Importance of Pattern Standard Deviation of Humphrey 10-2 Visual Field to Evaluate Central Visual Function in Patients with Early-Stage Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Optical Coherence Tomography
2.4. Pattern Electroretinogram
2.5. VF Testing
2.6. Definition of Mean Deviation (MD) and Pattern Standard Deviation (PSD)
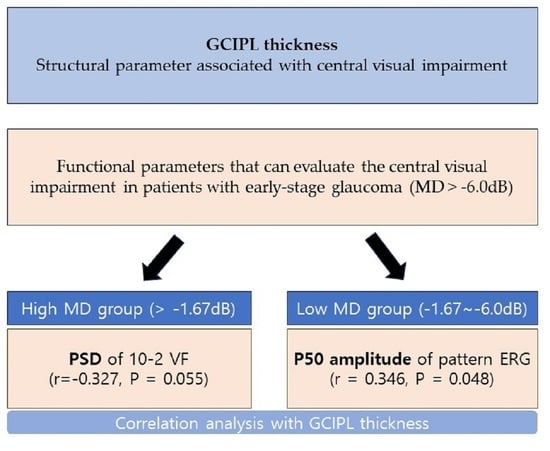
2.7. Classifying into High and Low MD Groups
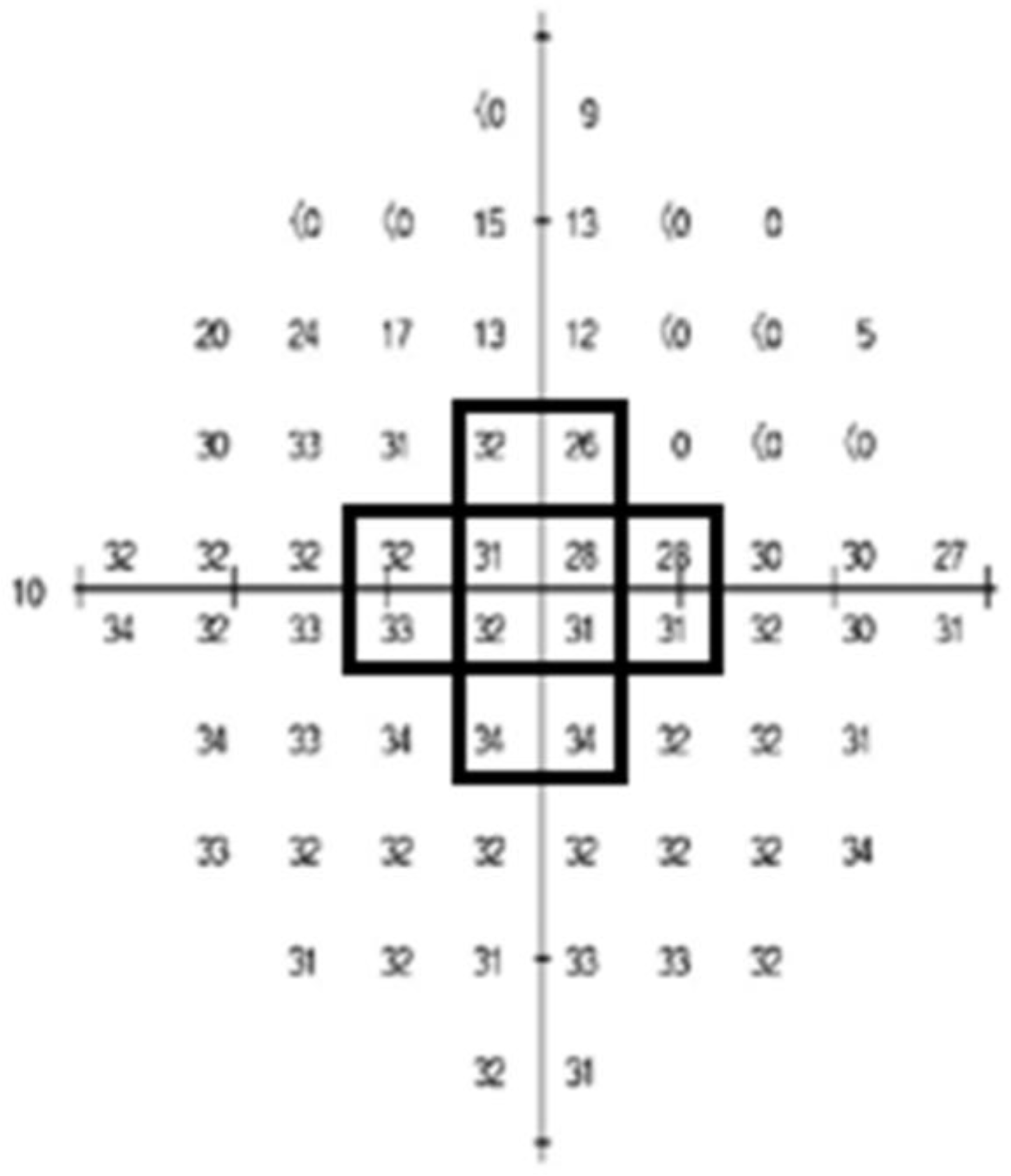
2.8. Creation of Threshold-Sensitive Points (Total and Center)
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, N.; Yücel, Y.H. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007, 18, 110–114. [Google Scholar] [CrossRef]
- GBD 2019 Blindness; Vision Impairment Collaborators; the Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.B.; Hobley, A.J. Frequency Distribution of Early Glaucomatous Visual Field Defects. Am. J. Optom. Physiol. Opt. 1986, 63, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Liebmann, J.M.; Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef]
- Hood, D.C.; Slobodnick, A.; Raza, A.S.; de Moraes, C.G.; Teng, C.C.; Ritch, R. Early Glaucoma Involves Both Deep Local, and Shallow Widespread, Retinal Nerve Fiber Damage of the Macular Region. Investig. Opthalmol. Vis. Sci. 2014, 55, 632–649. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kato, S.; Kobayashi, M.; Watanabe, M.; Ochiai, M. Relationship between vision-related quality of life and different types of existing visual fields in Japanese patients. Int. Ophthalmol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- De Moraes, C.G.; Hood, D.C.; Thenappan, A.; Girkin, C.A.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M. 24-2 Visual Fields Miss Central Defects Shown on 10-2 Tests in Glaucoma Suspects, Ocular Hypertensives, and Early Glaucoma. Ophthalmology 2017, 124, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Grillo, L.M.; Wang, D.L.; Ramachandran, R.; Ehrlich, A.C.; De Moraes, C.G.; Ritch, R.; Hood, D.C. The 24-2 Visual Field Test Misses Central Macular Damage Confirmed by the 10-2 Visual Field Test and Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- West, M.E.; Sharpe, G.P.; Hutchison, D.M.; Rafuse, P.E.; Shuba, L.M.; Nicolela, M.T.; Vianna, J.R.; Chauhan, B.C. Value of 10-2 Visual Field Testing in Glaucoma Patients with Early 24-2 Visual Field Loss. Ophthalmology 2021, 128, 545–553. [Google Scholar] [CrossRef]
- Wu, Z.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M. Performance of the 10-2 and 24-2 Visual Field Tests for Detecting Central Visual Field Abnormalities in Glaucoma. Am. J. Ophthalmol. 2018, 196, 10–17. [Google Scholar] [CrossRef]
- Chakravarti, T.; Moghimi, S.; De Moraes, C.G.; Weinreb, R.N. Central-most Visual Field Defects in Early Glaucoma. J. Glaucoma 2021, 30, e68–e75. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.L.; Hwang, B.-E.; Shin, H.-Y.; Park, C.K. Clinical Clues to Predict the Presence of Parafoveal Scotoma on Humphrey 10-2 Visual Field Using a Humphrey 24-2 Visual Field. Am. J. Ophthalmol. 2016, 161, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Baek, M.S.; Lee, J.Y.; Song, M.K.; Shin, J.W.; Kook, M.S. Comparison of the 24-2 and 24-2C Visual Field Grids in Determining the Macular Structure-Function Relationship in Glaucoma. J. Glaucoma 2021, 30, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Phu, J.; Kalloniatis, M. Comparison of 10-2 and 24-2C Test Grids for Identifying Central Visual Field Defects in Glaucoma and Suspect Patients. Ophthalmology 2021, 128, 1405–1416. [Google Scholar] [CrossRef]
- Falsini, B.; Marangoni, D.; Salgarello, T.; Stifano, G.; Montrone, L.; Campagna, F.; Aliberti, S.; Balestrazzi, E.; Colotto, A. Structure–function relationship in ocular hypertension and glaucoma: Interindividual and interocular analysis by OCT and pattern ERG. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1153–1162. [Google Scholar] [CrossRef]
- Turkey, E.; Elsanabary, Z.S.; Elshazly, L.H.M.; Osman, M.H. Role of Pattern Electroretinogram in Ocular Hypertension and Early Glaucoma. J. Glaucoma 2019, 28, 871–877. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Rabiolo, A.; Fu, Q.; Morales, E.; Coleman, A.L.; Law, S.K.; Caprioli, J.; Nouri-Mahdavi, K. Longitudinal Macular Structure–Function Relationships in Glaucoma. Ophthalmology 2020, 127, 888–900. [Google Scholar] [CrossRef]
- Bambo, M.P.; Güerri, N.; Ferrandez, B.; Cameo, B.; Fuertes, I.; Polo, V.; Garcia-Martin, E. Evaluation of the Macular Ganglion Cell-Inner Plexiform Layer and the Circumpapillary Retinal Nerve Fiber Layer in Early to Severe Stages of Glaucoma: Correlation with Central Visual Function and Visual Field Indexes. Ophthalmic Res. 2017, 57, 216–223. [Google Scholar] [CrossRef]
- Kim, K.E.; Park, K.H.; Jeoung, J.W.; Kim, S.H.; Kim, D.M. Severity-dependent association between ganglion cell inner plexiform layer thickness and macular mean sensitivity in open-angle glaucoma. Acta Ophthalmol. 2014, 92, e650–e656. [Google Scholar] [CrossRef]
- Hood, D.C.; Tsamis, E.; Bommakanti, N.K.; Joiner, D.B.; Al-Aswad, L.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; De Moraes, C.G. Structure-Function Agreement Is Better Than Commonly Thought in Eyes with Early Glaucoma. Investig. Opthalmology Vis. Sci. 2019, 60, 4241–4248. [Google Scholar] [CrossRef]
- Mwanza, J.-C.; Oakley, J.D.; Budenz, D.L.; Chang, R.T.; Knight, O.J.; Feuer, W.J. Macular Ganglion Cell–Inner Plexiform Layer: Automated Detection and Thickness Reproducibility with Spectral Domain–Optical Coherence Tomography in Glaucoma. Investig. Opthalmol. Vis. Sci. 2022, 52, 8323–8329. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Park, H.-Y.L.; Jung, K.I.; Park, C.K. Comparative Study of Macular Ganglion Cell–Inner Plexiform Layer and Peripapillary Retinal Nerve Fiber Layer Measurement: Structure–Function Analysis. Investig. Opthalmol. Vis. Sci. 2022, 54, 7344–7353. [Google Scholar] [CrossRef]
- Jeon, S.J.; Park, H.-Y.L.; Jung, K.I.; Park, C.K. Relationship between pattern electroretinogram and optic disc morphology in glaucoma. PLoS ONE 2019, 14, e0220992. [Google Scholar] [CrossRef]
- Jung, K.I.; Jeon, S.; Shin, D.Y.; Lee, J.; Park, C.K. Pattern Electroretinograms in Preperimetric and Perimetric Glaucoma. Am. J. Ophthalmol. 2020, 215, 118–126. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Garway-Heath, D.F.; Goni, F.J.; Rossetti, L.; Bengtsson, B.; Viswanathan, A.C.; Heijl, A. Practical recommendations for measuring rates of visual field change in glaucoma. Br. J. Ophthalmol. 2008, 92, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Aung, T. Automated static perimetry: The influence of myopia and its method of correction. Ophthalmology 2001, 108, 290–295. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. Influence of cataract surgery on automated perimetry in patients with glaucoma. Am. J. Ophthalmol. 2001, 132, 41–46. [Google Scholar] [CrossRef]
- Blumenthal, E.Z.; Sapir-Pichhadze, R. Misleading statistical calculations in far-advanced glaucomatous visual field loss. Ophthalmology 2003, 110, 196–200. [Google Scholar] [CrossRef]
- Heo, D.W.; Kim, K.N.; Lee, M.W.; Lee, S.B.; Kim, C.-S. Properties of pattern standard deviation in open-angle glaucoma patients with hemi-optic neuropathy and bi-optic neuropathy. PLoS ONE 2017, 12, e0171960. [Google Scholar] [CrossRef]
- Gardiner, S.K.; Demirel, S.; Johnson, C.A. Perimetric Indices as Predictors of Future Glaucomatous Functional Change. Optom. Vis. Sci. 2011, 88, 56–62. [Google Scholar] [CrossRef]
- Artes, P.H.; Nicolela, M.T.; LeBlanc, R.P.; Chauhan, B.C. Visual Field Progression in Glaucoma: Total Versus Pattern Deviation Analyses. Investig. Opthalmol. Vis. Sci. 2005, 46, 4600–4606. [Google Scholar] [CrossRef]
- Casas-Llera, P.; Rebolleda, G.; Munoz-Negrete, F.J.; Arnalich-Montiel, F.; Perez-Lopez, M.; Fernandez-Buenaga, R. Visual field index rate and event-based glaucoma progression analysis: Comparison in a glaucoma population. Br. J. Ophthalmol. 2009, 93, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Ganekal, S.; Dorairaj, S. Pattern Electroretinography Changes in Patients with Established or Suspected Primary Open Angle Glaucoma. J. Curr. Glaucoma Pract. 2013, 7, 39–42. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.; Lee, J. Measurement of macular structure-function relationships using spectral domain-optical coherence tomography (SD-OCT) and pattern electroretinograms (PERG). PLoS ONE 2017, 12, e0178004. [Google Scholar] [CrossRef]
| Variables | Description |
|---|---|
| Age (y) | 52.91 ± 15.06 |
| Female, no. (%) | 35 (50%) |
| Axial length (mm) | 24.98 ± 2.20 |
| Central corneal thickness (μm) | 538.27 ± 42.39 |
| Average pRNFL thickness (μm) | 82.29 ± 9.50 |
| Average mGC/IPL thickness (μm) | 74.30 ± 8.33 |
| MD of VF 24-2 (dB) | −2.15 ± 2.32 |
| PSD of VF 24-2 (dB) | 3.32 ± 2.53 |
| MD of VF 10-2 (dB) | −1.76 ± 2.99 |
| PSD of VF 10-2 (dB) | 2.51 ± 2.93 |
| Threshold sensitive points (center) | 32.47 ± 3.38 |
| Threshold sensitive points (total) | 31.23 ± 2.93 |
| P50 amplitude of PERG | 3.12 ± 1.31 |
| N95 amplitude of PERG | 5.35 ± 1.47 |
| Variables | High MD Group | Low MD Group | p Value |
|---|---|---|---|
| Age (y) | 51.7 ± 14.2 | 54.2 ± 16.0 | 0.489 * |
| Female, no. (%) | 20 (57.1%) | 20 (57.1%) | 1.000 † |
| Axial length (mm) | 24.9 ± 1.9 | 25.4 ± 2.4 | 0.447 * |
| Central corneal thickness (μm) | 547.6 ± 43.9 | 529.6 ± 40.2 | 0.094 * |
| Average pRNFL thickness (μm) | 85.8 ± 8.8 | 78.7 ± 8.9 | 0.001 * |
| Average mGC/IPL thickness (μm) | 76.3 ± 6.0 | 72.3 ± 9.8 | 0.046 * |
| MD of VF 24-2 (dB) | −0.3 ± 1.0 | −4.0 ± 1.8 | 0.000 * |
| PSD of VF 24-2 (dB) | 2.0 ± 1.0 | 4.6 ± 2.9 | 0.000 * |
| MD of VF 10-2 (dB) | −0.6 ± 1.4 | −2.9 ± 3.7 | 0.001 * |
| PSD of VF 10-2 (dB) | 1.7 ± 1.7 | 3.3 ± 3.6 | 0.022 * |
| Threshold sensitive points (center) | 33.5 ± 1.8 | 31.5 ± 4.2 | 0.012 * |
| Threshold sensitive points (total) | 32.3 ± 1.6 | 30.1 ± 3.5 | 0.001 * |
| P50 amplitude of PERG | 3.3 ± 1.5 | 2.9 ± 1.1 | 0.248 * |
| N95 amplitude of PERG | 5.7 ± 1.7 | 5.0 ± 1.1 | 0.047 * |
| RNFL Thickness | GCIPL Thickness | |||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| P50 amplitude | 0.093 | 0.445 | 0.236 | 0.052 |
| N95 amplitude | 0.041 | 0.738 | 0.093 | 0.448 |
| SITA 24-2MD | 0.356 | 0.002 | 0.240 | 0.049 |
| SITA 24-2PSD | −0.269 | 0.024 | −0.194 | 0.114 |
| SITA 10-2MD | 0.217 | 0.071 | 0.437 | 0.000 |
| SITA 10-2PSD | −0.250 | 0.037 | −0.459 | 0.000 |
| Threshold sensitive points (center) | 0.180 | 0.136 | 0.385 | 0.001 |
| Threshold sensitive points (total) | 0.191 | 0.113 | 0.381 | 0.001 |
| RNFL Thickness | GCIPL Thickness | |||||||
|---|---|---|---|---|---|---|---|---|
| High MD | Low MD | High MD | Low MD | |||||
| r | p Value | r | p Value | r | p Value | r | p Value | |
| P50 amplitude | −0.073 | 0.678 | 0.202 | 0.245 | 0.107 | 0.540 | 0.347 | 0.048 |
| N95 amplitude | −0.118 | 0.500 | 0.040 | 0.821 | −0.038 | 0.830 | 0.141 | 0.432 |
| SITA 24-2MD | −0.019 | 0.915 | 0.177 | 0.308 | −0.013 | 0.942 | 0.181 | 0.313 |
| SITA 24-2PSD | 0.039 | 0.824 | −0.152 | 0.382 | −0.101 | 0.564 | −0.108 | 0.551 |
| SITA 10-2MD | −0.155 | 0.375 | 0.191 | 0.273 | 0.053 | 0.763 | 0.500 | 0.003 |
| SITA 10-2PSD | −0.085 | 0.628 | −0.216 | 0.212 | −0.327 | 0.055 | −0.466 | 0.006 |
| Threshold sensitive points (center) | −0.039 | 0.826 | 0.131 | 0.455 | 0.062 | 0.724 | 0.441 | 0.010 |
| Threshold sensitive points (total) | −0.051 | 0.773 | 0.110 | 0.531 | 0.072 | 0.683 | 0.430 | 0.013 |
| SITA 10-2MD | SITA 10-2PSD | |||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| SITA 24-2MD | 0.390 | 0.001 | −0.293 | 0.014 |
| SITA 24-2PSD | −0.305 | 0.010 | 0.445 | 0.000 |
| Threshold sensitive points (center) | 0.921 | 0.000 | −0.638 | 0.000 |
| Threshold sensitive points (total) | 0.942 | 0.000 | −0.734 | 0.000 |
| P50 amplitude | 0.002 | 0.986 | −0.062 | 0.611 |
| N95 amplitude | 0.125 | 0.301 | −0.163 | 0.179 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (y) | 1.02 (0.97–1.08) | 0.418 | ||
| Female, no. (%) | 0.61 (0.09–4.02) | 0.614 | 0.47 (0.21–1.04) | 0.070 |
| Axial length (mm) | 0.92 (0.6–1.4) | 0.700 | 0.63 (0.51–0.79) | 0.0002 |
| Central corneal thickness (μm) | 0.99 (0.97–1.02) | 0.610 | ||
| Average pRNFL thickness (μm) | 0.93 (0.84–1.02) | 0.131 | ||
| Average mGC/IPL thickness (μm) | 0.81 (0.74–0.89) | <0.0001 | 0.90 (0.85–0.95) | 0.001 |
| MD of VF 24-2 (dB) | 0.72 (0.49–1.05) | 0.095 | 1.67 (1.32–2.13) | 0.0001 |
| PSD of VF 24-2 (dB) | 1.82 (1.31–2.54) | 0.001 | 1.43 (1.13–1.80) | 0.009 |
| MD of VF 10-2 (dB) | 0.44 (0.37–0.51) | <0.0001 | 0.24 (0.17–0.33) | <0.0001 |
| Threshold sensitive points (center) | 0.55 (0.46–0.67) | <0.0001 | 1.96 (1.47–2.59) | <0.0001 |
| Threshold sensitive points (total) | 0.44 (0.36–0.54) | <0.0001 | ||
| P50 amplitude of PERG | 0.85 (0.44–1.66) | 0.640 | 0.80 (0.60–1.08) | 0.149 |
| N95 amplitude of PERG | 0.70 (0.39–1.24) | 0.225 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.J.; Oh, S.E.; Park, C.K.; Park, H.-Y.L. Importance of Pattern Standard Deviation of Humphrey 10-2 Visual Field to Evaluate Central Visual Function in Patients with Early-Stage Glaucoma. J. Clin. Med. 2023, 12, 5091. https://doi.org/10.3390/jcm12155091
Shin HJ, Oh SE, Park CK, Park H-YL. Importance of Pattern Standard Deviation of Humphrey 10-2 Visual Field to Evaluate Central Visual Function in Patients with Early-Stage Glaucoma. Journal of Clinical Medicine. 2023; 12(15):5091. https://doi.org/10.3390/jcm12155091
Chicago/Turabian StyleShin, Hee Jong, Si Eun Oh, Chan Kee Park, and Hae-Young Lopilly Park. 2023. "Importance of Pattern Standard Deviation of Humphrey 10-2 Visual Field to Evaluate Central Visual Function in Patients with Early-Stage Glaucoma" Journal of Clinical Medicine 12, no. 15: 5091. https://doi.org/10.3390/jcm12155091
APA StyleShin, H. J., Oh, S. E., Park, C. K., & Park, H.-Y. L. (2023). Importance of Pattern Standard Deviation of Humphrey 10-2 Visual Field to Evaluate Central Visual Function in Patients with Early-Stage Glaucoma. Journal of Clinical Medicine, 12(15), 5091. https://doi.org/10.3390/jcm12155091