Implementation and Assessment of a Laparotomy-Assisted Three-Port Fetoscopic Spina Bifida Repair Program
Abstract
1. Objective
2. What Is the Aim of This Current Study?
3. Material and Methods
4. Results
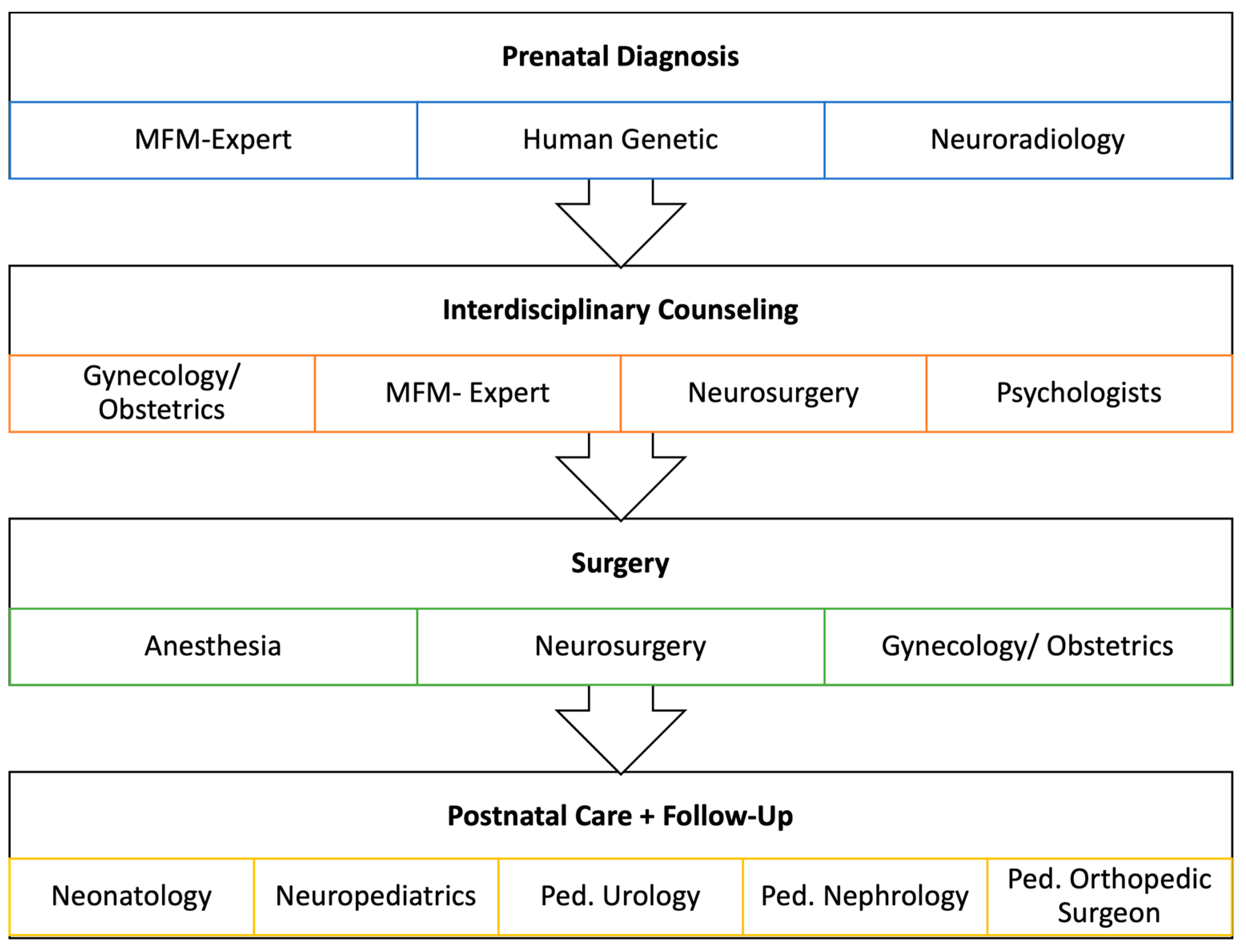
4.1. Diagnostics
4.2. Parental Counseling
4.3. OSB Surgery
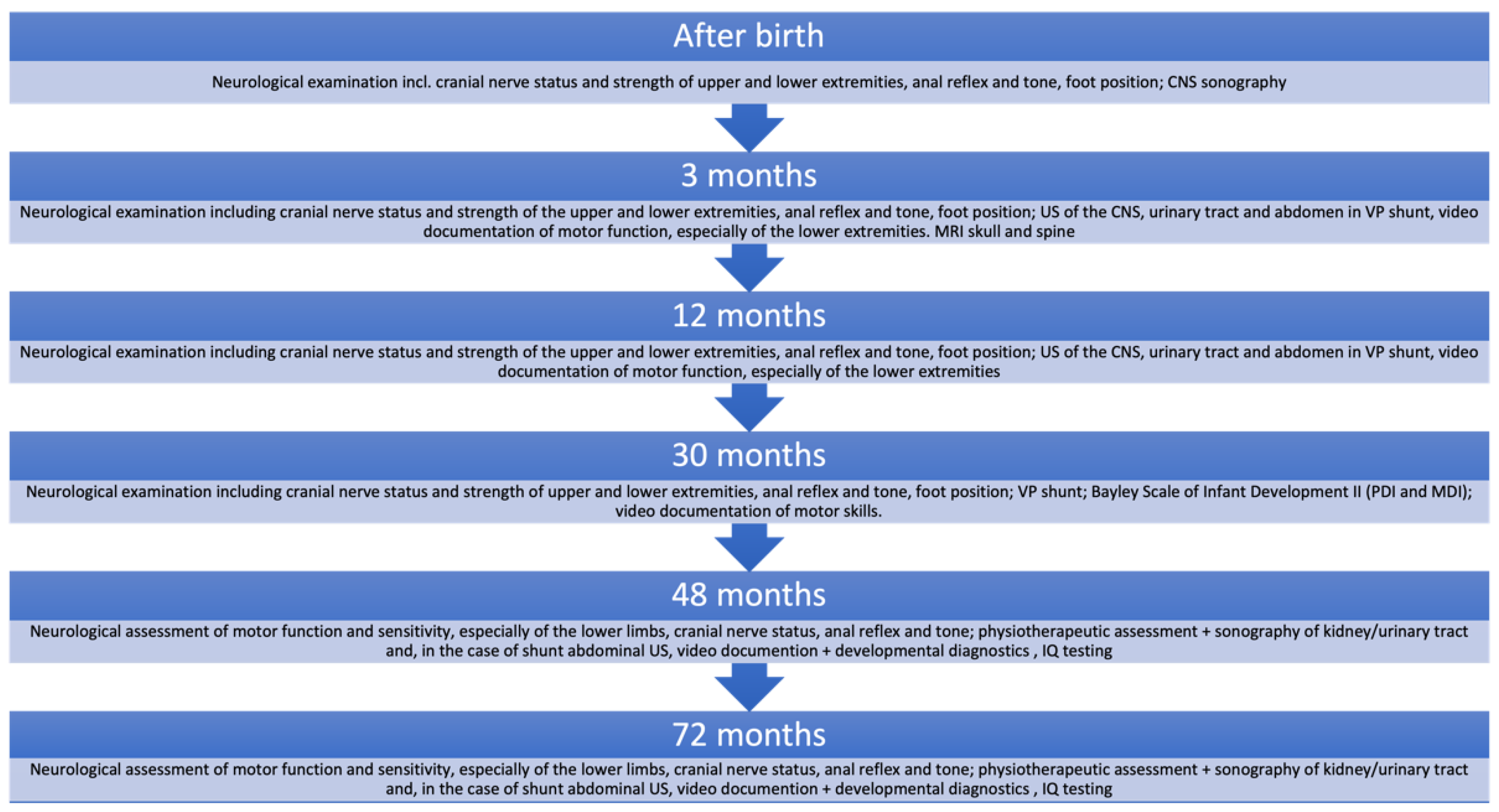
4.4. Delivery and Postnatal Care
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoshnood, B.; Loane, M.; Walle, H.; Arriola, L.; Addor, M.-C.; Barisic, I.; Beres, J.; Bianchi, F.; Dias, C.; Draper, E.; et al. Long term trends in prevalence of neural tube defects in Europe: Population based study. BMJ 2015, 351, h5949. [Google Scholar] [CrossRef]
- Canfield, M.A.; Mai, C.T.; Wang, Y.; O’Halloran, A.; Marengo, L.K.; Olney, R.S.; Borger, C.L.; Rutkowski, R.; Fornoff, J.; Irwin, N.; et al. The Association Between Race/Ethnicity and Major Birth Defects in the United States, 1999–2007. Am. J. Public Health 2014, 104, e14–e23. [Google Scholar] [CrossRef]
- Bowman, R.M.; McLone, D.G.; Grant, J.A.; Tomita, T.; Ito, J.A. Spina Bifida Outcome: A 25-Year Prospective. Pediatr. Neurosurg. 2001, 34, 114–120. [Google Scholar] [CrossRef]
- Meuli, M.; Meuli-Simmen, C.; Yingling, C.D.; Hutchins, G.M.; Hoffman, K.M.; Harrison, M.R.; Adzick, N.S. Creation of myelomeningocele in utero: A model of functional damage from spinal cord exposure in fetal sheep. J. Pediatr. Surg. 1995, 30, 1028–1033. [Google Scholar] [CrossRef]
- Meuli, M.; Meuli-Simmen, C.; Hutchins, G.M.; Seller, M.J.; Harrison, M.R.; Adzick, N.S. The spinal cord lesion in human fetuses with myelomeningocele: Implications for fetal surgery. J. Pediatr. Surg. 1997, 32, 448–452. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Tulipan, N.; Wellons, J.C.; Thom, E.A.; Gupta, N.; Sutton, L.N.; Burrows, P.K.; Farmer, D.; Walsh, W.; Johnson, M.P.; Rand, L.; et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. PED 2015, 16, 613–620. [Google Scholar] [CrossRef]
- Farmer, D.L.; Thom, E.A.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Gupta, N.; Adzick, N.S. The Management of Myelomeningocele Study: Full cohort 30-month pediatric outcomes. Am. J. Obstet. Gynecol. 2018, 218, 256.e1–256.e13. [Google Scholar] [CrossRef]
- Johnson, M.P.; Bennett, K.A.; Rand, L.; Burrows, P.K.; Thom, E.A.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Brock, J.W.; Farmer, D.L.; et al. The Management of Myelomeningocele Study: Obstetrical outcomes and risk factors for obstetrical complications following prenatal surgery. Am. J. Obstet. Gynecol. 2016, 215, 778.e1–778.e9. [Google Scholar] [CrossRef]
- Wilson, R.D.; Johnson, M.P.; Flake, A.W.; Crombleholme, T.M.; Hedrick, H.L.; Wilson, J.; Adzick, N.S. Reproductive outcomes after pregnancy complicated by maternal-fetal surgery. Am. J. Obstet. Gynecol. 2004, 191, 1430–1436. [Google Scholar] [CrossRef]
- Soni, S.; Moldenhauer, J.S.; Spinner, S.S.; Rendon, N.; Khalek, N.; Martinez-Poyer, J.; Johnson, M.P.; Adzick, N.S. Chorioamniotic membrane separation and preterm premature rupture of membranes complicating in utero myelomeningocele repair. Am. J. Obstet. Gynecol. 2016, 214, 647.e1–647.e7. [Google Scholar] [CrossRef]
- Lapa, D.A.; Chmait, R.H.; Gielchinsky, Y.; Yamamoto, M.; Persico, N.; Santorum, M.; Gil, M.M.; Trigo, L.; Quintero, R.A.; Nicolaides, K.H. Percutaneous fetoscopic spina bifida repair: Effect on ambulation and need for postnatal cerebrospinal fluid diversion and bladder catheterization. Ultrasound Obstet. Gyne 2021, 58, 582–589. [Google Scholar] [CrossRef]
- Kohl, T. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part I: Surgical technique and perioperative outcome. Ultrasound Obs. Gynecol. 2014, 44, 515–524. [Google Scholar] [CrossRef]
- Belfort, M.A.; Whitehead, W.E.; Shamshirsaz, A.A.; Bateni, Z.H.; Olutoye, O.O.; Olutoye, O.A.; Mann, D.G.; Espinoza, J.; Williams, E.; Lee, T.C.; et al. Fetoscopic Open Neural Tube Defect Repair: Development and Refinement of a Two-Port, Carbon Dioxide Insufflation Technique. Obstet. Gynecol. 2017, 129, 734–743. [Google Scholar] [CrossRef]
- Graf, K.; Kohl, T.; Neubauer, B.A.; Dey, F.; Faas, D.; Wanis, F.A.; Reinges, M.H.T.; Uhl, E.; Kolodziej, M.A. Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part III: Neurosurgical intervention in the first postnatal year: Fetoscopic surgery for SBA: Part III. Ultrasound Obstet. Gynecol. 2016, 47, 158–161. [Google Scholar] [CrossRef]
- Verweij, E.J.; Vries, M.C.; Oldekamp, E.J.; Eggink, A.J.; Oepkes, D.; Slaghekke, F.; Spoor, J.K.H.; Deprest, J.A.; Miller, J.L.; Baschat, A.A.; et al. Fetoscopic myelomeningocoele closure: Is the scientific evidence enough to challenge the gold standard for prenatal surgery? Prenat. Diagn. 2021, 41, 949–956. [Google Scholar] [CrossRef]
- Joyeux, L.; De Bie, F.; Danzer, E.; Russo, F.M.; Javaux, A.; Peralta, C.F.A.; De Salles, A.A.F.; Pastuszka, A.; Olejek, A.; Van Mieghem, T.; et al. Learning curves of open and endoscopic fetal spina bifida closure: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2020, 55, 730–739. [Google Scholar] [CrossRef]
- Joyeux, L.; De Bie, F.; Danzer, E.; Van Mieghem, T.; Flake, A.W.; Deprest, J. Safety and efficacy of fetal surgery techniques to close a spina bifida defect in the fetal lamb model: A systematic review. Prenat. Diagn. 2018, 38, 231–242. [Google Scholar] [CrossRef]
- Chmait, R.H.; Monson, M.A.; Chon, A.H. Advances in Fetal Surgical Repair of Open Spina Bifida. Obstet. Gynecol. 2023, 141, 505–521. [Google Scholar] [CrossRef]
- Sanz Cortes, M.; Lapa, D.A.; Acacio, G.L.; Belfort, M.; Carreras, E.; Maiz, N.; Peiro, J.L.; Lim, F.Y.; Miller, J.; Baschat, A.; et al. Proceedings of the First Annual Meeting of the International Fetoscopic Myelomeningocele Repair Consortium: International Fetoscopic Myelomeningocele Repair Consortium. Ultrasound Obstet. Gynecol. 2019, 53, 855–863. [Google Scholar] [CrossRef]
- Sanz Cortes, M.; Chmait, R.H.; Lapa, D.A.; Belfort, M.A.; Carreras, E.; Miller, J.L.; Brawura Biskupski Samaha, R.; Sepulveda Gonzalez, G.; Gielchinsky, Y.; Yamamoto, M.; et al. Experience of 300 cases of prenatal fetoscopic open spina bifida repair: Report of the International Fetoscopic Neural Tube Defect Repair Consortium. Am. J. Obstet. Gynecol. 2021, 225, 678.e1–678.e11. [Google Scholar] [CrossRef]
- Hirst, A.; Philippou, Y.; Blazeby, J.; Campbell, B.; Campbell, M.; Feinberg, J.; Rovers, M.; Blencowe, N.; Pennell, C.; Quinn, T.; et al. No Surgical Innovation Without Evaluation: Evolution and Further Development of the IDEAL Framework and Recommendations. Ann. Surg. 2019, 269, 211–220. [Google Scholar] [CrossRef]
- Miller, J.L.; Groves, M.L.; Ahn, E.S.; Berman, D.J.; Murphy, J.D.; Rosner, M.K.; Wolfson, D.; Jelin, E.B.; Korth, S.A.; Keiser, A.M.; et al. Implementation Process and Evolution of a Laparotomy-Assisted 2-Port Fetoscopic Spina Bifida Closure Program. Fetal Diagn. Ther. 2021, 48, 603–610. [Google Scholar] [CrossRef]
- Arthuis, C.; James, S.; Bussieres, L.; Hovhannisyan, S.; Corroenne, R.; Ville, Y.; Stirnemann, J.J. Laparotomy-Assisted 2-Port Fetoscopic Repair of Spina Bifida Aperta: Report of a Single-Center Experience in Paris, France. Fetal Diagn. Ther. 2022, 49, 377–384. [Google Scholar] [CrossRef]
- Pastuszka, A.; Zamłyński, M.; Horzelski, T.; Zamłyński, J.; Horzelska, E.; Maruniak-Chudek, I.; Marzec, A.; Paprocka, J.; Gazy, P.; Koszutski, T.; et al. Fetoscopic Myelomeningocele Repair with Complete Release of the Tethered Spinal Cord Using a Three-Port Technique: Twelve-Month Follow-Up—A Case Report. Diagnostics 2022, 12, 2978. [Google Scholar] [CrossRef]
- Degenhardt, J.; Schürg, R.; Winarno, A.; Oehmke, F.; Khaleeva, A.; Kawecki, A.; Enzensberger, C.; Tinneberg, H.-R.; Faas, D.; Ehrhardt, H.; et al. Percutaneous minimal-access fetoscopic surgery for spina bifida aperta. Part II: Maternal management and outcome. Ultrasound Obstet. Gynecol. 2014, 44, 525–531. [Google Scholar] [CrossRef]
- Joyeux, L.; Basurto, D.; Eastwood, M.; Javaux, A.; De Bie, F.; Vergote, S.; Devlieger, R.; De Catte, L.; Van Calenbergh, F.; Belfort, M.A.; et al. OC10.01: A novel training program for fetoscopic spina bifida repair. Ultrasound Obstet. Gynecol. 2020, 56, 27. [Google Scholar] [CrossRef]
- Carreras, E.; Maroto, A.; Illescas, T.; Meléndez, M.; Arévalo, S.; Peiró, J.L.; García-Fontecha, C.G.; Belfort, M.; Cuxart, A. Prenatal ultrasound evaluation of segmental level of neurological lesion in fetuses with myelomeningocele: Development of a new technique: Functional ultrasound in MMC. Ultrasound Obstet. Gynecol. 2016, 47, 162–167. [Google Scholar] [CrossRef]
- Ushakov, F.; Sacco, A.; Andreeva, E.; Tudorache, S.; Everett, T.; David, A.L.; Pandya, P.P. Crash sign: New first-trimester sonographic marker of spina bifida. Ultrasound Obstet. Gynecol. 2019, 54, 740–745. [Google Scholar] [CrossRef]
- Chaoui, R.; Benoit, B.; Mitkowska-Wozniak, H.; Heling, K.S.; Nicolaides, K.H. Assessment of intracranial translucency (IT) in the detection of spina bifida at the 11–13-week scan. Ultrasound Obstet. Gynecol. 2009, 34, 249–252. [Google Scholar] [CrossRef]
- Sirico, A.; Raffone, A.; Lanzone, A.; Saccone, G.; Travaglino, A.; Sarno, L.; Rizzo, G.; Zullo, F.; Maruotti, G.M. First trimester detection of fetal open spina bifida using BS/BSOB ratio. Arch. Gynecol. Obstet. 2020, 301, 333–340. [Google Scholar] [CrossRef]
- Trigo, L.; Eixarch, E.; Bottura, I.; Dalaqua, M.; Barbosa, A.A.; De Catte, L.; Demaerel, P.; Dymarkowski, S.; Deprest, J.; Lapa, D.A.; et al. Prevalence of supratentorial anomalies assessed by magnetic resonance imaging in fetuses with open spina bifida. Ultrasound Obstet. Gynecol. 2022, 59, 804–812. [Google Scholar] [CrossRef]
- Mufti, N.; Sacco, A.; Aertsen, M.; Ushakov, F.; Ourselin, S.; Thomson, D.; Deprest, J.; Melbourne, A.; David, A.L. What brain abnormalities can magnetic resonance imaging detect in foetal and early neonatal spina bifida: A systematic review. Neuroradiology 2022, 64, 233–245. [Google Scholar] [CrossRef]
- Egloff, A.; Bulas, D. Magnetic Resonance Imaging Evaluation of Fetal Neural Tube Defects. Semin. Ultrasound CT MRI 2015, 36, 487–500. [Google Scholar] [CrossRef]
- Davidson, J.R.; Uus, A.; Matthew, J.; Egloff, A.M.; Deprez, M.; Yardley, I.; De Coppi, P.; David, A.; Carmichael, J.; Rutherford, M.A. Fetal body MRI and its application to fetal and neonatal treatment: An illustrative review. Lancet Child. Adolesc. Health 2021, 5, 447–458. [Google Scholar] [CrossRef]
- Zarutskie, A.; Guimaraes, C.; Yepez, M.; Torres, P.; Shetty, A.; Sangi-Haghpeykar, H.; Lee, W.; Espinoza, J.; Shamshirsaz, A.A.; Nassr, A.; et al. Prenatal brain imaging for predicting need for postnatal hydrocephalus treatment in fetuses that had neural tube defect repair in utero. Ultrasound Obstet. Gynecol. 2019, 53, 324–334. [Google Scholar] [CrossRef]
- Flint, G.A.; Lammers, W.; Mitnick, D.G. Emotional Freedom Techniques. J. Aggress. Maltreatment Trauma 2006, 12, 125–150. [Google Scholar] [CrossRef]
- Craig, G. The EFT manual. EFT: Emotional Freedom Technique: A Universal Healing Aid. Retrieved 15 January 2001. Available online: http://www.emofree.com/freestuff.htm (accessed on 6 August 2023).
- Sacco, A.; Ushakov, F.; Thompson, D.; Peebles, D.; Pandya, P.; De Coppi, P.; Wimalasundera, R.; Attilakos, G.; David, A.L.; Deprest, J. Fetal surgery for open spina bifida. Obstet. Gynecol. 2019, 21, 271–282. [Google Scholar] [CrossRef]
- Espinoza, J.; Shamshirsaz, A.A.; Sanz Cortes, M.; Pammi, M.; Nassr, A.A.; Donepudi, R.; Whitehead, W.E.; Castillo, J.; Johnson, R.; Meshinchi, N.; et al. Two-port, exteriorized uterus, fetoscopic meningomyelocele closure has fewer adverse neonatal outcomes than open hysterotomy closure. Am. J. Obstet. Gynecol. 2021, 225, 327.e1–327.e9. [Google Scholar] [CrossRef]
- Ferschl, M.; Ball, R.; Lee, H.; Rollins, M.D. Anesthesia for In Utero Repair of Myelomeningocele. Anesthesiology 2013, 118, 1211–1223. [Google Scholar] [CrossRef]
- Ring, L.E.; Ginosar, Y. Anesthesia for Fetal Surgery and Fetal Procedures. Clin. Perinatol. 2019, 46, 801–816. [Google Scholar] [CrossRef]
- Hoagland, M.A.; Chatterjee, D. Anesthesia for fetal surgery. Paediatr. Anaesth. 2017, 27, 346–357. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Surgical Step | Problem | Solution |
|---|---|---|
| Low transverse laparotomy, externalisation of the uterus | Laparotomy is too small, which can lead to compression of the uterine artery, affecting feto-placental perfusion | Extend laparotomy |
| Placing 3 Ports (12 French, 10 French) | Incorrect port placement will result in difficulty in visualising and accessing the fetal OSB and may compromise surgical closure. | Careful port placement considering placenta, fetal position and operative environment. |
| Alteration of placenta/fetus/myometral vessels | Careful sonographic visualisation of (intra-)uterine structures (placenta, fetus, vessels) | |
| Removing amniotic fluid | Can lead to fetal heart alteration | Careful monitoring of fetal heart rate, slow remove |
| Uterine carbon dioxide insufflation (10 ± 2 mm Hg) | Gas needs to be humidified and warmed, otherwise there is an increased risk of premature rupture of the membranes. | Additional Intermittent irrigation of membranes with sterile solution during OSB repair |
| Moderate uterine pressure and moisturisation prevents fetal acidaemia | ||
| Fetal positioning | Can lead to fetal heart alteration, lack of positioning can complicate surgery or make it impossible. | Immediate cessation of fetal manipulation, ongoing fetal monitoring |
| Intrauterine resuscitation of the fetus by administration of epinephrine (0.01 mg/kg) | ||
| Fetal anesthesia | Can lead to fetal heart alteration, fetal death caused by inccorect dose/application | Assesment of estimated preoperative fetal weight by experienced investigator |
| (fentanyl 10 ug/kg, cis-atracurium 0.6 mg/kg, atropine 0.03 mg/kg)- every 60 min during OSB repair | Monitoring and administration of fetal anaesthesia using four-eyes principle (MFM and anesthesia) | |
| Subcutaneous administration under direct vision only | ||
| Releasing spinal cord | Too close to skin | May develop inclusion cysts causing severe functional deterioration |
| Excessive distance to skin | Injury to the neuronal structures | |
| Preparation muscle flap | Not enough muscle mobilised/present | Close as much muscle as possible |
| Preparation skin flap | Not enough skin mobilised/present | Relaxing skin incisions lateral of the defect |
| Placing bovine dura patch | Patch too small/wide/big | Patch preparation according to defect size from MRI/US |
| Single sutures muscle | Low proportion of muscle | Close as much muscle as possible |
| Continous closure of the skin | Thin/fragile skin | Relaxing skin incisions lateral of the defect |
| End of CO2-insufflation and instillation of sterile solution | Too much gas/too less sterile solution | Sonographic monitoring of refill |
| Removal of 3 ports and closure of the myometrial defect | Suture of fetal structures during closure | Controlled suture under sonographic guidance |
| Insufficient closure | Uterine loss of amniotic fluid | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keil, C.; Köhler, S.; Sass, B.; Schulze, M.; Kalmus, G.; Belfort, M.; Schmitt, N.; Diehl, D.; King, A.; Groß, S.; et al. Implementation and Assessment of a Laparotomy-Assisted Three-Port Fetoscopic Spina Bifida Repair Program. J. Clin. Med. 2023, 12, 5151. https://doi.org/10.3390/jcm12155151
Keil C, Köhler S, Sass B, Schulze M, Kalmus G, Belfort M, Schmitt N, Diehl D, King A, Groß S, et al. Implementation and Assessment of a Laparotomy-Assisted Three-Port Fetoscopic Spina Bifida Repair Program. Journal of Clinical Medicine. 2023; 12(15):5151. https://doi.org/10.3390/jcm12155151
Chicago/Turabian StyleKeil, Corinna, Siegmund Köhler, Benjamin Sass, Maximilian Schulze, Gerald Kalmus, Michael Belfort, Nicolas Schmitt, Daniele Diehl, Alice King, Stefanie Groß, and et al. 2023. "Implementation and Assessment of a Laparotomy-Assisted Three-Port Fetoscopic Spina Bifida Repair Program" Journal of Clinical Medicine 12, no. 15: 5151. https://doi.org/10.3390/jcm12155151
APA StyleKeil, C., Köhler, S., Sass, B., Schulze, M., Kalmus, G., Belfort, M., Schmitt, N., Diehl, D., King, A., Groß, S., Sutton, C. D., Joyeux, L., Wege, M., Nimsky, C., Whitehead, W. E., Uhl, E., Huisman, T. A. G. M., Neubauer, B. A., Weber, S., ... Bedei, I. (2023). Implementation and Assessment of a Laparotomy-Assisted Three-Port Fetoscopic Spina Bifida Repair Program. Journal of Clinical Medicine, 12(15), 5151. https://doi.org/10.3390/jcm12155151







