Initial Temporal Muscle Thickness and Area: Poor Predictors of Neurological Outcome in Aneurysmal Subarachnoid Hemorrhage in a Central European Patient Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
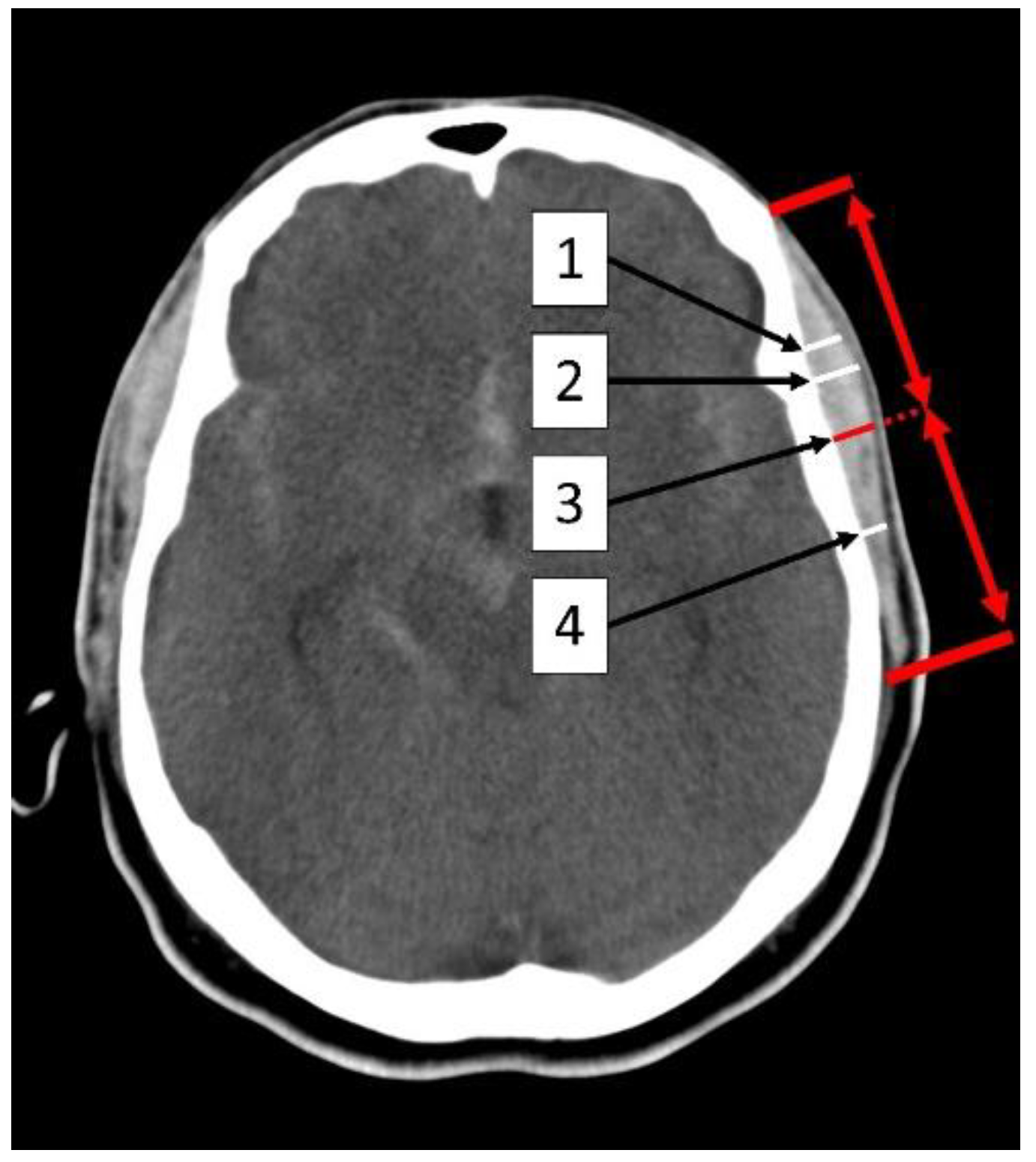
2.2. Measurement of the Temporal Muscle
2.3. Data Management and Definition of Outcome Measures
2.4. Statistical Analysis and Significance Level
3. Results
3.1. Patients
3.2. Established Outcome Parameters
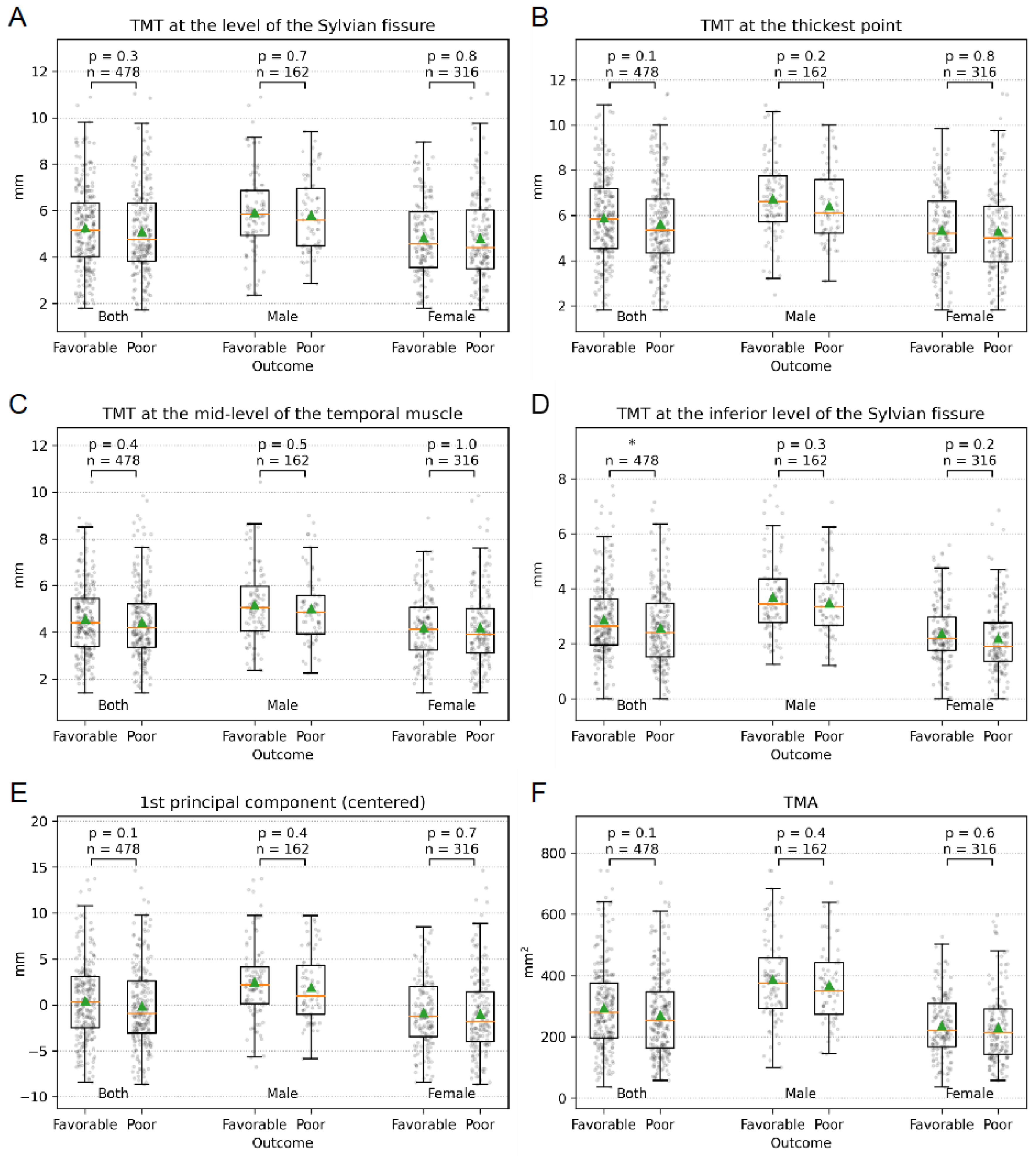
3.3. Temporal Muscle Thickness
3.4. Temporal Muscle Area
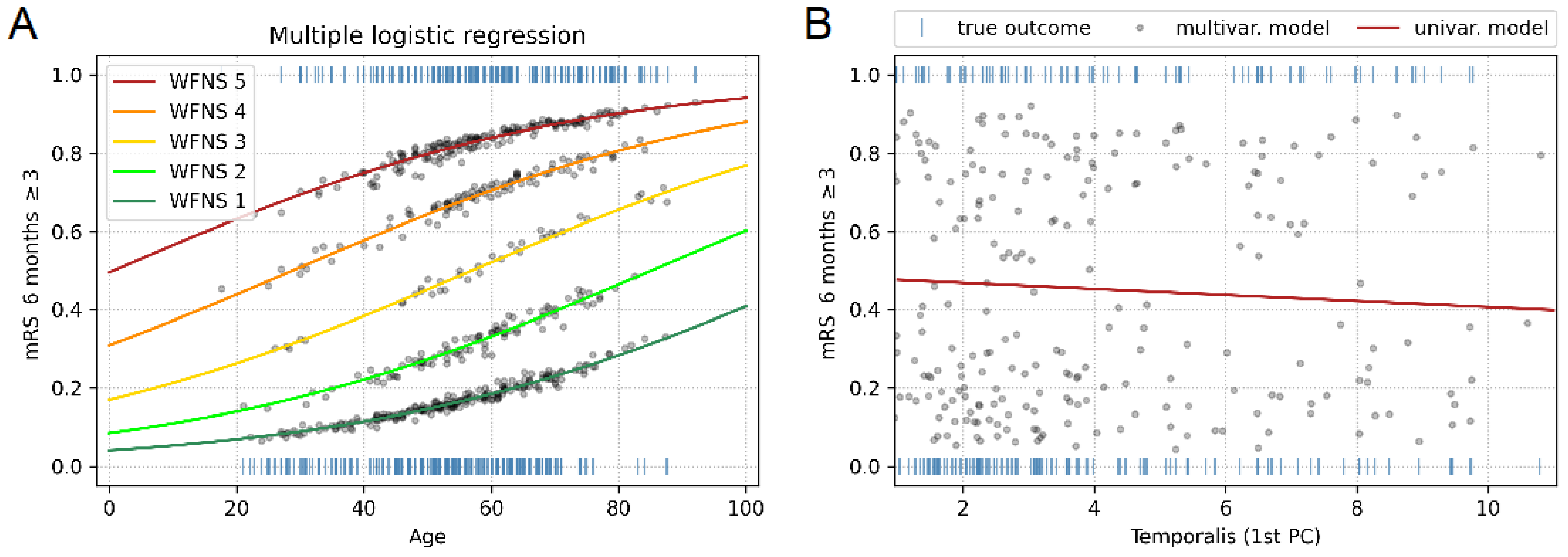
3.5. Multiple Logistic Regression Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Lieshout, J.H.; Dibue-Adjei, M.; Cornelius, J.F.; Slotty, P.J.; Schneider, T.; Restin, T.; Boogaarts, H.D.; Steiger, H.J.; Petridis, A.K.; Kamp, M.A. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2018, 41, 917–930. [Google Scholar] [CrossRef]
- Stegmayr, B.; Eriksson, M.; Asplund, K. Declining mortality from subarachnoid hemorrhage: Changes in incidence and case fatality from 1985 through 2000. Stroke 2004, 35, 2059–2063. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Anderson, C.S. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003, 2, 43–53. [Google Scholar] [CrossRef]
- Rinkel, G.J.E.; Algra, A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011, 10, 349–356. [Google Scholar] [CrossRef]
- de Winkel, J.; Cras, T.Y.; Dammers, R.; van Doormaal, P.J.; van der Jagt, M.; Dippel, D.W.J.; Lingsma, H.F.; Roozenbeek, B. Early predictors of functional outcome in poor-grade aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. BMC Neurol. 2022, 22, 239. [Google Scholar] [CrossRef]
- Jaja, B.N.R.; Cusimano, M.D.; Etminan, N.; Hanggi, D.; Hasan, D.; Ilodigwe, D.; Lantigua, H.; Le Roux, P.; Lo, B.; Louffat-Olivares, A. Clinical prediction models for aneurysmal subarachnoid hemorrhage: A systematic review. Neurocritical Care 2013, 18, 143–153. [Google Scholar] [CrossRef]
- Van Donkelaar, C.E.; Bakker, N.A.; Birks, J.; Veeger, N.J.G.M.; Metzemaekers, J.D.M.; Molyneux, A.J.; Groen, R.J.M.; van Dijk, J.M.C. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage. Stroke 2019, 50, 837–844. [Google Scholar] [CrossRef]
- Etminan, N.; Beseoglu, K.; Heiroth, H.J.; Turowski, B.; Steiger, H.J.; Hanggi, D. Early perfusion computerized tomography imaging as a radiographic surrogate for delayed cerebral ischemia and functional outcome after subarachnoid hemorrhage. Stroke 2013, 44, 1260–1266. [Google Scholar] [CrossRef]
- Caspers, J.; Rubbert, C.; Turowski, B.; Martens, D.; Reichelt, D.C.; May, R.; Aissa, J.; Hanggi, D.; Etminan, N.; Mathys, C. Timing of Mean Transit Time Maximization is Associated with Neurological Outcome After Subarachnoid Hemorrhage. Clin. Neuroradiol. 2017, 27, 15–22. [Google Scholar] [CrossRef]
- Hofmann, B.B.; Donaldson, D.M.; Fischer, I.; Karadag, C.; Neyazi, M.; Piedade, G.S.; Abusabha, Y.; Muhammad, S.; Rubbert, C.; Hänggi, D.; et al. Blood Pressure Affects the Early CT Perfusion Imaging in Patients with aSAH Reflecting Early Disturbed Autoregulation. Neurocritical Care 2023. [Google Scholar] [CrossRef]
- Hofmann, B.B.; Fischer, I.; Engel, A.; Jannusch, K.; Donaldson, D.M.; Karadag, C.; van Lieshout, J.H.; Beseoglu, K.; Muhammad, S.; Turowski, B.; et al. MTT Heterogeneity in Perfusion CT Imaging as a Predictor of Outcome after Aneurysmal SAH. Am. J. Neuroradiol. 2021, 42, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.B.; Fischer, I.; Donaldson, D.M.; Abusabha, Y.; Karadag, C.; Muhammad, S.; Beseoglu, K.; Hänggi, D.; Turowski, B.; Rubbert, C.; et al. Evaluation of MTT Heterogeneity of Perfusion CT Imaging in the Early Brain Injury Phase: An Insight into aSAH Pathopysiology. Brain Sci. 2023, 13, 824. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, J.H.; Mijderwijk, H.J.; Nieboer, D.; Lingsma, H.F.; Ahmadi, S.A.; Karadag, C.; Muhammad, S.; Porčnik, A.; Wasilewski, D.; Wessels, L.; et al. Development and Internal Validation of the ARISE Prediction Models for Rebleeding after Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2022, 91, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.R.; Saposnik, G.; Lingsma, H.F.; Macdonald, E.; Thorpe, K.E.; Mamdani, M.; Steyerberg, E.W.; Molyneux, A.; Manoel, A.L.O.; Schatlo, B.; et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: The SAHIT multinational cohort study. BMJ 2018, 360, j5745. [Google Scholar] [CrossRef]
- Kim, Y.I.; Shin, J.Y.; Yang, S.H.; Kim, H.H.; Shim, B.Y.; Ahn, S. Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Curr. Oncol. 2022, 29, 6463–6471. [Google Scholar] [CrossRef]
- Li, Y.X.; Hou, J.; Liu, W.Y. Long-term prognostic significance of sarcopenia in acute ischemic stroke. Medicine 2022, 101, e30031. [Google Scholar] [CrossRef]
- Furtner, J.; Weller, M.; Weber, M.; Gorlia, T.; Nabors, B.; Reardon, D.A.; Tonn, J.C.; Stupp, R.; Preusser, M. Temporal Muscle Thickness as a Prognostic Marker in Patients with Newly Diagnosed Glioblastoma: Translational Imaging Analysis of the CENTRIC EORTC 26071-22072 and CORE Trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 129–136. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Rabelo, N.N.; Telles, J.P.M.; Solla, D.J.F.; Coelho, A.; Jacobsen Teixeira, M.; Figueiredo, E.G. Sarcopenia as a Predictor of the Functional Outcome in Patients with Intracranial Aneurysms. Gerontology 2023, 69, 65–72. [Google Scholar] [CrossRef]
- Lee, B.; Bae, Y.J.; Jeong, W.J.; Kim, H.; Choi, B.S.; Kim, J.H. Temporalis muscle thickness as an indicator of sarcopenia predicts progression-free survival in head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 19717. [Google Scholar] [CrossRef]
- Dubinski, D.; Won, S.Y.; Behmanesh, B.; Cantré, D.; Mattes, I.; Trnovec, S.; Baumgarten, P.; Schuss, P.; Freiman, T.M.; Gessler, F. Significance of Temporal Muscle Thickness in Chronic Subdural Hematoma. J. Clin. Med. 2022, 11, 6456. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Terjimanian, M.; Lisiecki, J.; Rinkinen, J.; Mukkamala, A.; Brownley, C.; Buchman, S.R.; Wang, S.C.; Levi, B. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J. Surg. Res. 2014, 186, 246–252. [Google Scholar] [CrossRef]
- Katsuki, M.; Kakizawa, Y.; Nishikawa, A.; Yamamoto, Y.; Uchiyama, T. Temporal muscle thickness and area are an independent prognostic factors in patients aged 75 or younger with aneurysmal subarachnoid hemorrhage treated by clipping. Surg. Neurol. Int. 2021, 12, 151. [Google Scholar] [CrossRef]
- Katsuki, M.; Yamamoto, Y.; Uchiyama, T.; Wada, N.; Kakizawa, Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? Clin. Neurol. Neurosurg. 2019, 186, 105535. [Google Scholar] [CrossRef]
- Katsuki, M.; Suzuki, Y.; Kunitoki, K.; Sato, Y.; Sasaki, K.; Mashiyama, S.; Matsuoka, R.; Allen, E.; Saimaru, H.; Sugawara, R.; et al. Temporal Muscle as an Indicator of Sarcopenia is Independently Associated with Hunt and Kosnik Grade on Admission and the Modified Rankin Scale Score at 6 Months of Patients with Subarachnoid Hemorrhage Treated by Endovascular Coiling. World Neurosurg. 2020, 137, e526–e534. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.X.; Lim, Y.G.; Kumar, A.; Cheong, T.M.; Han, J.X.; Chen, M.W.; Wen, D.; Lim, W.; Ng, I.H.B.; Ng, V.Y.P.; et al. Relevance of presenting risks of frailty, sarcopaenia and osteopaenia to outcomes from aneurysmal subarachnoid haemorrhage. BMC Geriatr. 2022, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- Steindl, A.; Leitner, J.; Schwarz, M.; Nenning, K.H.; Asenbaum, U.; Mayer, S.; Woitek, R.; Weber, M.; Schöpf, V.; Berghoff, A.S.; et al. Sarcopenia in neurological patients: Standard values for temporal muscle thickness and muscle strength evaluation. J. Clin. Med. 2020, 9, 1272. [Google Scholar] [CrossRef]
- Nagano, A.; Shimizu, A.; Maeda, K.; Ueshima, J.; Inoue, T.; Murotani, K.; Ishida, Y.; Mori, N. Predictive value of temporal muscle thickness for sarcopenia after acute stroke in older patients. Nutrients 2022, 14, 5048. [Google Scholar] [CrossRef]
- Nozoe, M.; Kubo, H.; Kanai, M.; Yamamoto, M.; Okakita, M.; Suzuki, H.; Shimada, S.; Mase, K. Reliability and validity of measuring temporal muscle thickness as the evaluation of sarcopenia risk and the relationship with functional outcome in older patients with acute stroke. Clin. Neurol. Neurosurg. 2021, 201, 106444. [Google Scholar] [CrossRef]
- An, G.; Ahn, S.; Park, J.S.; Jeun, S.S.; Hong, Y.K. Association between temporal muscle thickness and clinical outcomes in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 2021, 147, 901–909. [Google Scholar] [CrossRef]
- Broen, M.P.G.; Beckers, R.; Willemsen, A.C.H.; Huijs, S.M.H.; Pasmans, R.; Eekers, D.B.P.; Ackermans, L.; Beckervordersandforth, J.; van Raak, E.P.M.; Verduin, M.; et al. Temporal muscle thickness as an independent prognostic imaging marker in newly diagnosed glioblastoma patients: A validation study. Neuro-Oncol. Adv. 2022, 4, vdac038. [Google Scholar] [CrossRef]
- Wende, T.; Kasper, J.; Prasse, G.; Glass, Ä.; Kriesen, T.; Freiman, T.M.; Meixensberger, J.; Henker, C. Newly Diagnosed IDH-Wildtype Glioblastoma and Temporal Muscle Thickness: A Multicenter Analysis. Cancers 2021, 13, 5610. [Google Scholar] [CrossRef]
- Furtner, J.; Berghoff, A.S.; Schöpf, V.; Reumann, R.; Pascher, B.; Woitek, R.; Asenbaum, U.; Pelster, S.; Leitner, J.; Widhalm, G.; et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J. Neuro-Oncol. 2018, 140, 173–178. [Google Scholar] [CrossRef]
- Furtner, J.; Nenning, K.H.; Roetzer, T.; Gesperger, J.; Seebrecht, L.; Weber, M.; Grams, A.; Leber, S.L.; Marhold, F.; Sherif, C.; et al. Evaluation of the Temporal Muscle Thickness as an Independent Prognostic Biomarker in Patients with Primary Central Nervous System Lymphoma. Cancers 2021, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Levolger, S.; Zaid Al-Kaylani, A.H.A.; Uyttenboogaart, M.; van Donkelaar, C.E.; Van Dijk, J.M.C.; Viddeleer, A.R.; Bokkers, R.P.H. Skeletal muscle atrophy and myosteatosis are not related to long-term aneurysmal subarachnoid hemorrhage outcome. PLoS ONE 2022, 17, e0264616. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.; Reitmeir, P.; Glodny, B.; Rass, V.; Lindner, A.; Ianosi, B.A.; Gaasch, M.; Schiefecker, A.J.; Putnina, L.; Beer, R. The Loss of Temporal Muscle Volume is Associated with Poor Outcome in Patients with Subarachnoid Hemorrhage: An Observational Cohort Study. Neurocrit. Care 2023. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total n = 478 | mRS 0–2 n = 247 | mRS 3–6 n = 231 | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 162 (34%) | 95 (38%) | 67 (29%) | |
| Female | 316 (66%) | 152 (62%) | 164 (71%) | |
| Age (years) | ||||
| mean ± SD | 56 ± 13 | 53 ± 13 | 60 ± 13 | <0.001 |
| <20 | 7 (1%) | 6 (2%) | 1 (0%) | |
| <30 | 29 (6%) | 20 (8%) | 9 (4%) | |
| <40 | 50 (10%) | 32 (13%) | 18 (8%) | |
| <50 | 129 (27%) | 80 (32%) | 49 (21%) | |
| <60 | 145 (30%) | 73 (30%) | 72 (31%) | |
| <70 | 77 (16%) | 31 (13%) | 46 (20%) | |
| <80 | 35 (7%) | 4 (2%) | 31 (13%) | |
| <90 | 6 (1%) | 1 (0%) | 5 (2%) | |
| Aneurysm location | ||||
| Acom | 175(37%) | 99 (40%) | 76 (33%) | |
| ACA | 11 (2%) | 7 (3%) | 4 (2%) | |
| PCA | 17 (4%) | 9 (4%) | 8 (3%) | |
| MCA | 107 (22%) | 56 (23%) | 51 (22%) | |
| ICA | 32 (7%) | 14 (6%) | 18 (8%) | |
| Pcom | 50 (10%) | 31 (13%) | 19 (8%) | |
| BA | 38 (8%) | 12 (5%) | 26 (11%) | |
| VA | 10 (2%) | 3 (1%) | 7 (3%) | |
| PICA | 18 (4%) | 7 (3%) | 11 (5%) | |
| SCA | 1 (0.2%) | 1 (0.4%) | 0 (0) | |
| Multiple locations | 19 | 8 | 11 | |
| WFNS scale | ||||
| mean ± SD | 2.9 ± 1.7 | 2 ± 1.4 | 3.9 ± 1.4 | <0.001 |
| 1–3 | 261 (55%) | 196 (79%) | 65 (28%) | |
| 4–5 | 217 (45%) | 51 (21%) | 166 (72%) | |
| Fisher scale | ||||
| mean ± SD | 3.1 ± 0.9 | 2.8 ± 1 | 3.5 ± 0.6 | <0.001 |
| 0–2 | 64 (13%) | 59 (24%) | 5 (2%) | |
| 3–4 | 414 (87%) | 188 (76%) | 226 (98%) | |
| Treatment | ||||
| 1 Surgical | 259 (54%) | 144 (58%) | 115 (50%) | |
| 2 Endovascular | 164 (34%) | 96 (39%) | 68 (29%) | |
| 3 None | 55 (12%) | 7 (3%) | 48 (21%) | |
| mRS at discharge | ||||
| mean ± SD | 3.5 ± 2.1 | 1.8 ± 1.3 | 5.3 ± 1 | <0.001 |
| 0–2 | 187 (39%) | 186 (76%) | 1 (0%) | |
| 3–6 | 290 (61%) | 60 (24%) | 230 (100%) | |
| No information | 1 | 1 | 0 | |
| mRS 6 months | ||||
| mean ± SD | 2.9 ± 2.4 | 0.8 ± 0.7 | 5.1 ± 1.2 | <0.001 |
| 0–2 | 247 (52%) | 247 (100%) | 0 (0%) | |
| 3–6 | 231 (48%) | 0 (0%) | 231 (100%) | |
| TMA | ||||
| Total | 283.8 ± 136.1 | 295.5 ± 136.6 | 271.3 ± 134.8 | 0.1 |
| Female | 234.5 ± 107.9 | 237.8 ± 99.7 | 231.4 ± 115.3 | 0.6 |
| Male | 380.0 ± 134.1 | 387.9 ± 137.2 | 368.9 ± 129.8 | 0.4 |
| TMT Sylvian fissure | ||||
| Total | 5.2 ± 1.8 | 5.3 ±1.8 | 5.1 ± 1.8 | 0.3 |
| Female | 4.8 ±1.8 | 4.8 ± 1.7 | 4.8 ± 1.8 | 0.8 |
| Male | 5.9 ± 1.7 | 5.9 ±1.8 | 5.8 ±1.6 | 0.7 |
| TMT at the thickest point | ||||
| Total | 5.8 ± 1.8 | 5.9 ± 1.8 | 5.6 ± 1.9 | 0.1 |
| Female | 5.3 ± 1.8 | 5.4 ± 1.7 | 5.3 ± 1.9 | 0.8 |
| Male | 6.6 ± 1.7 | 6.7 ± 1.7 | 6.4 ± 1.6 | 0.2 |
| TMT at the mid-level of the muscle | ||||
| Total | 4.5 ± 1.6 | 4.6 ± 1.5 | 4.4 ± 1.6 | 0.4 |
| Female | 4.2 ± 1.5 | 4.2 ± 1.4 | 4.2 ± 1.6 | 1 |
| Male | 5.1 ± 1.6 | 5.2 ± 1.6 | 5.0 ± 1.5 | 0.5 |
| TMT at the inferior level of the Sylvian fissure | ||||
| Total | 2.7 ±1.4 | 2.9 ± 1.4 | 2.6 ± 1.4 | 0.02 |
| Female | 2.3 ± 1.2 | 2.4 ± 1.1 | 2.2 ± 1.3 | 0.2 |
| Male | 3.6 ± 1.4 | 3.7 ± 1.4 | 3.5 ± 1.3 | 0.3 |
| Baseline Characteristics | Total n = 478 | Low-Volume < Median n = 244 | High-Volume ≥ Median n = 234 | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 162 (34%) | 53 (22%) | 109 (47%) | |
| Female | 316 (66%) | 191 (78%) | 125 (53%) | |
| Age (years) | ||||
| mean ± SD | 56 ± 13 | 59 ± 12 | 53 ± 14 | <0.001 |
| <20 | 7 (1%) | 2 (1%) | 5 (2%) | |
| <30 | 29 (6%) | 8 (3%) | 21 (9%) | |
| <40 | 50 (10%) | 13 (5%) | 37 (17%) | |
| <50 | 129 (27%) | 68 (28%) | 61 (27%) | |
| <60 | 145 (30%) | 80 (33%) | 65 (26%) | |
| <70 | 77 (16%) | 46 (19%) | 31 (13%) | |
| <80 | 35 (7%) | 25 (10%) | 11 (5%) | |
| <90 | 6 (1%) | 2 (1%) | 3 (1%) | |
| WFNS scale | ||||
| mean ± SD | 2.9 ± 1.7 | 3 ± 1.7 | 2.8 ± 1.7 | 0.42 |
| 1–3 | 261 (55%) | 128 (52%) | 133 (57%) | |
| 4–5 | 217 (45%) | 116 (48%) | 101 (43%) | |
| Fisher scale | ||||
| mean ± SD | 3.1 ± 0.9 | 3.2 ± 0.8 | 3.1 ± 0.9 | 0.8 |
| 0–2 | 64 (13%) | 32 (13%) | 32 (14%) | |
| 3–4 | 414 (87%) | 212 (87%) | 202 (86%) | |
| Treatment | ||||
| 1 Surgical | 259 (54%) | 124 (51%) | 135 (58%) | |
| 2 Endovascular | 164 (34%) | 82 (34%) | 82 (35%) | |
| 3 None | 55 (12%) | 38 (16%) | 17 (7%) | |
| mRS at discharge | ||||
| mean ± SD | 3.5 ± 2.1 | 3.7 ± 2.1 | 3.2 ± 2.1 | 0.1 |
| 0–2 | 187 (39%) | 86 (35%) | 101 (43%) | |
| 3–6 | 290 (61%) | 157 (65%) | 133 (57%) | |
| No information | 1 | 1 | 0 | |
| mRS 6 months | ||||
| mean ± SD | 2.9 ± 2.4 | 3.2 ± 2.4 | 2.7 ± 2.3 | 0.08 |
| 0–2 | 247 (52%) | 116 (48%) | 131 (56%) | |
| 3–6 | 231 (48%) | 128 (52%) | 103 (44%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadag, C.; Kamp, M.A.; Fischer, I.; Boogaarts, H.D.; Beseoglu, K.; Muhammad, S.; Cornelius, J.F.; Hofmann, B.B. Initial Temporal Muscle Thickness and Area: Poor Predictors of Neurological Outcome in Aneurysmal Subarachnoid Hemorrhage in a Central European Patient Cohort. J. Clin. Med. 2023, 12, 5210. https://doi.org/10.3390/jcm12165210
Karadag C, Kamp MA, Fischer I, Boogaarts HD, Beseoglu K, Muhammad S, Cornelius JF, Hofmann BB. Initial Temporal Muscle Thickness and Area: Poor Predictors of Neurological Outcome in Aneurysmal Subarachnoid Hemorrhage in a Central European Patient Cohort. Journal of Clinical Medicine. 2023; 12(16):5210. https://doi.org/10.3390/jcm12165210
Chicago/Turabian StyleKaradag, Cihat, Marcel A. Kamp, Igor Fischer, Hieronymus D. Boogaarts, Kerim Beseoglu, Sajjad Muhammad, Jan F. Cornelius, and Björn B. Hofmann. 2023. "Initial Temporal Muscle Thickness and Area: Poor Predictors of Neurological Outcome in Aneurysmal Subarachnoid Hemorrhage in a Central European Patient Cohort" Journal of Clinical Medicine 12, no. 16: 5210. https://doi.org/10.3390/jcm12165210
APA StyleKaradag, C., Kamp, M. A., Fischer, I., Boogaarts, H. D., Beseoglu, K., Muhammad, S., Cornelius, J. F., & Hofmann, B. B. (2023). Initial Temporal Muscle Thickness and Area: Poor Predictors of Neurological Outcome in Aneurysmal Subarachnoid Hemorrhage in a Central European Patient Cohort. Journal of Clinical Medicine, 12(16), 5210. https://doi.org/10.3390/jcm12165210








