Debridement, Antibiotics and Implant Retention: A Systematic Review of Strategies for Treatment of Early Infections after Revision Total Knee Arthroplasty
Abstract
1. Introduction
2. Methods
2.1. Data Source and Search
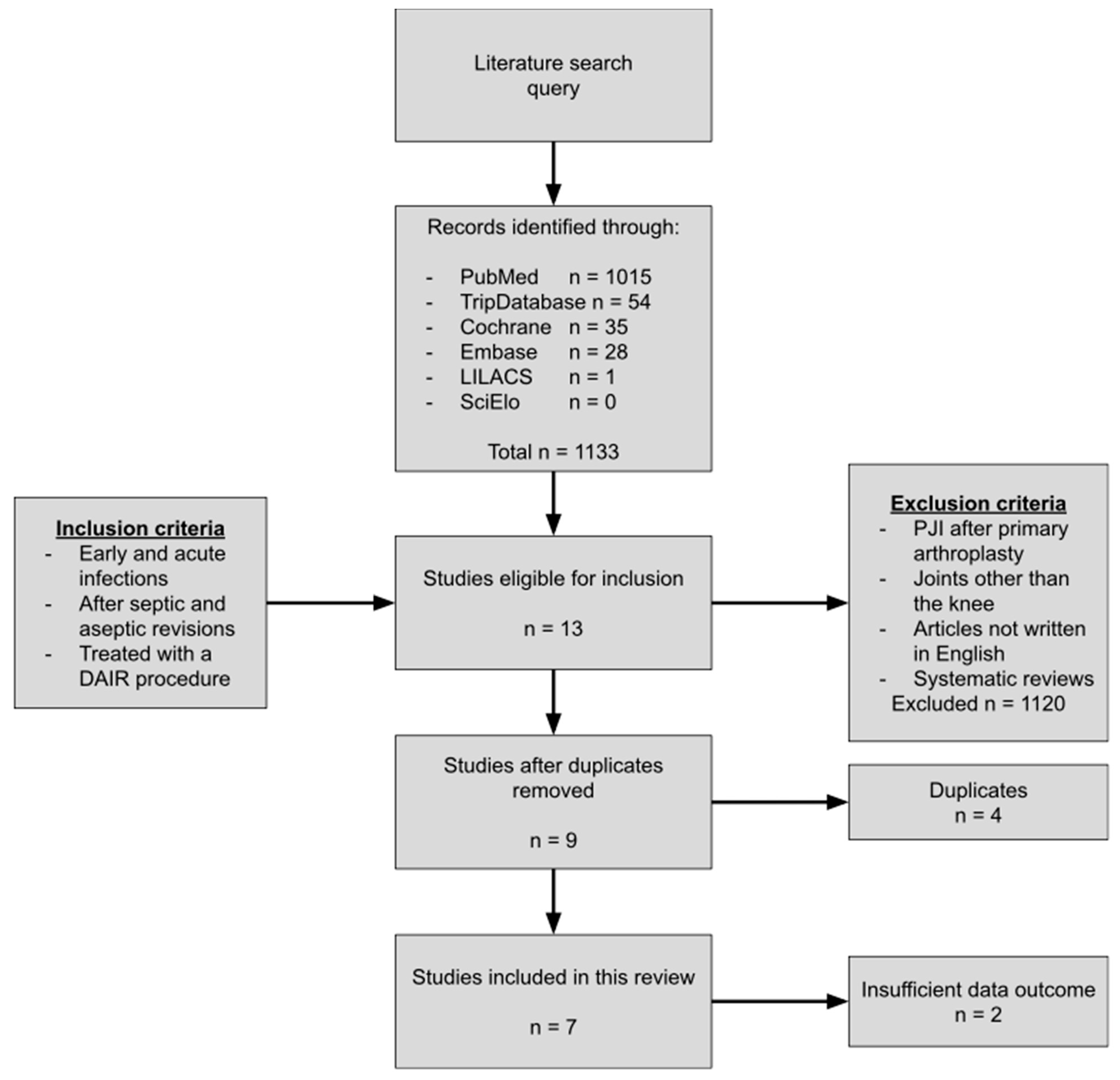
2.2. Study Selection
2.3. Definitions of Infection
3. Results
3.1. The DAIR Procedure
3.2. DAIR as Treatment for Early and Acute Infections after Revision Total Knee Arthroplasty
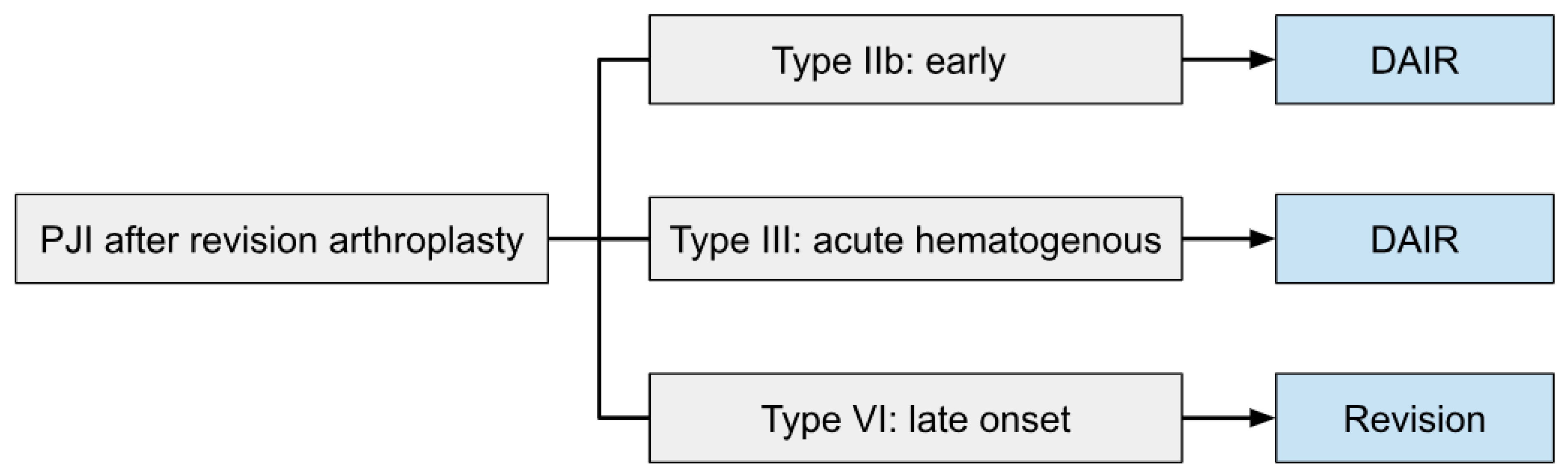
3.3. Proposed Treatment Algorithm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; de Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.; Beswick, A.; Toms, A.D.; Porter, M.L.; Blom, A.W.; National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: An analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open 2017, 7, e014056. [Google Scholar] [CrossRef]
- Goud, A.L.; Harlianto, N.I.; Ezzafzafi, S.; Veltman, E.S.; Bekkers, J.E.J.; van der Wal, B.C.H. Reinfection rates after one- and two-stage revision surgery for hip and knee arthroplasty: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2023, 143, 829–838. [Google Scholar] [CrossRef]
- Rajgor, H.; Dong, H.; Nandra, R.; Parry, M.; Stevenson, J.; Jeys, L. Repeat revision TKR for failed management of peri-prosthetic infection has long-term success but often require multiple operations: A case control study. Arch. Orthop. Trauma Surg. 2023, 143, 987–994. [Google Scholar] [CrossRef]
- Haddad, F.S.; Ngu, A.; Negus, J.J. Prosthetic Joint Infections and Cost Analysis? Adv. Exp. Med. Biol. 2017, 971, 93–100. [Google Scholar] [CrossRef]
- Pravizi, J.; Zmistowski, B. A Quarter of Patients Treated for PJI Dead within 5 Years. December 2012. Available online: https://www.healio.com/news/orthopedics/20130104/a-quarter-of-patients-treated-for-pji-dead-within-5-years (accessed on 7 March 2023).
- Sandifrod, N.; Frencescini, M.; Kendoff, D. The Burden of Prosthetic Joint Infection (PJI). July 2021. Available online: https://aoj.amegroups.com/article/view/6209/html#B5 (accessed on 7 March 2023).
- Tsukayama, D.T.; Goldberg, V.M.; Kyle, R. Diagnosis and management of infection after total knee arthroplasty. J. Bone Jt. Surg. 2003, 85 (Suppl. 1), S75–S80. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- de Vries, L.; van der Weegen, W.; Neve, W.; Das, H.; Ridwan, B.; Steens, J. The Effectiveness of Debridement, Antibiotics and Irrigation for Periprosthetic Joint Infections after Primary Hip and Knee Arthroplasty. A 15 Years Retrospective Study in Two Community Hospitals in the Netherlands. J. Bone Jt. Infect. 2016, 1, 20–24. [Google Scholar] [CrossRef]
- Barros, L.H.; Barbosa, T.A.; Esteves, J.; Abreu, M.A.; Soares, D.; Sousa, R. Early Debridement, antibiotics and implant retention (DAIR) in patients with suspected acute infection after hip or knee arthroplasty—Safe, effective and without negative functional impact. J. Bone Jt. Infect. 2019, 4, 300–305. [Google Scholar] [CrossRef]
- Iza, K.; Foruria, X.; Moreta, J.; Uriarte, I.; Loroño, A.; Aguirre, U.; Mozos, J.L.M.d.L. DAIR (Debridement, Antibiotics and Implant Retention) less effective in hematogenous total knee arthroplasty infections. J. Orthop. Surg. Res. 2019, 14, 278. [Google Scholar] [CrossRef]
- Gerritsen, M.; Khawar, A.; Scheper, H.; van der Wal, R.; Schoones, J.; de Boer, M.; Nelissen, R.; Pijls, B. Modular component exchange and outcome of DAIR for hip and knee periprosthetic joint infection: A systematic review and meta-regression analysis. Bone Jt. Open 2021, 2, 806–812. [Google Scholar] [CrossRef]
- Veerman, K.; Raessens, J.; Telgt, D.; Smulders, K.; Goosen, J.H.M. Debridement, antibiotics, and implant retention after revision arthroplasty: Antibiotic mismatch, timing, and repeated DAIR associated with poor outcome. Bone Jt. J. 2022, 104-B, 464–471. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Faschingbauer, M.; Boettner, F.; Bieger, R.; Weiner, C.; Reichel, H.; Kappe, T. Outcome of Irrigation and Debridement after Failed Two-Stage Reimplantation for Periprosthetic Joint Infection. BioMed Res. Int. 2018, 2018, 2875018. [Google Scholar] [CrossRef]
- Salmons, H.I.; Bettencourt, J.W.; Wyles, C.C.; Osmon, D.R.; Berry, D.J.; Abdel, M.P. Irrigation and Debridement with Chronic Antibiotic Suppression for the Management of Acutely Infected Aseptic Revision Total Joint Arthroplasties. J. Arthroplast. 2023. [Google Scholar] [CrossRef]
- Chiu, F.-Y.; Chen, C.-M. Surgical Débridement and Parenteral Antibiotics in Infected Revision Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2007, 461, 130–135. [Google Scholar] [CrossRef]
- Vahedi, H.; Aali-Rezaie, A.; Shahi, A.; Conway, J.D. Irrigation, Débridement, and Implant Retention for Recurrence of Periprosthetic Joint Infection Following Two-Stage Revision Total Knee Arthroplasty: A Matched Cohort Study. J. Arthroplast. 2019, 34, 1772–1775. [Google Scholar] [CrossRef]
- Bongers, J.; Jacobs, A.M.; Smulders, K.; Van Hellemondt, G.G.; Goosen, J.H. Reinfection and re-revision rates of 113 two-stage revisions in infected TKA. J. Bone Jt. Infect. 2020, 5, 137–144. [Google Scholar] [CrossRef]
- Cochrane, N.H.; Wellman, S.S.; Lachiewicz, P.F. Early Infection After Aseptic Revision Knee Arthroplasty: Prevalence and Predisposing Risk Factors. J. Arthroplast. 2022, 37, S281–S285. [Google Scholar] [CrossRef]
- Argenson, J.N.; Arndt, M.; Babis, G.; Battenberg, A.; Budhiparama, N.; Catani, F.; Chen, F.; de Beaubien, B.; Ebied, A.; Esposito, S.; et al. Hip and Knee Section, Treatment, Debridement and Retention of Implant: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S399–S419. [Google Scholar] [CrossRef]
- Weston, J.T.; Watts, C.D.; Mabry, T.M.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Irrigation and debridement with chronic antibiotic suppression for the management of infected total knee arthroplasty: A Contemporary Analysis. Bone Jt. J. 2018, 100-B, 1471–1476. [Google Scholar] [CrossRef]
- Chung, A.S.; Niesen, M.C.; Graber, T.J.; Schwartz, A.J.; Beauchamp, C.P.; Clarke, H.D.; Spangehl, M.J. Two-Stage Debridement With Prosthesis Retention for Acute Periprosthetic Joint Infections. J. Arthroplast. 2019, 34, 1207–1213. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Löwik, C.A.; Ploegmakers, J.J.; Knobben, B.A.; Dijkstra, B.; de Vries, A.J.; Mithoe, G.; Kampinga, G.; Zijlstra, W.P.; Jutte, P.C.; et al. A Second Surgical Debridement for Acute Periprosthetic Joint Infections Should Not Be Discarded. J. Arthroplast. 2020, 35, 2204–2209. [Google Scholar] [CrossRef]
- Vilchez, F.; Martínez-Pastor, J.; García-Ramiro, S.; Bori, G.; Maculé, F.; Sierra, J.; Font, L.; Mensa, J.; Soriano, A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin. Microbiol. Infect. 2011, 17, 439–444. [Google Scholar] [CrossRef]
- Antonios, J.K.; Bozic, K.J.; Clarke, H.D.; Spangehl, M.J.; Bingham, J.S.; Schwartz, A.J. Cost-effectiveness of Single vs Double Debridement and Implant Retention for Acute Periprosthetic Joint Infections in Total Knee Arthroplasty: A Markov Model. Arthroplast. Today 2021, 11, 187–195. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Deng, W.; Li, R.; Shao, H.; Yu, B.; Chen, J.; Zhou, Y. Comparison of the success rate after debridement, antibiotics and implant retention (DAIR) for periprosthetic joint infection among patients with or without a sinus tract. BMC Musculoskelet. Disord. 2021, 22, 895. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Xu, W. Risk factors affect success rate of debridement, antibiotics and implant retention (DAIR) in periprosthetic joint infection. Arthroplasty 2020, 2, 37. [Google Scholar] [CrossRef]
- Puhto, A.-P.; Puhto, T.; Syrjala, H. Short-course antibiotics for prosthetic joint infections treated with prosthesis retention. Clin. Microbiol. Infect. 2012, 18, 1143–1148. [Google Scholar] [CrossRef][Green Version]
- Bernard, L.; Legout, L.; Zürcher-Pfund, L.; Stern, R.; Rohner, P.; Peter, R.; Assal, M.; Lew, D.; Hoffmeyer, P.; Uçkay, I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J. Infect. 2010, 61, 125–132. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Berbari, E.F.; Suh, G.A.; Lahr, B.D.; Abdel, M.P.; Tande, A.J. Truth in DAIR: Duration of Therapy and the Use of Quinolone/Rifampin-Based Regimens after Debridement and Implant Retention for Periprosthetic Joint Infections. Open Forum Infect. Dis. 2022, 9, ofac363. [Google Scholar] [CrossRef]
- Miller, R.; Higuera, C.A.; Wu, J.; Klika, A.; Babic, M.; Piuzzi, N.S. Periprosthetic Joint Infection: A Review of Antibiotic Treatment. JBJS Rev. 2020, 8, e19.00224. [Google Scholar] [CrossRef]
- Tirumala, V.; Smith, E.; Box, H.; Kieboom, J.v.D.; Klemt, C.; Kwon, Y.-M. Outcome of Debridement, Antibiotics, and Implant Retention with Modular Component Exchange in Acute Culture-Negative Periprosthetic Joint Infections. J. Arthroplast. 2021, 36, 1087–1093. [Google Scholar] [CrossRef]
- van Eck, J.; Liu, W.-Y.; Goosen, J.H.M.; Rijnen, W.H.C.; van der Zwaard, B.C.; Heesterbeek, P.; van der Weegen, W.; The Further Members of Regional Prosthetic Joint Infection Group. Higher 1-year risk of implant removal for culture-positive than for culture-negative DAIR patients following 359 primary hip or knee arthroplasties. J. Bone Jt. Infect. 2022, 7, 143–149. [Google Scholar] [CrossRef]
- Li, F.; Qiao, Y.; Zhang, H.; Cao, G.; Zhou, S. Comparable clinical outcomes of culture-negative and culture-positive periprosthetic joint infections: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 210. [Google Scholar] [CrossRef]
| Surgical Procedure | Chiu et al. [18] | Faschingbauer et al. [16] | Vahedi et al. [19] | Bongers et al. [20] | Cochrane et al. [21] | Veerman et al. [14] | Salomons et al. [17] |
|---|---|---|---|---|---|---|---|
| Opening via pre-existing incision | Yes | Yes | Did not mention | Yes | Did not mention | Yes | Did not mention |
| Synovectomy (taking cultures) | Yes | Yes | Did not mention | Yes | Did not mention | Yes | Did not mention |
| Debridement of infected soft tissue (taking cultures) | Yes | Yes | Yes | Did not mention | Did not mention | Yes | Yes |
| Replacement of modular parts | Yes | Yes | Yes | Yes | Yes | Yes | Did not mention |
| Irrigation of implants | Antibiotic solution using pulsed lavage | 10 L of anti-infectious irrigation | Did not mention | 3 L betadine saline solution and 3 L saline | Did not mention | 6 L of saline using pulsed lavage | 6–9 L of saline, in some cases along with antibiotic and/or betadine solution |
| Autor | Study Design | Study Size | Mean Age (Years) | Reason for Index Revision | Type of (Re-)Infection | Causative Pathogen | Prophylactic/Preoperative Antibiotic Regime | Postoperative Culture-Directed Antibiotic Therapy | Mean Follow-Up (Months) | Overall Success Rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiu 2007 [18] | Prospective | 40 knees | 72.7 (range 59–85) | Aseptic loosening 70% Wear 30% | 25% Early 50% Late (>4 weeks) 25% Acute hematogenous | 13 MRSA 12 CoNS 5 Multiple organisms 2 GBS 2 GGS 1 E. coli | 1 S. epidermidis 1 S. aureus 1 C. parapsilosis 1 C. glabrata 1 P. aeruginosa | Did not mention | Parenteral for 6 weeks | 79 (range 36–143) | 30 | |
| Faschingbauer 2018 [16] | Retrospective | 7 knees | 67.3 (range 45–84) | PJI 100% | Early or acute hematogenous | 7 MRSE 3 E. coli 2 S. aureus 2 Multiple organisms | 1 S. intermedius 1 Enterococcus 1 Enterobacter 1 Culture negative | Successful cases 7 Le, Ri 3 no antibiotics 1 Va, Ti, Ri 1 Fl, Ri | Failed cases 2 no antibiotics 2 Le, Ri 1 Cl, Ri 1 Me | Parenteral, oral or both for 2 weeks | 39 (range 24–90) | 57.1 |
| Vahedi 2019 [19] | Retrospective matched cohort study | 24 knees | 64 (range 43–77) | PJI 100% | Acute | 7 S. aureus 4 S. epidermidis 4 Gram negative | 2 MRSA 2 Multiple organisms 5 Culture negative | Did not mention | Parenteral for 6 weeks + oral for 6 months | 46 (range 29–86) | 71 (50 for second DAIR) | |
| Bongers 2020 [20] | Retrospective | 11 knees | 67 (range 46–86) | PJI 100% | Early (<6 weeks) or acute hematogenous | Did not mention | Before 2-stage revision: 3 g Ce daily until culture results, Va in case of resistance of allergy or antibiotics based on previously cultivated susceptibility | Not specifically given for DAIR | 94 (range 24–172) | 50 | ||
| Cochrane 2021 [21] | Retrospective | 11 knees | 65 (SD 7.3) | Mechanical failure 100% | Early or acute hematogenous | 4 MRSA 2 CoNS 2 Enterobac. | 1 S. aureus 1 Enterococcus 1 P. acnes | Before 2-stage revision: 2 or 3 g of Ce before incision | Not specifically given for DAIR | 46 (SD 34) | 64 | |
| Veerman 2022 [14] | Retrospective | 35 knees | 66 (SD 11) | Mechanical failure 100% | Early or acute hematogenous | 37 Staphylococcus species 18 Gram negative bacilli 17 Multiple organisms 33 Culture negative | One dose of 2 g Ce followed by 3 × 1 g for 5 days or until culture samples are available; Ri was added for susceptible Staphylococcus species | Parenteral for 5 days + oral or iv for 3 months | 24 | 62 (50 for second DAIR) | ||
| Salomons 2023 [17] | Retrospective | 12 knees | 71 (range 41–90) | Aseptic loosening 42% Instability 25% Periprosthetic fracture 17% Arthrofibrosis 17% | Early (8%) or acute hematogenous (92%) | 3 S. aureus 3 S. mitis, S. viridans, S. agalactiae 1 S. aureus + Enterococcus 1 CoNS 4 Culture negative | Did not mention | Parenteral for 3–6 weeks followed by culture directed suppressive antibiotics for the life of the implant | 84 (range 24–180) | 92 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulleman, C.W.J.; de Windt, T.S.; Veerman, K.; Goosen, J.H.M.; Wagenaar, F.-C.B.M.; van Hellemondt, G.G. Debridement, Antibiotics and Implant Retention: A Systematic Review of Strategies for Treatment of Early Infections after Revision Total Knee Arthroplasty. J. Clin. Med. 2023, 12, 5026. https://doi.org/10.3390/jcm12155026
Hulleman CWJ, de Windt TS, Veerman K, Goosen JHM, Wagenaar F-CBM, van Hellemondt GG. Debridement, Antibiotics and Implant Retention: A Systematic Review of Strategies for Treatment of Early Infections after Revision Total Knee Arthroplasty. Journal of Clinical Medicine. 2023; 12(15):5026. https://doi.org/10.3390/jcm12155026
Chicago/Turabian StyleHulleman, Caspar W. J., Tommy S. de Windt, Karin Veerman, Jon H. M. Goosen, Frank-Christiaan B. M. Wagenaar, and Gijs G. van Hellemondt. 2023. "Debridement, Antibiotics and Implant Retention: A Systematic Review of Strategies for Treatment of Early Infections after Revision Total Knee Arthroplasty" Journal of Clinical Medicine 12, no. 15: 5026. https://doi.org/10.3390/jcm12155026
APA StyleHulleman, C. W. J., de Windt, T. S., Veerman, K., Goosen, J. H. M., Wagenaar, F.-C. B. M., & van Hellemondt, G. G. (2023). Debridement, Antibiotics and Implant Retention: A Systematic Review of Strategies for Treatment of Early Infections after Revision Total Knee Arthroplasty. Journal of Clinical Medicine, 12(15), 5026. https://doi.org/10.3390/jcm12155026






