Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Variables
2.3. Data Sources and Measurements
2.4. Statistical Methods
3. Results
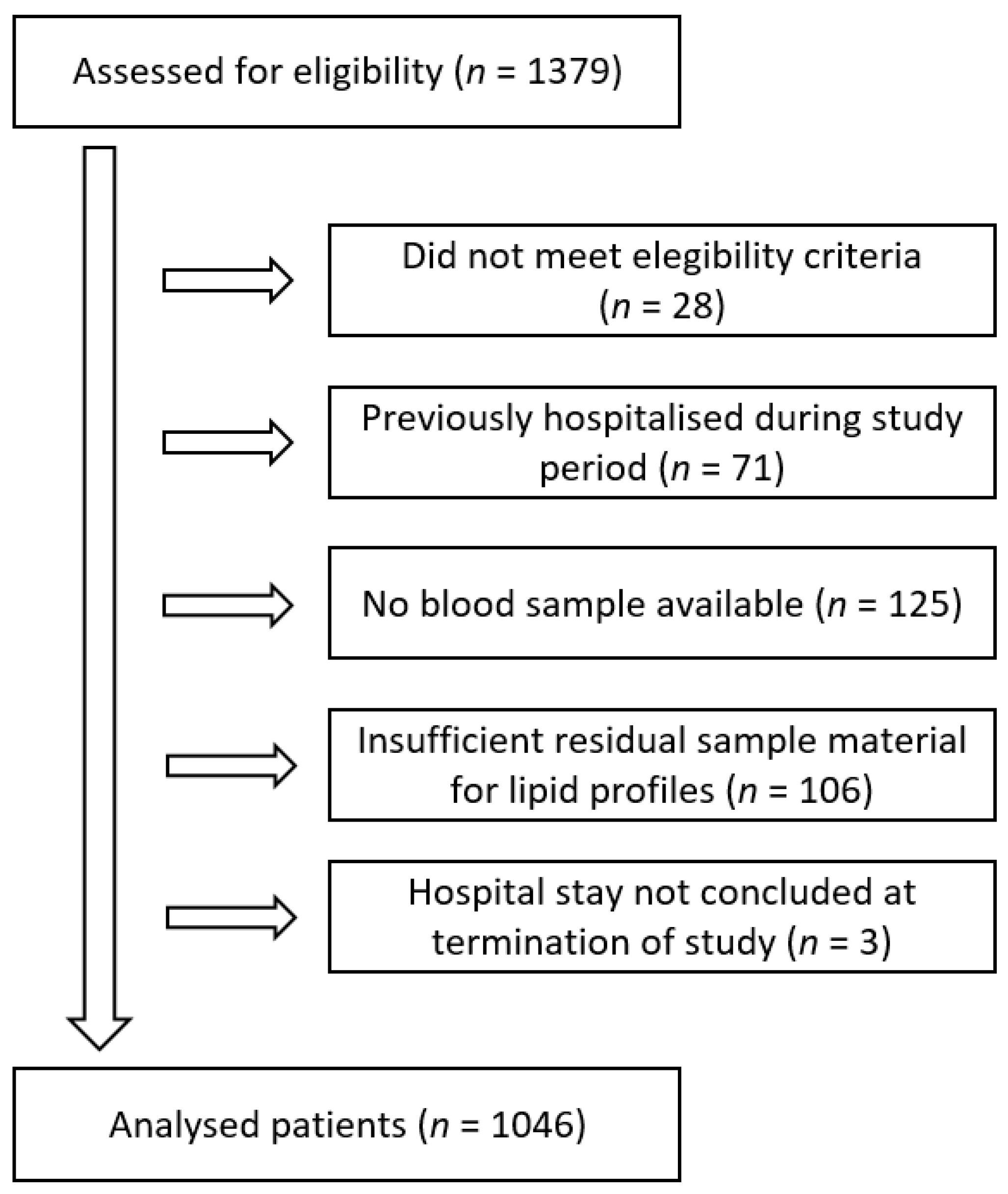
3.1. Participants
3.2. Risk of Outcome by Lipid Levels
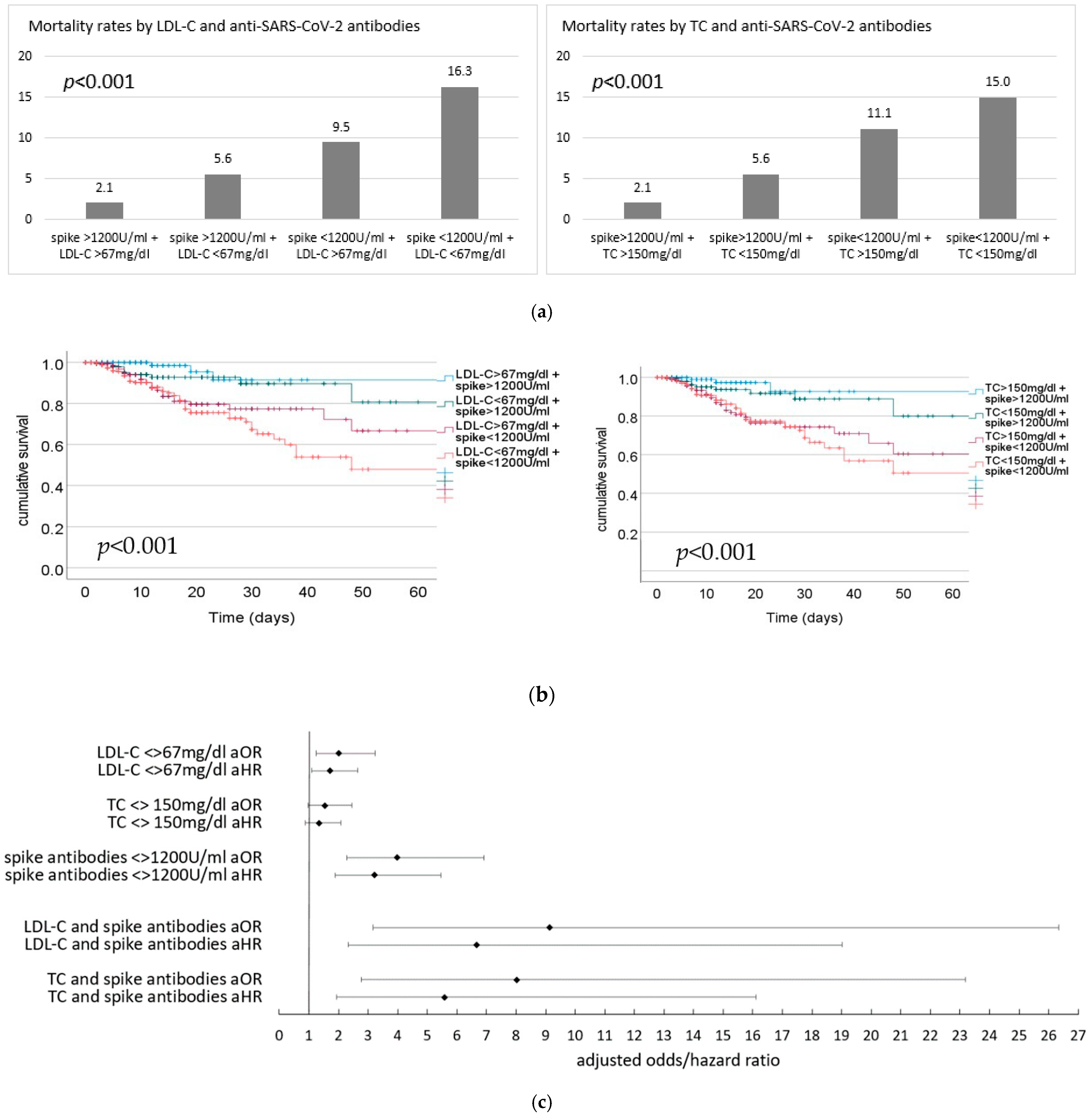
3.3. Risk of Outcome by Lipid Levels and Anti-SARS-CoV-2 Antibodies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| PCR | polymerase chain reaction |
| ICU | intensive care unit |
| OR, aOR, HR | odds ratio, adjusted odds ratio, hazard ratio |
| CI | confidence interval |
| LDL-C | low density lipoprotein |
| HDL-C | high density lipoprotein |
| BMI | body mass index |
| COPD | chronic obstructive pulmonary disease |
| CAD | coronary artery disease |
| TIA | transient ischemic attack |
| CVD | cerebrovascular disease |
References
- Tanaka, S.; Couret, D.; Tran-Dinh, A.; Duranteau, J.; Montravers, P.; Schwendeman, A.; Meilhac, O. High-density lipoproteins during sepsis: From bench to bedside. Crit. Care 2020, 24, 134. [Google Scholar] [CrossRef]
- Trinder, M.; Boyd, J.H.; Brunham, L.R. Molecular regulation of plasma lipid levels during systemic inflammation and sepsis. Curr. Opin. Lipidol. 2019, 30, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Levels, J.H.; Abraham, P.R.; van den Ende, A.; van Deventer, S.J. Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 2001, 69, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Biller, K.; Fae, P.; Germann, R.; Drexel, H.; Walli, A.K.; Fraunberger, P. Cholesterol rather than procalcitonin or C-reactive protein predicts mortality in patients with infection. Shock 2014, 42, 129–132. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef]
- Marsh, M.; Helenius, A. Virus entry: Open sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Bagam, P.; Singh, D.P.; Inda, M.E.; Batra, S. Unraveling the role of membrane microdomains during microbial infections. Cell Biol. Toxicol. 2017, 33, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Taube, S.; Jiang, M.; Wobus, C.E. Glycosphingolipids as receptors for non-enveloped viruses. Viruses 2010, 2, 1011–1049. [Google Scholar] [CrossRef]
- Chen, P.; Wu, M.; He, Y.; Jiang, B.; He, M.-L. Metabolic alterations upon SARS-CoV-2 infection and potential therapeutic targets against coronavirus infection. Signal Transduct. Target Ther. 2023, 8, 237. [Google Scholar] [CrossRef]
- Nagy, P.D.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. Building Viral Replication Organelles: Close Encounters of the Membrane Types. PLoS Pathog. 2016, 12, e1005912. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jericó, C.; Guerrero, C.; Miret, M.; Näf, S.; Pardo, A.; et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Jablonski, S.M.; Jablonski, J.; Hobson, R.; Valente, S.; Reddy, C.B.; Hansen, S.B. The role of high cholesterol in age-related COVID19 lethality. Cold Spring Harbor Laboratory. bioRxiv 2021. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2023, 11, 120–128. [Google Scholar] [CrossRef]
- Durrington, P. Blood lipids after COVID-19 infection. Lancet Diabetes Endocrinol. 2023, 11, 68–69. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Yin, Y.; Chen, W.; Li, X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): A meta-analysis. Virol. J. 2021, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Ceasovschih, A.; Sorodoc, V.; Shor, A.; Haliga, R.E.; Roth, L.; Lionte, C.; Aursulesei, V.O.; Sirbu, O.; Culis, N.; Shapieva, A.; et al. Distinct Features of Vascular Diseases in COVID-19. J. Inflamm. Res. 2023, 16, 2783–2800. [Google Scholar] [CrossRef]
- Chidambaram, V.; Geetha, H.S.; Kumar, A.; Majella, M.G.; Sivakumar, R.K.; Voruganti, D.; Mehta, J.L.; Karakousis, P.C. Association of Lipid Levels With COVID-19 Infection, Disease Severity and Mortality: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 862999. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Fois, A.G.; Solidoro, P.; Carru, C.; Mangoni, A.A. Cholesterol and Triglyceride Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis With Meta-Regression. Front. Public Health 2021, 9, 705916. [Google Scholar] [CrossRef]
- Aparisi, Á.; Martín-Fernández, M.; Ybarra-Falcón, C.; Gil, J.F.; Carrasco-Moraleja, M.; Martínez-Paz, P.; Cusácovich, I.; Gonzalo-Benito, H.; Fuertes, R.; Marcos-Mangas, M.; et al. Dyslipidemia and Inflammation as Hallmarks of Oxidative Stress in COVID-19: A Follow-Up Study. Int. J. Mol. Sci. 2022, 23, 15350. [Google Scholar] [CrossRef] [PubMed]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A.; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Cohen, M.S.; Nirula, A.; Mulligan, M.J.; Novak, R.M.; Marovich, M.; Yen, C.; Stemer, A.; Mayer, S.M.; Wohl, D.; Brengle, B.; et al. Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial. JAMA 2021, 326, 46–55. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhang, X. Neutralizing antibodies for the prevention and treatment of COVID-19. Cell Mol. Immunol. 2021, 18, 2293–2306. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, D.-K.; Lee, J.; Kim, Y.-I.; Seo, J.-M.; Kim, Y.-G.; Jeong, J.-H.; Kim, M.; Kim, J.-I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, 386, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Cossiga, V.; Capasso, M.; Guarino, M.; Loperto, I.; Brusa, S.; Cutolo, F.M.; Attanasio, M.R.; Lieto, R.; Portella, G.; Morisco, F. Safety and Immunogenicity of Anti-SARS-CoV-2 Booster Dose in Patients with Chronic Liver Disease. J. Clin. Med. 2023, 12, 2281. [Google Scholar] [CrossRef] [PubMed]
- Valeanu, L.; Andrei, S.; Morosanu, B.; Longrois, D.; Bubenek-Turconi, S.-I.; Covati-Ro, C. The COVID-19 Vaccination Coverage in ICU Patients with Severe COVID-19 Infection in a Country with Low Vaccination Coverage-A National Retrospective Analysis. J. Clin. Med. 2023, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; List, W.; Hoefle, G.; Frick, M.; Suessenbacher, A.; Winder, T.; Fetz, C.; Boesl, A.; Saely, C.H.; Drexel, H.; et al. Evaluation of SARS-CoV-2 antibody levels on hospital admission as a correlate of protection against mortality. J. Intern. Med. 2023, 293, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.H.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; de Angelis, M.H.; et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef] [PubMed]
- Drexel, H.; Rosano, G.M.C.; Lewis, B.S.; Huber, K.; Vonbank, A.; Dopheide, J.F.; Mader, A.; Niessner, A.; Savarese, G.; Wassmann, S.; et al. The age of randomized clinical trials: Three important aspects of randomized clinical trials in cardiovascular pharmacotherapy with examples from lipid and diabetes trials. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 97–103. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef]
| Whole Cohort n = 1152 | LDL-C <67 mg/dL n = 525 | LDL-C >67 mg/dL n = 520 | p-Value | TC <150 mg/dL n = 520 | TC >150 mg/dL n = 526 | p-Value | |
|---|---|---|---|---|---|---|---|
| age (years) | 66.8 ± 20.3 | 67.7 ± 19.8 | 66.0 ± 19.9 | 0.116 | 68.2 ± 19.1 | 65.5 ± 20.5 | 0.038 |
| male gender (%) | 53.2 | 56.6 | 49.6 | 0.024 | 44.5 | 61.9 | <0.001 |
| BMI (kg/m2) | 27.1 ± 6.5 | 27.7 ± 6.9 | 26.6 ± 5.8 | 0.012 | 27.4 ± 6.7 | 26.8 ± 6.1 | 0.135 |
| DM (%) | 23.9 | 30.5 | 17.3 | <0.001 | 29.2 | 18.6 | <0.001 |
| hypertension (%) | 50.5 | 55.0 | 45.8 | 0.003 | 56.0 | 44.9 | <0.001 |
| CAD (%) | 21.6 | 25.5 | 16.3 | <0.001 | 26.9 | 15.0 | <0.001 |
| heart failure (%) | 7.2 | 8.0 | 6.0 | 0.196 | 7.7 | 6.3 | 0.368 |
| COPD (%) | 9.6 | 11.2 | 8.3 | 0.106 | 10.8 | 8.7 | 0.270 |
| asthma (%) | 2.4 | 2.3 | 2.7 | 0.673 | 2.3 | 2.7 | 0.713 |
| renal disease (%) | 22.9 | 27.7 | 18.6 | <0.001 | 27.2 | 19.1 | 0.002 |
| stroke/TIA/CVD (%) | 11.7 | 15.2 | 8.8 | 0.002 | 16.5 | 7.6 | <0.001 |
| mortality (%) | 10.2 | 12.6 | 6.7 | <0.001 | 11.7 | 7.6 | 0.024 |
| CT value | 21.3 ± 6.6 | 21.3 ± 6.5 | 21.2 ± 6.5 | 0.652 | 21.5 ± 6.6 | 21.0 ± 6.5 | 0.224 |
| total cholesterol | 155.6 ± 50.3 | 120.2 ± 2.2 | 191.4 ± 42.3 | <0.001 | 116.9 ± 22.7 | 193.9 ± 39.7 | <0.001 |
| LDL-C | 71.0 ± 37.0 | 42.7 ± 15.6 | 99.5 ± 29.6 | <0.001 | 44.9 ± 18.1 | 96.8 ± 32.4 | <0.001 |
| HDL-C | 37.7 ± 14.8 | 34.2 ± 13.2 | 41.4 ± 15.4 | <0.001 | 32.8 ± 11.3 | 42.7 ± 16.1 | <0.001 |
| triglycerides | 139.8 ± 83.8 | 132.7 ± 80.6 | 146.9 ± 86.5 | <0.001 | 119.0 ± 51.7 | 160.3 ± 103 | <0.001 |
| statin therapy (%) | 25.7 | 36.0 | 16.5 | <0.001 | 35.4 | 17.3 | <0.001 |
| OR, 95%CI | p-Value | aOR, 95%CI | p-Value | OR z-Scores, 95%CI | p-Value | aOR z-Scores, 95%CI | p-Value | |
|---|---|---|---|---|---|---|---|---|
| LDL-C | LDL-C | |||||||
| mortality | 1.16, 1.08–1.24 | <0.001 | 1.16, 1.07–1.25 | <0.001 | 1.73, 1.34–2.23 | <0.001 | 1.73, 1.30–2.31 | <0.001 |
| mortality, HR | 1.04, 1.02–1.06 | <0.001 | 1.04, 1.02–1.06 | <0.001 | 1.16, 1.09–1.23 | <0.001 | 1.15, 1.08–1.23 | <0.001 |
| HDL-C | HDL-C | |||||||
| mortality | 1.23, 1.05–1.44 | 0.012 | 1.28, 1.07–1.53 | 0.008 | 1.35, 1.07–1.71 | 0.012 | 1.44, 1.10–1.88 | 0.008 |
| mortality, HR | 1.11, 1.06–1.16 | <0.001 | 1.12, 1.07–1.17 | <0.001 | 1.16, 1.09–1.24 | <0.001 | 1.18, 1.10–1.26 | <0.001 |
| total cholesterol | total cholesterol | |||||||
| mortality | 1.09, 1.04–1.14 | <0.001 | 1.08, 1.03–1.14 | 0.003 | 1.51, 1.20–1.92 | <0.001 | 1.49, 1.14–1.94 | 0.003 |
| mortality, HR | 1.03, 1.02–1.04 | <0.001 | 1.03, 1.01–1.04 | <0.001 | 1.15, 1.09–1.23 | <0.001 | 1.14, 1.07–1.22 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mink, S.; Saely, C.H.; Frick, M.; Leiherer, A.; Drexel, H.; Fraunberger, P. Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5068. https://doi.org/10.3390/jcm12155068
Mink S, Saely CH, Frick M, Leiherer A, Drexel H, Fraunberger P. Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(15):5068. https://doi.org/10.3390/jcm12155068
Chicago/Turabian StyleMink, Sylvia, Christoph H. Saely, Matthias Frick, Andreas Leiherer, Heinz Drexel, and Peter Fraunberger. 2023. "Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study" Journal of Clinical Medicine 12, no. 15: 5068. https://doi.org/10.3390/jcm12155068
APA StyleMink, S., Saely, C. H., Frick, M., Leiherer, A., Drexel, H., & Fraunberger, P. (2023). Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study. Journal of Clinical Medicine, 12(15), 5068. https://doi.org/10.3390/jcm12155068





