; Shah, G.K. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology 2013, 120, 1102–1103.e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Fujinami, K.; Watanabe, K.; Tsunoda, K.; Noda, T. Internal Limiting Membrane Peeling to Prevent Post-vitrectomy Epiretinal Membrane Development in Retinal Detachment. Am. J. Ophthalmol. 2016, 171, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Foveau, P.; Leroy, B.; Berrod, J.-P.; Conart, J.-B. Internal Limiting Membrane Peeling in Macula-off Retinal Detachment Complicated by Grade B Proliferative Vitreoretinopathy. Am. J. Ophthalmol. 2018, 191, 1–6. [Google Scholar] [CrossRef]
- Shroff, D.; Saha, I.; Bhatia, G.; Dutta, R.; Gupta, C.; Shrof, C.M. Tug of war: A bimanual technique for anterior circumferential proliferative vitreoretinopathy in recurrent retinal detachment. Indian J. Ophthalmol. 2020, 68, 2155–2158. [Google Scholar] [CrossRef]
- Abrams, G.W.; Azen, S.P.; McCuen, B.W.; Flynn, H.W.; Lai, M.Y.; Ryan, S.J. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: Results of additional and long-term follow-up. Silicone Study report 11. Arch. Ophthalmol. 1997, 115, 335–344. [Google Scholar] [CrossRef]
- Storey, P.; Alshareef, R.; Khuthaila, M.; London, N.; Leiby, B.; DeCroos, C.; Kaiser, R.; Wills PVR Study Group. Pars plana vitrectomy and scleral buckle versus pars plana vitrectomy alone for patients with rhegmatogenous retinal detachment at high risk for proliferative vitreoretinopathy. Retina 2014, 34, 1945–1951. [Google Scholar] [CrossRef]
- Kasetty, V.M.; Aye, J.; Patel, N.; Tripathi, N.; Hessburg, T.; Kumar, N.; Desai, U.R.; Hamad, A.E. Outcomes and complications of primary rhegmatogenous retinal detachment repair with pars plana vitrectomy in young adults. Int. J. Retina Vitr. 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, T.; Molle, A.; Carlà, M.M.; Picardi, S.M.; Gambini, G.; Scampoli, A.; Governatori, L.; Bernardinelli, P.; Rizzo, S. Applications of Human Amniotic Membrane Patching Assisted Vitrectomy in the Management of Postoperative PVR in Complex Retinal Detachments. J. Clin. Med. 2023, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Zuzic, M.; Rojo Arias, J.E.; Wohl, S.G.; Busskamp, V. Retinal miRNA Functions in Health and Disease. Genes 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpineto, P.; Licata, A.M.; Ciancaglini, M. Proliferative Vitreoretinopathy: A Reappraisal. J. Clin. Med. 2023, 12, 5287. https://doi.org/10.3390/jcm12165287
Carpineto P, Licata AM, Ciancaglini M. Proliferative Vitreoretinopathy: A Reappraisal. Journal of Clinical Medicine. 2023; 12(16):5287. https://doi.org/10.3390/jcm12165287
Chicago/Turabian StyleCarpineto, Paolo, Arturo Maria Licata, and Marco Ciancaglini. 2023. "Proliferative Vitreoretinopathy: A Reappraisal" Journal of Clinical Medicine 12, no. 16: 5287. https://doi.org/10.3390/jcm12165287
APA StyleCarpineto, P., Licata, A. M., & Ciancaglini, M. (2023). Proliferative Vitreoretinopathy: A Reappraisal. Journal of Clinical Medicine, 12(16), 5287. https://doi.org/10.3390/jcm12165287






