CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration
Abstract
1. Introduction
2. Materials and Methods
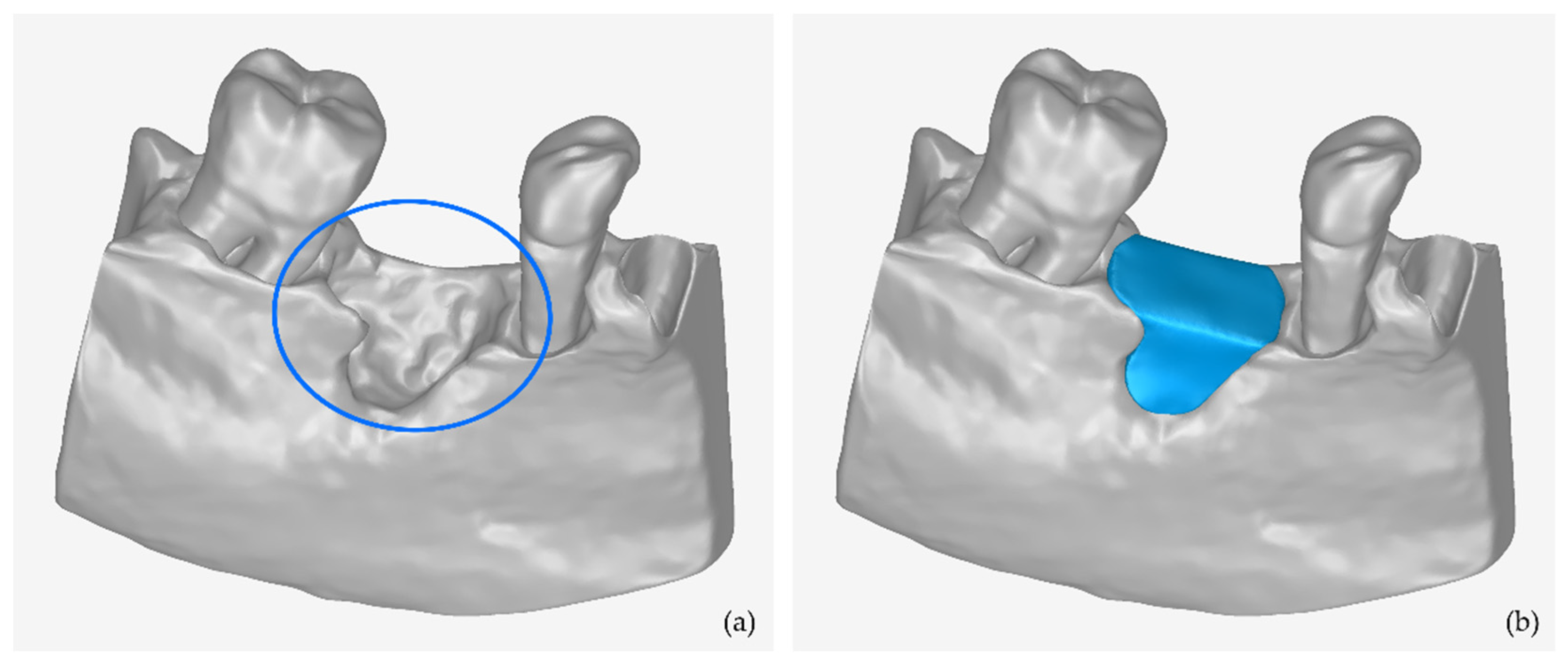
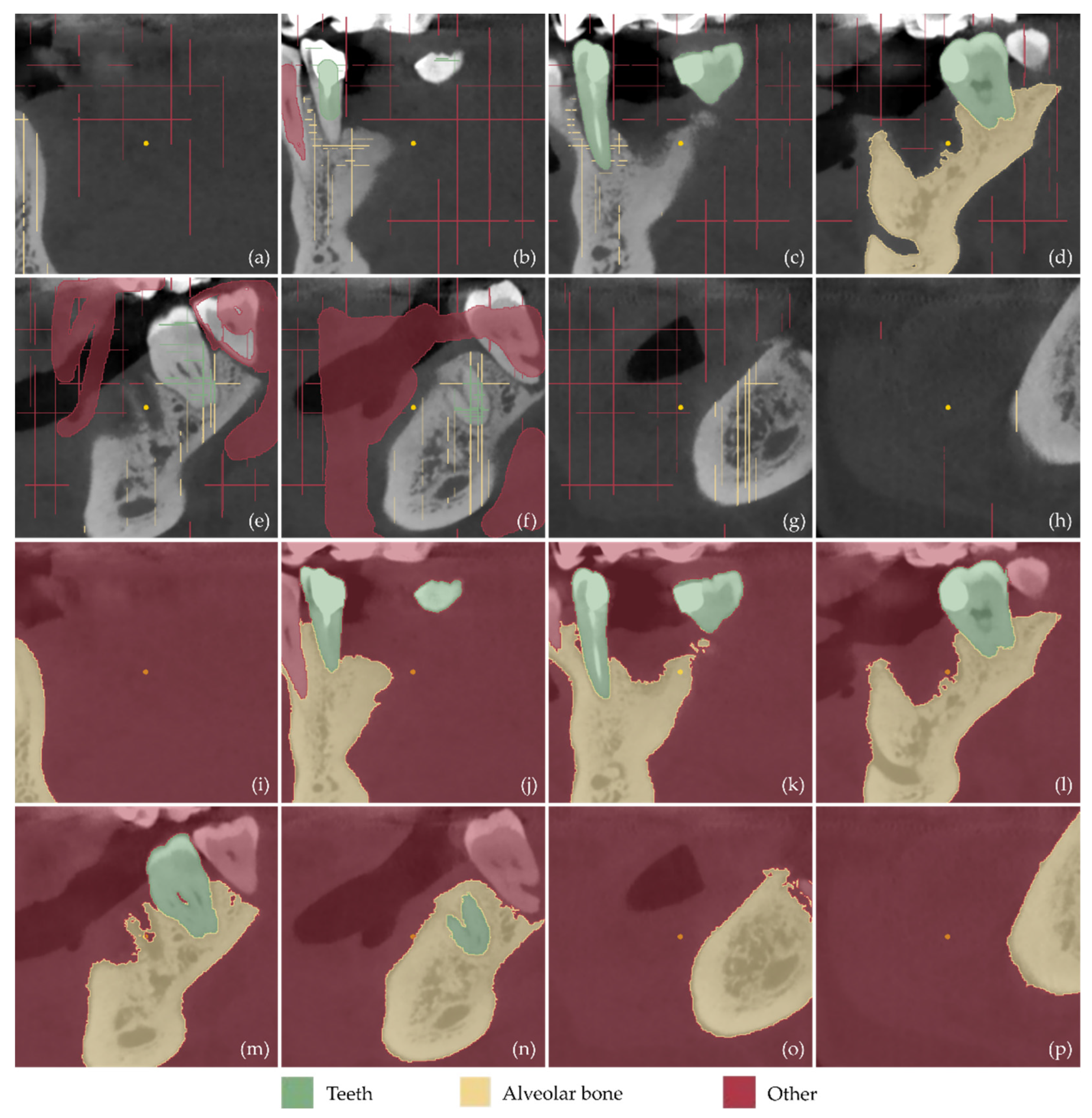
2.1. 3D Modeling of Periodontal Defects
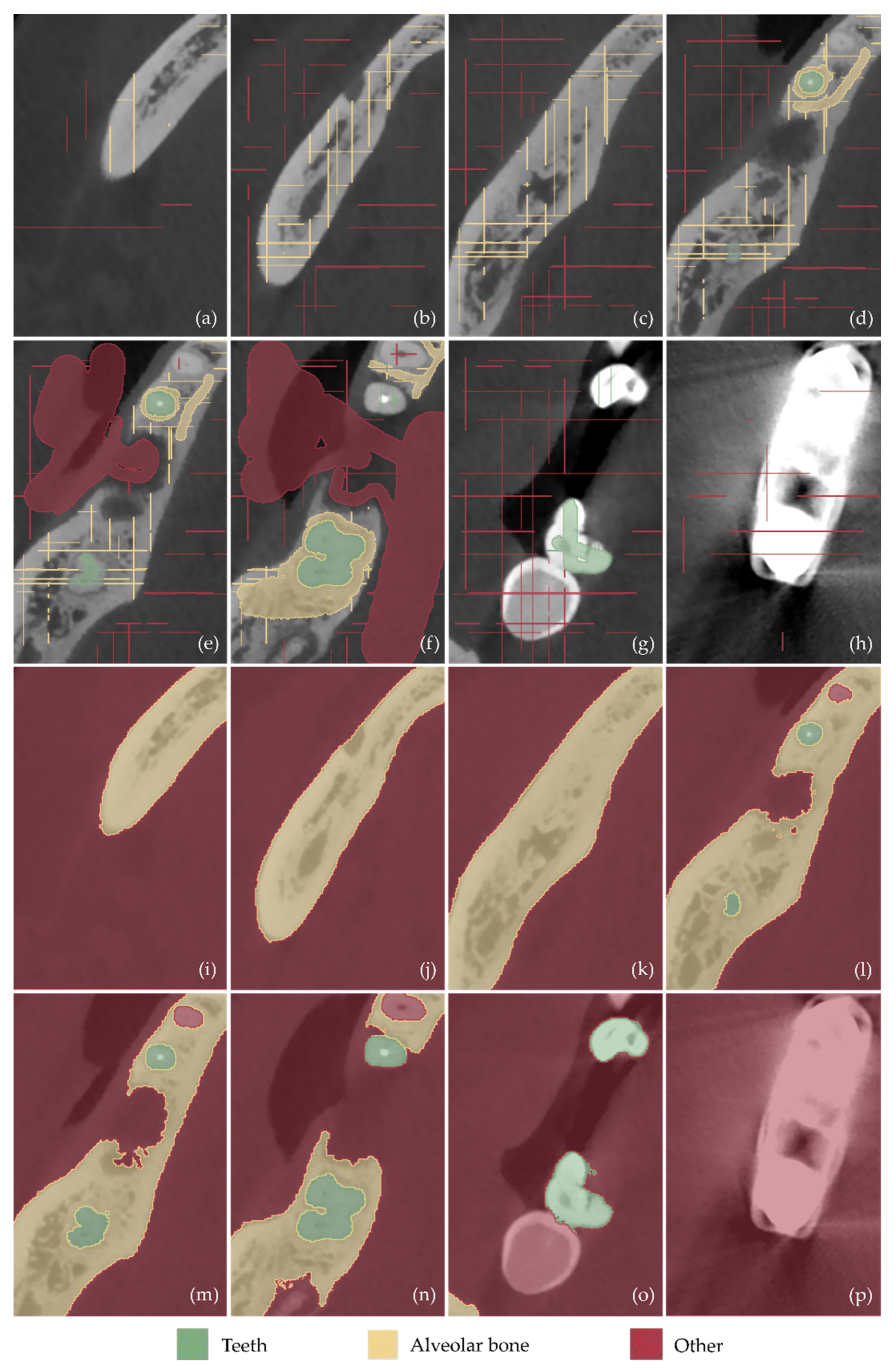
- Definition of the region of interest by cropping the corresponding volume defined in the CBCT dataset.
- Definition of three segments: Teeth, alveolar bone, and “other”, including all the other areas of the oral system, depicted in the CBCT cropped images.
- Definition of samples for the aforementioned segments for a small subset of CBCT images (e.g., 30 images: 10 per reference plane), through a combination of automatic (thresholding) and manual procedures.
- Initialization of the “Grow from seeds” method [52].
- Iterative correction and update of the result of the “Grow from seeds” method until the visual inspection of the result satisfies the user.
- Conversion of the segmentation results for the teeth and alveolar bone segments into 3D models (meshes) in STL format.
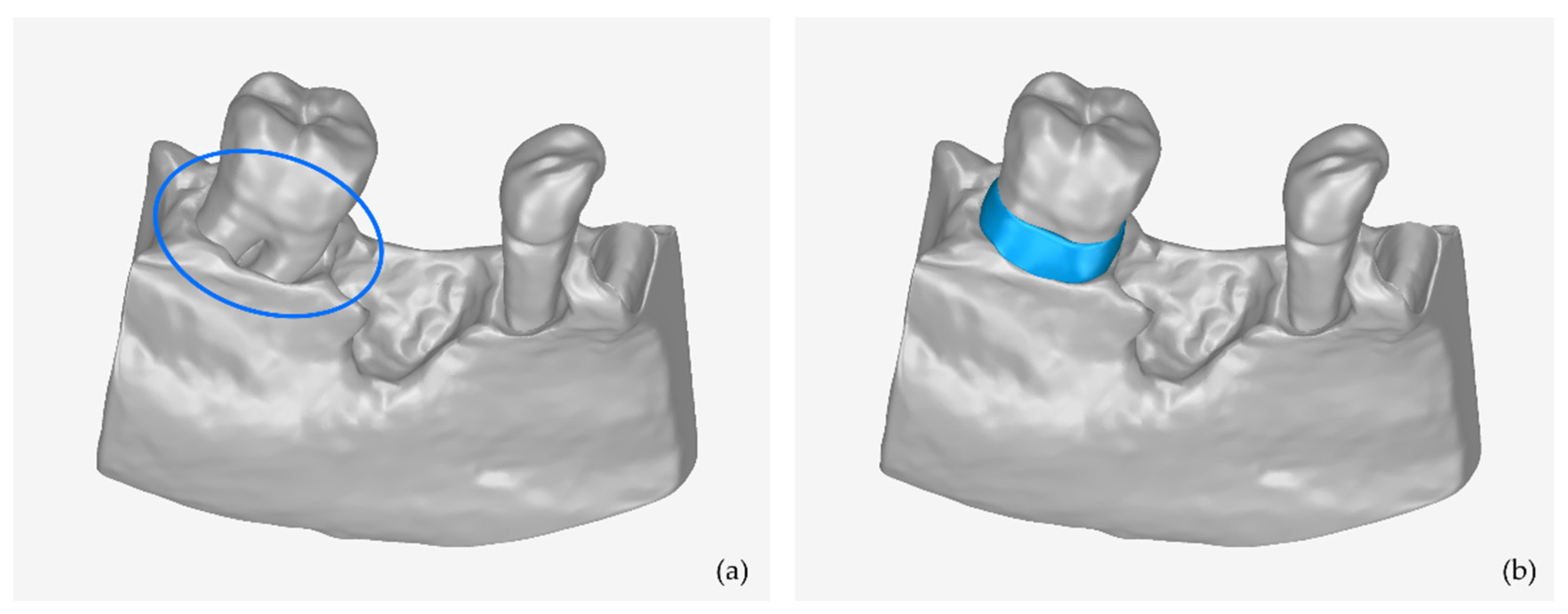
- Editing of the 3D models of teeth and alveolar bone, including, indicatively, the conversion of each 3D mesh to a point cloud; the editing of the point cloud; the creation of a 3D mesh (surface) using the edited point cloud; and the optional merging of the two 3D models into a single 3D model and storage in STL format.
2.2. Design of Periodontal Defect Customized Block Grafts
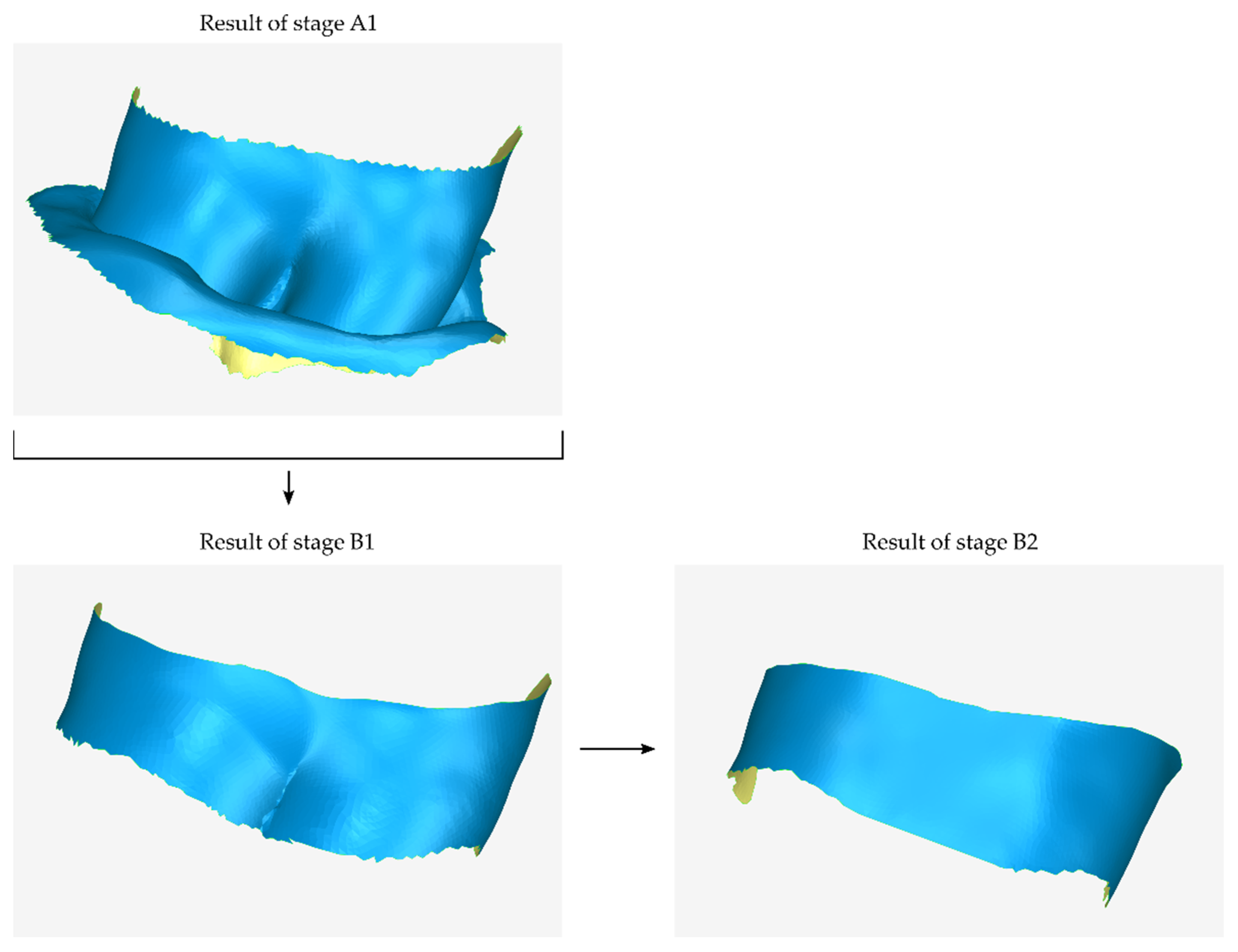
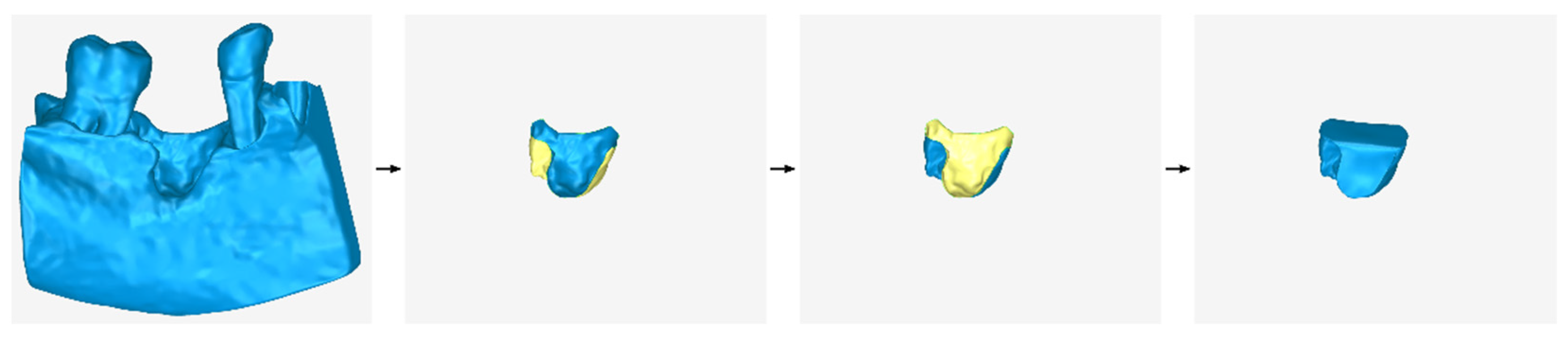
2.2.1. Stage A: Design of an Initial Approximation of the Scaffold Internal Surface
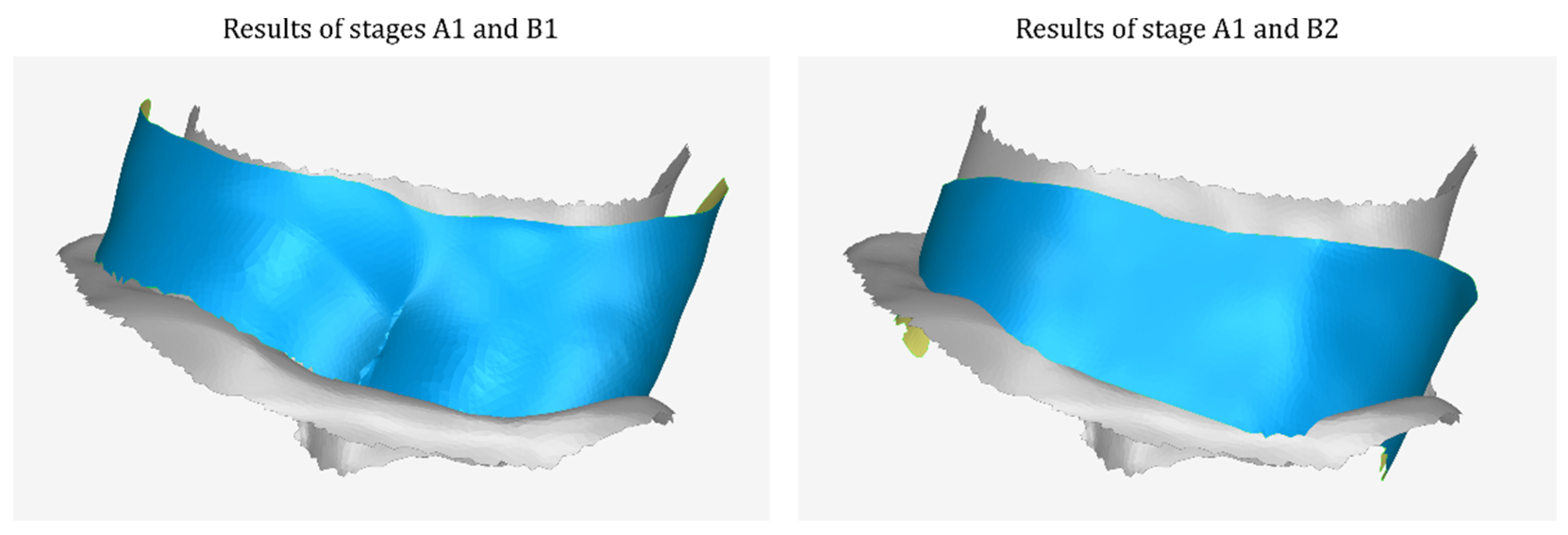
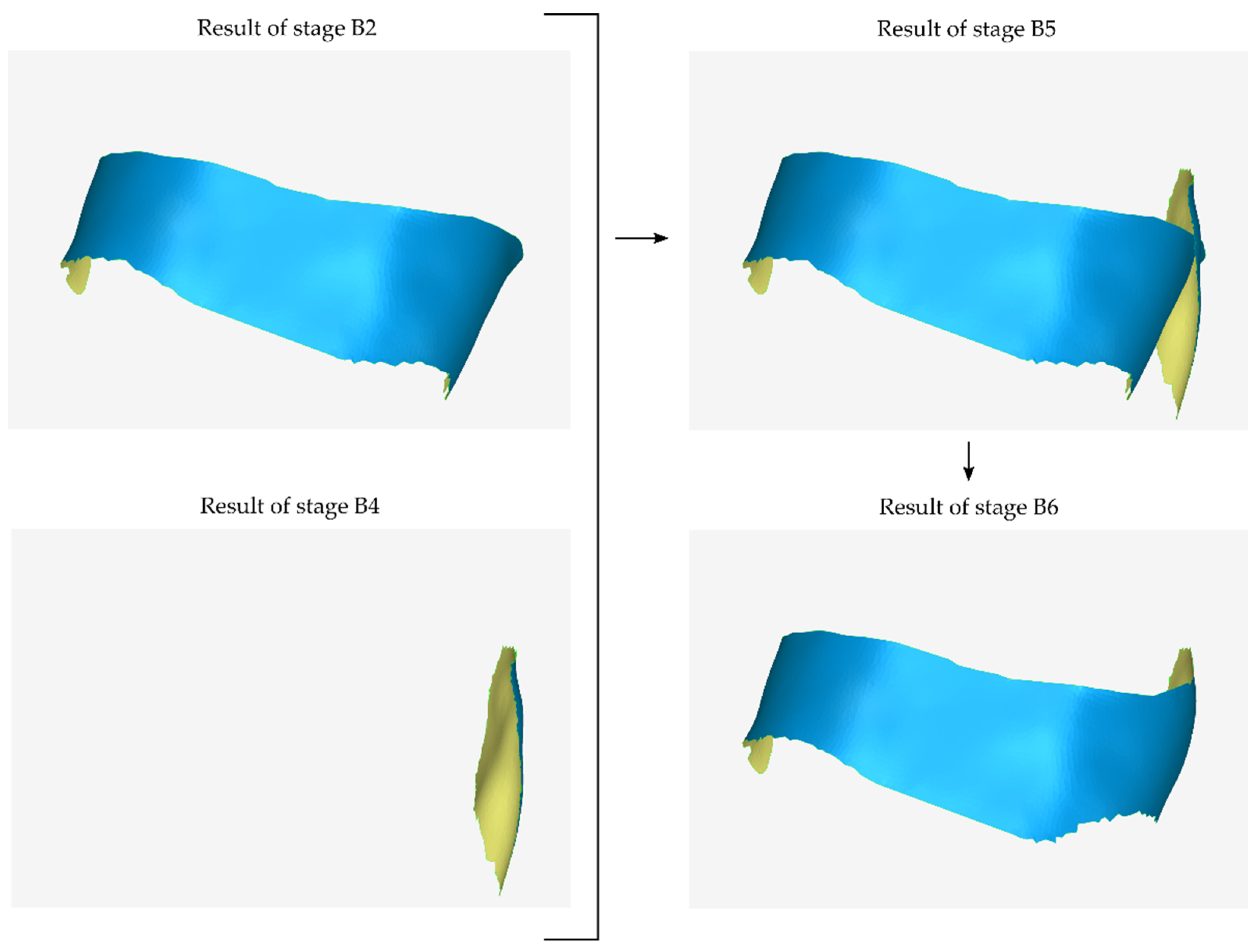
2.2.2. Stage B: Design of the Scaffold Outer Surface
- Copying the model that has been cut from the original 3D model of the part of the alveolar bone and the teeth in which the periodontal defect is concentrated (model that is the result of stage A1).
- Cutting the above model, so that its new surface covers approximately the area that will be occupied by the outer surface of the scaffold.
- Moving the above model by the intended distance according to the desired average thickness of the scaffold (e.g., 1 mm).
- Processing the above model (e.g., further cutting, filling gaps, etc.).
- The model that is the final result of stage B1—after further processing in subsequent steps of stage B—will constitute the outer surface of the scaffold.
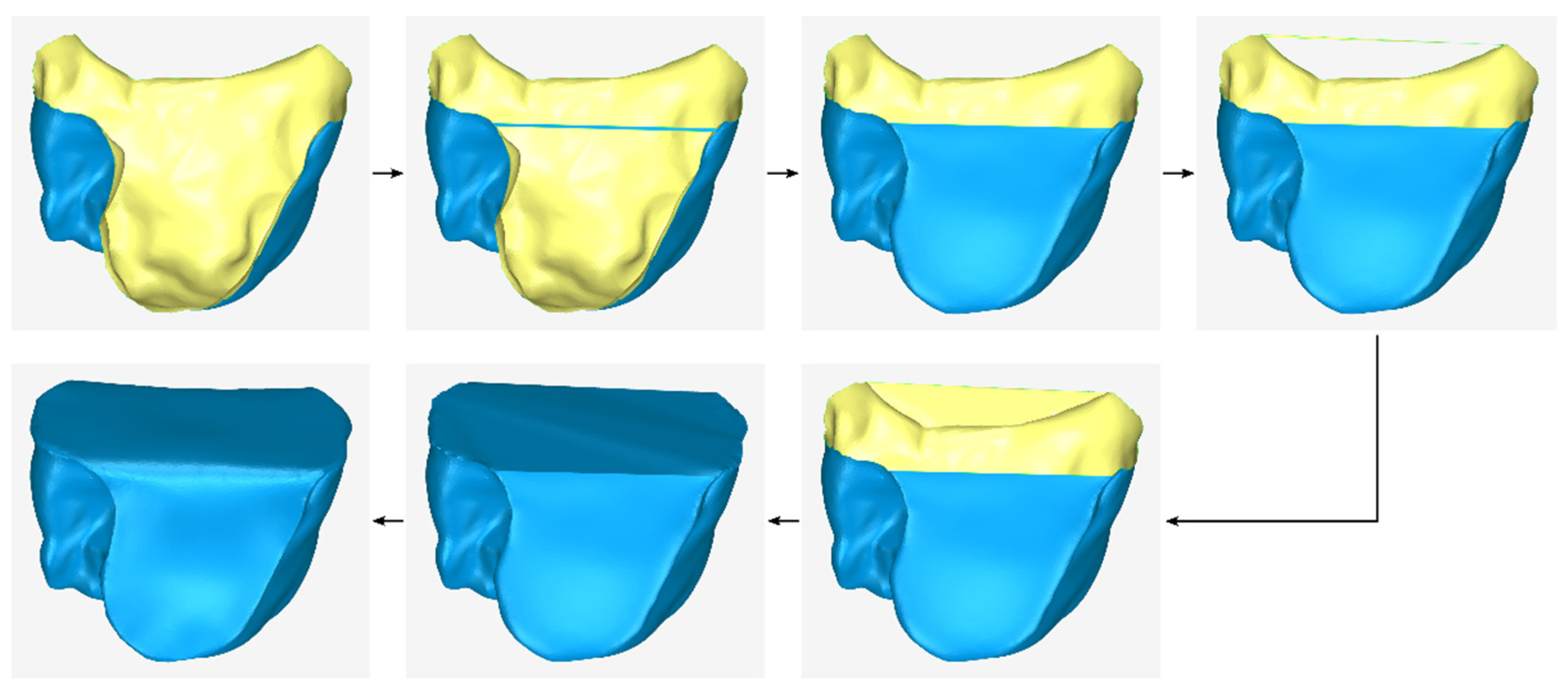
- Copying of the model that has undergone a cutting from the original 3D model of the periodontal defect (model that is the result of stage A1).
- Cutting of the above model, so that its new surface covers approximately the area that will be occupied by the lateral outer surface of the scaffold that is missing from the previous outer surface (i.e., from the result of stage B2) and is approximately parallel to the latter.
- Translation of the above model by the intended distance according to the desired average thickness of the scaffold.
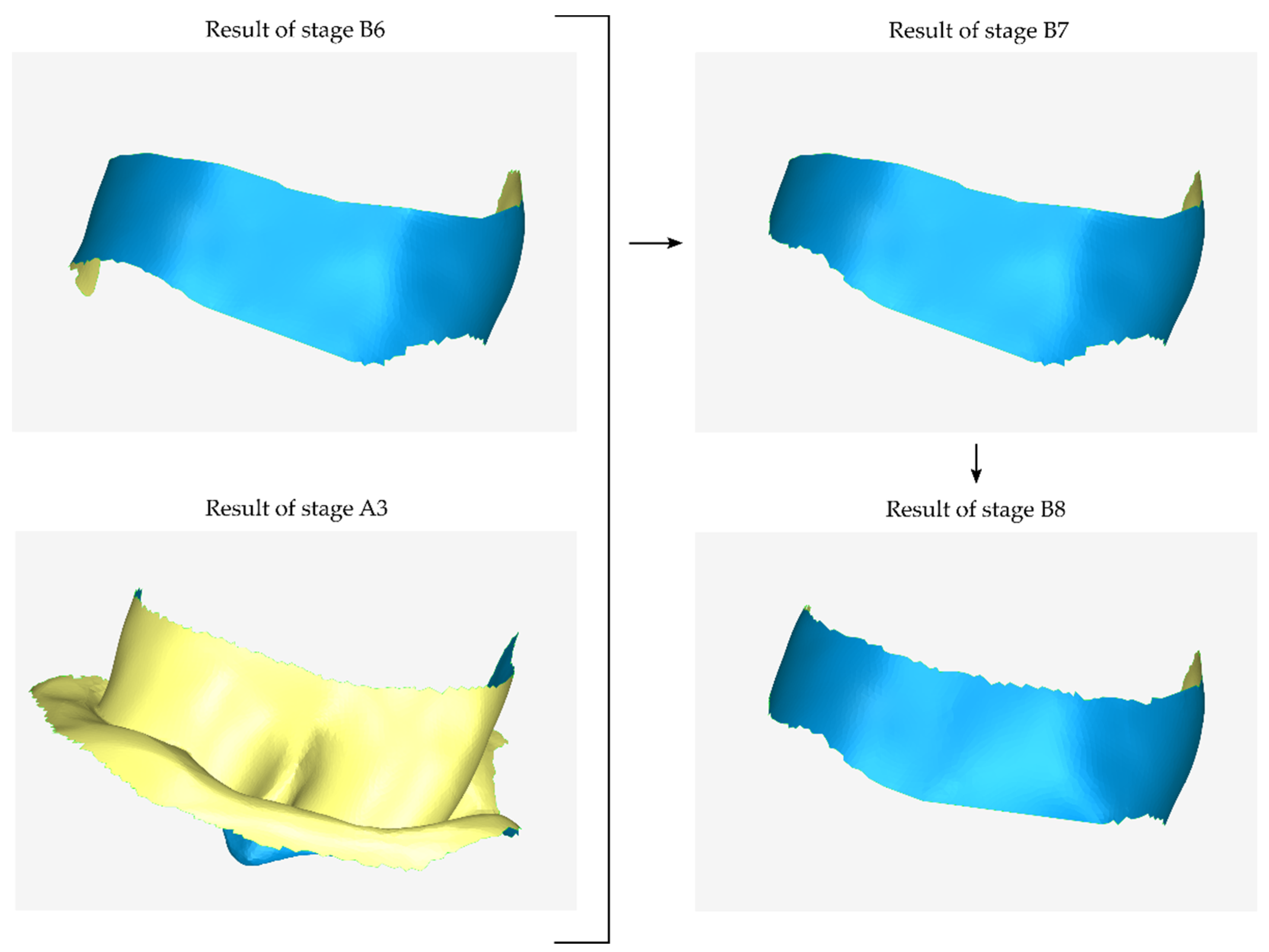
- Duplication of the model, which is the last approximation of the external surface of the scaffold (the result of stage B7).
- Cutting of the above model, so that its surface covers approximately the upper part of the missing surface.
- Translation of the above model by the intended distance upwards (to cover the upper part of the scaffold surface, if it is a mandibular scaffold) or downwards (to cover the lower part of the scaffold surface, if it is a maxillary scaffold).
- Merging of the above model with the model that is the last approximation of the outer surface of the scaffold (the result of stage B7).
- Processing of the merged model, which may include gap filling, smoothing, etc.
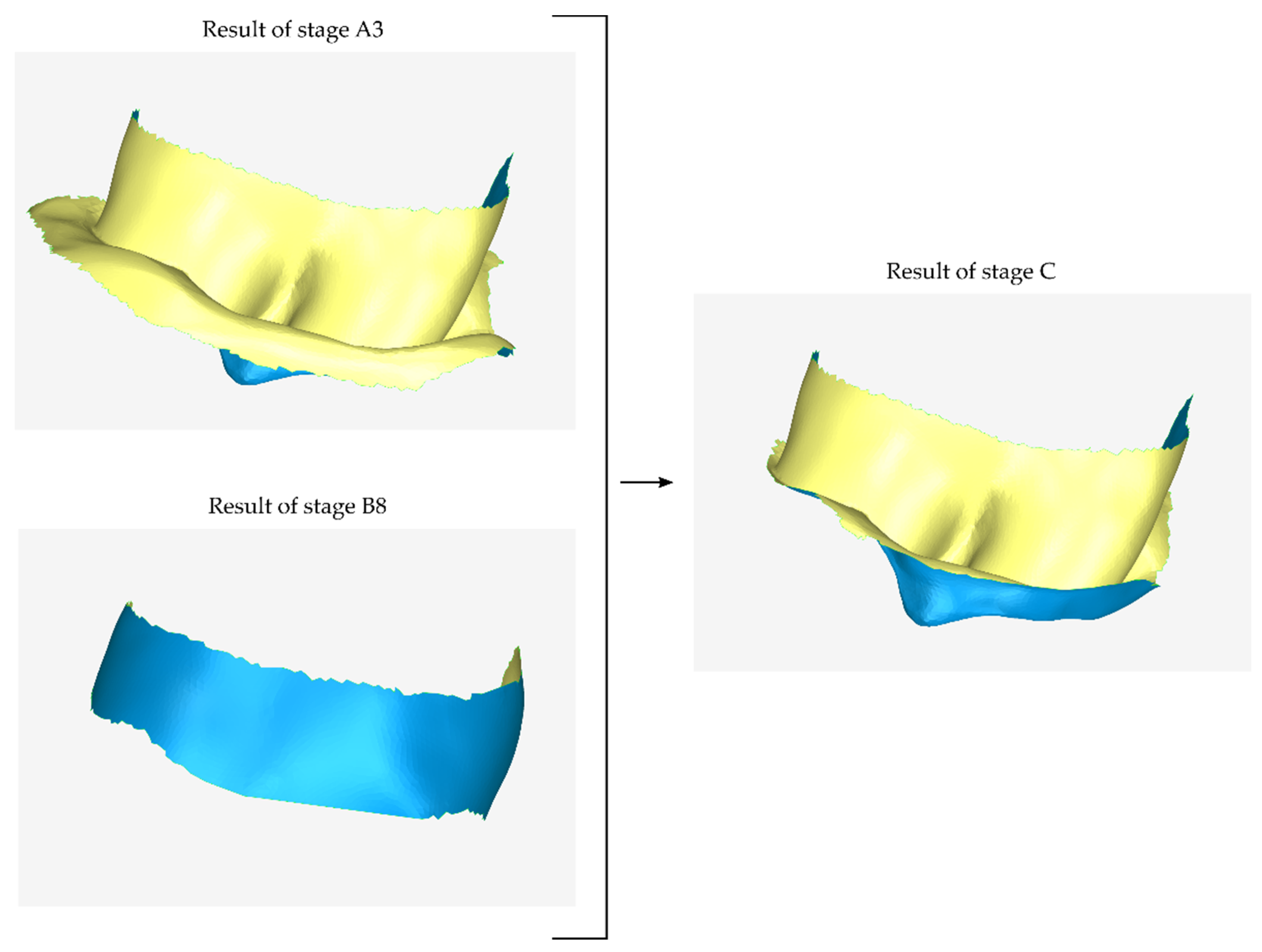
2.2.3. Stage C: Design of a Final Approximation of the Scaffold Internal Surface
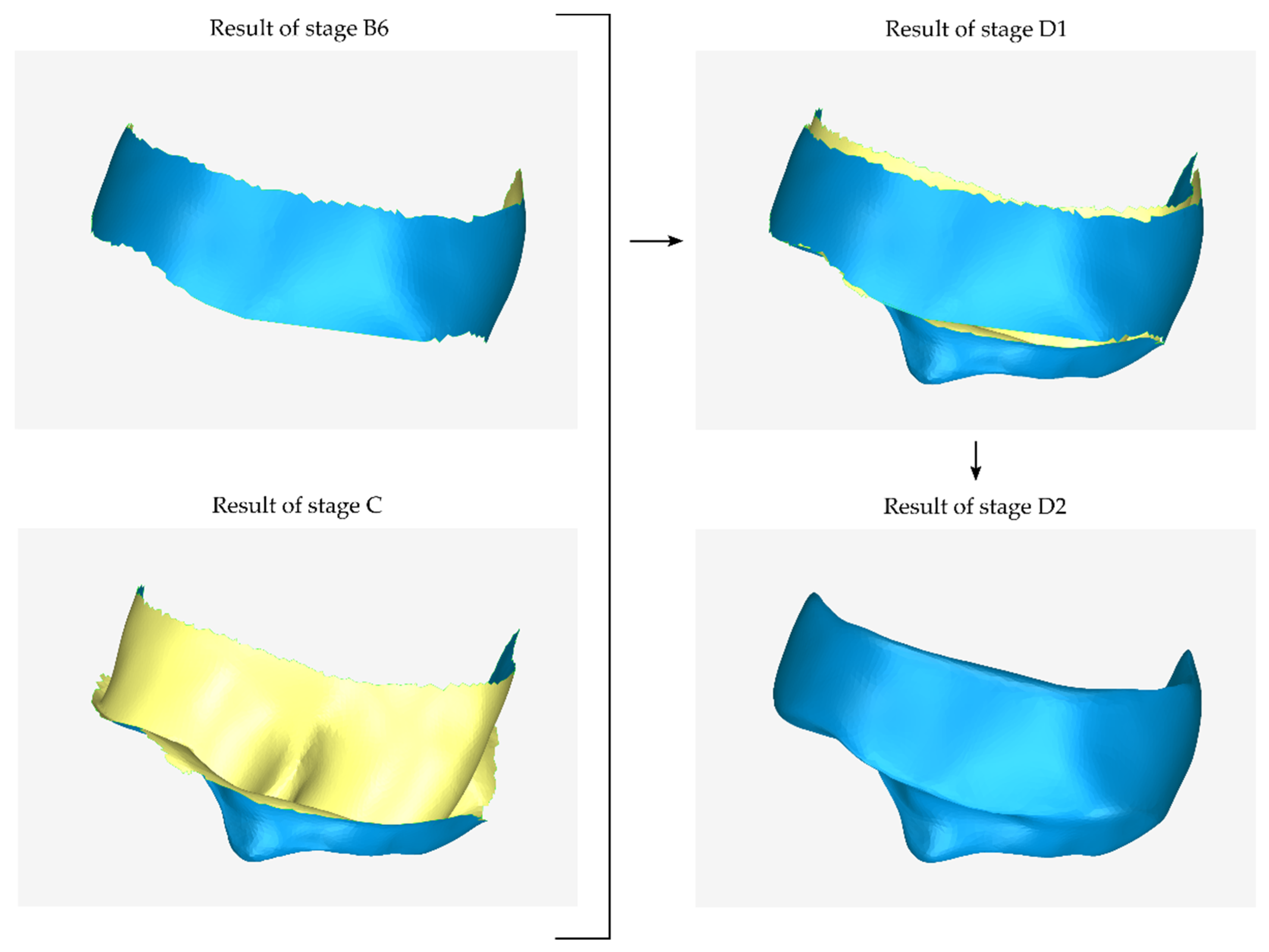
2.2.4. Stage D: Design of the Final Scaffold
2.3. Design of an Extraction Socket Preservation Customized Graft
2.3.1. Stage A: Design of the Scaffold Internal Surface
2.3.2. Stage B: Design of Final Scaffold
3. Results
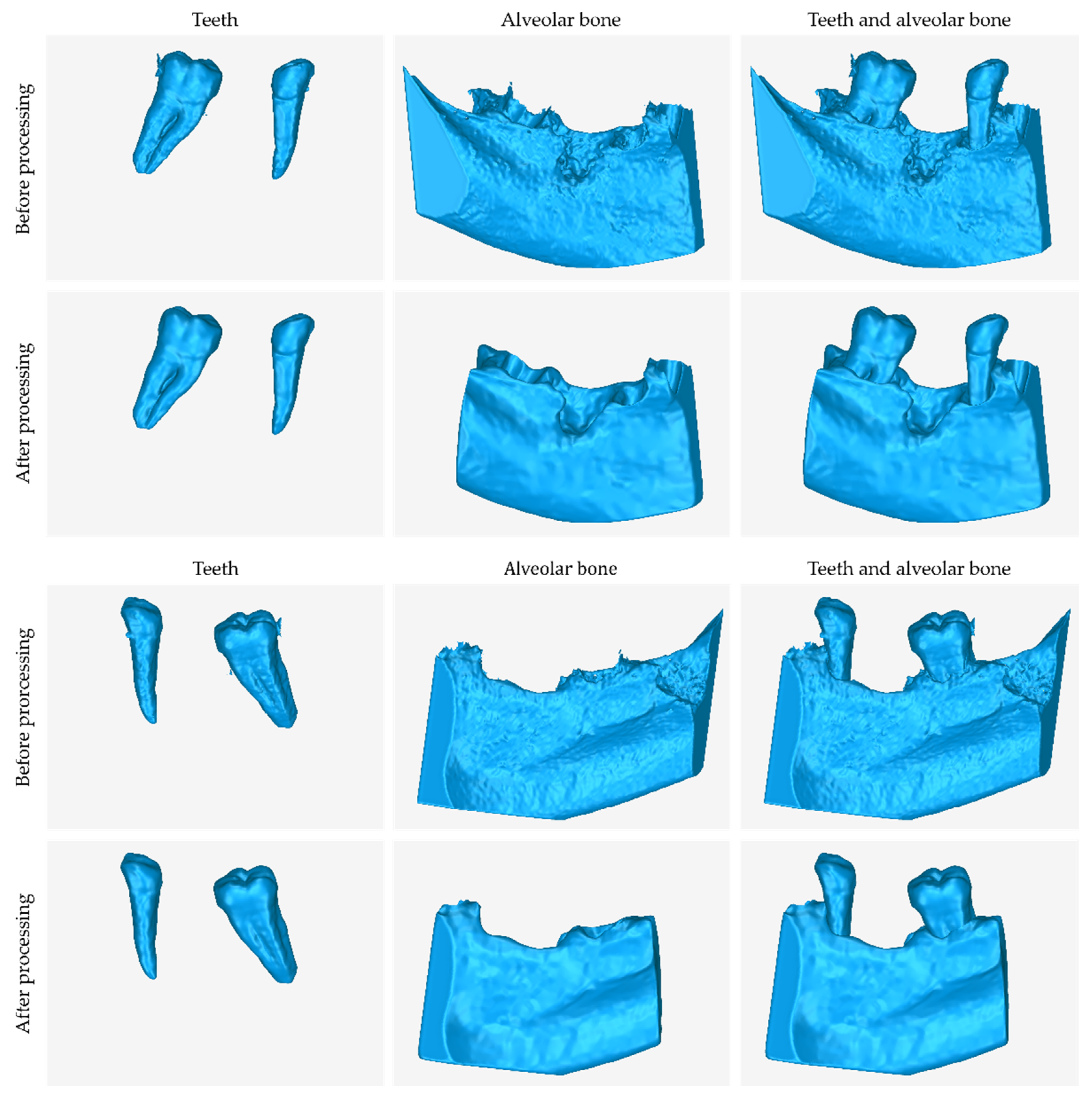
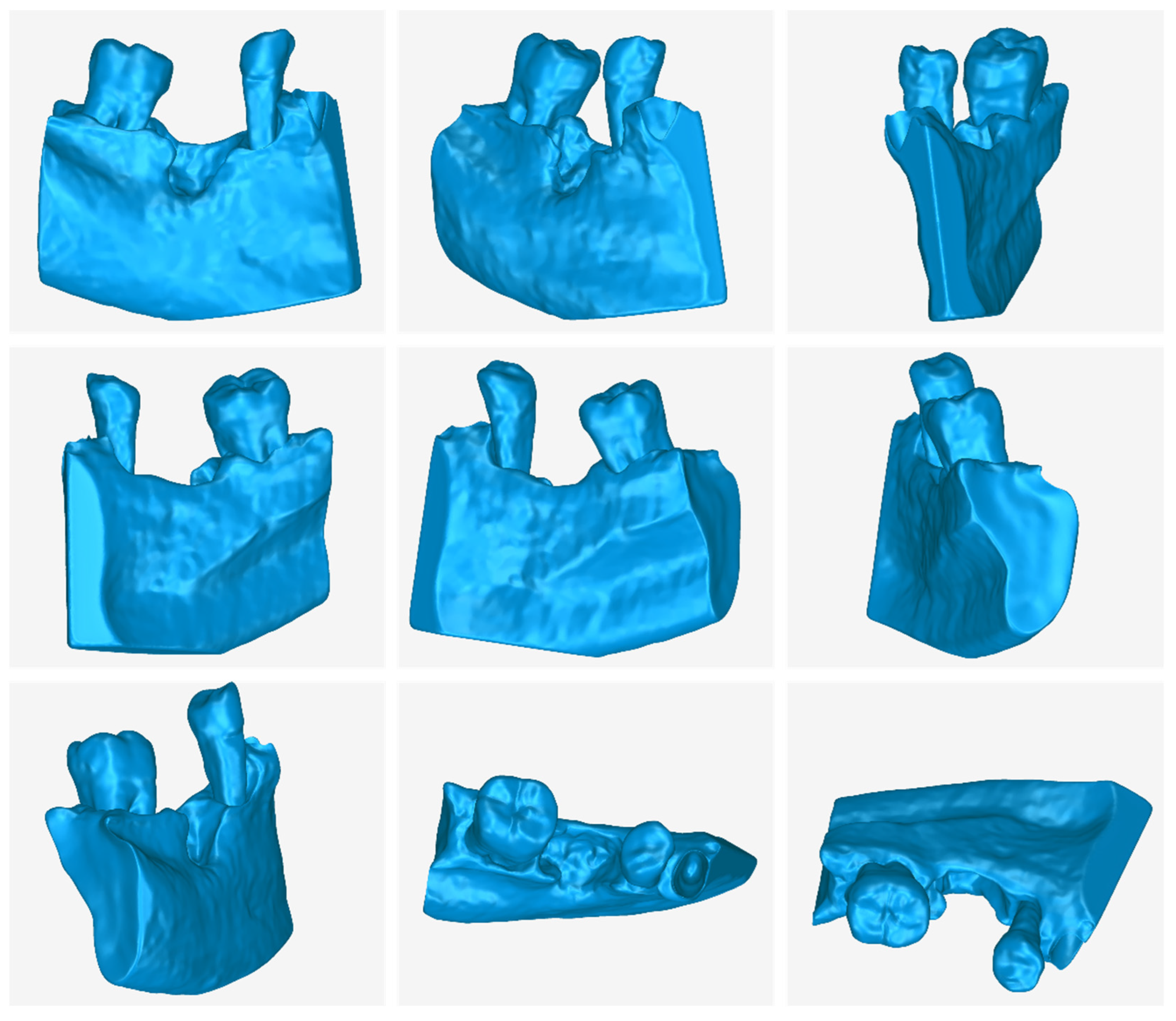
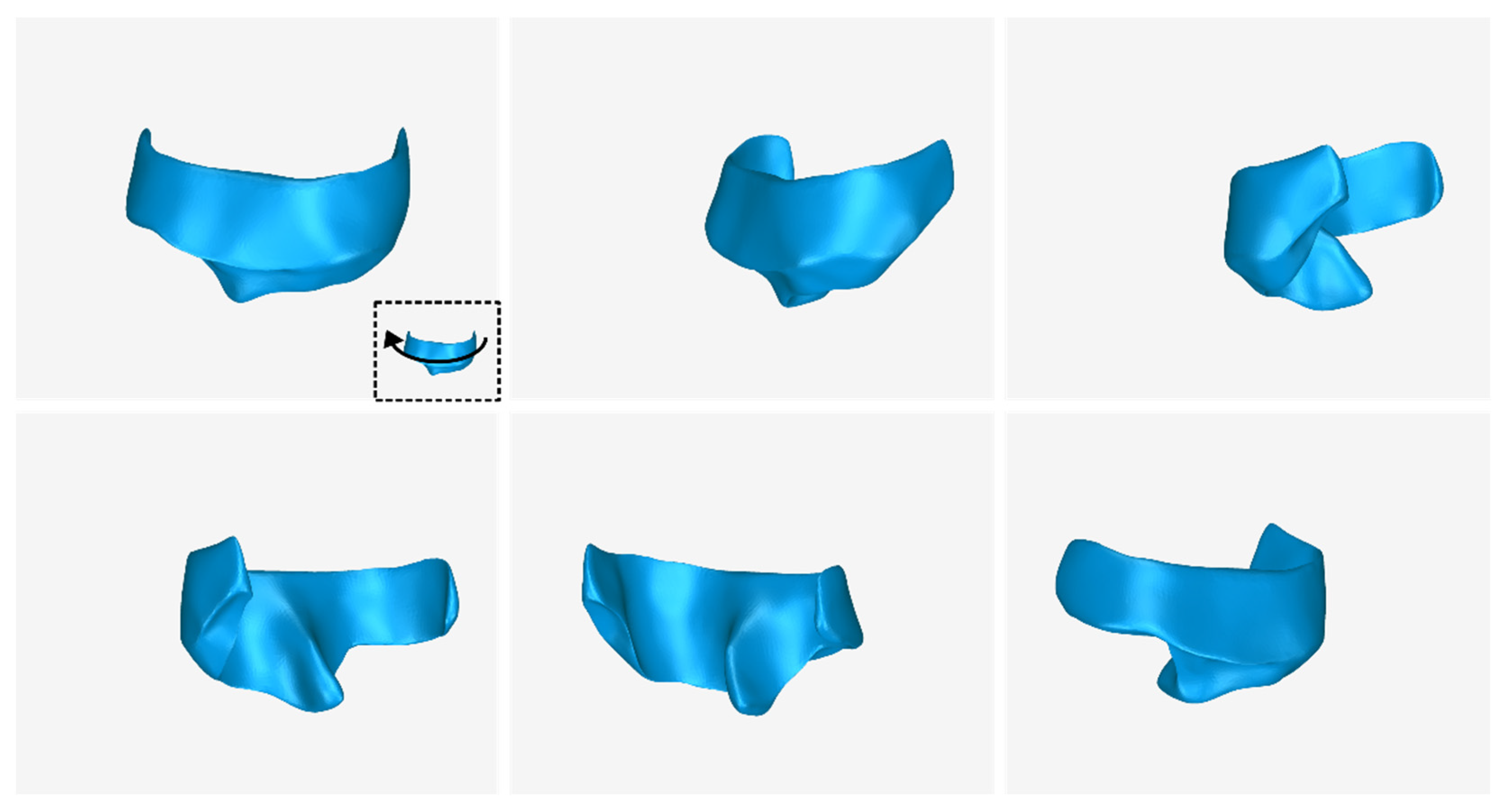
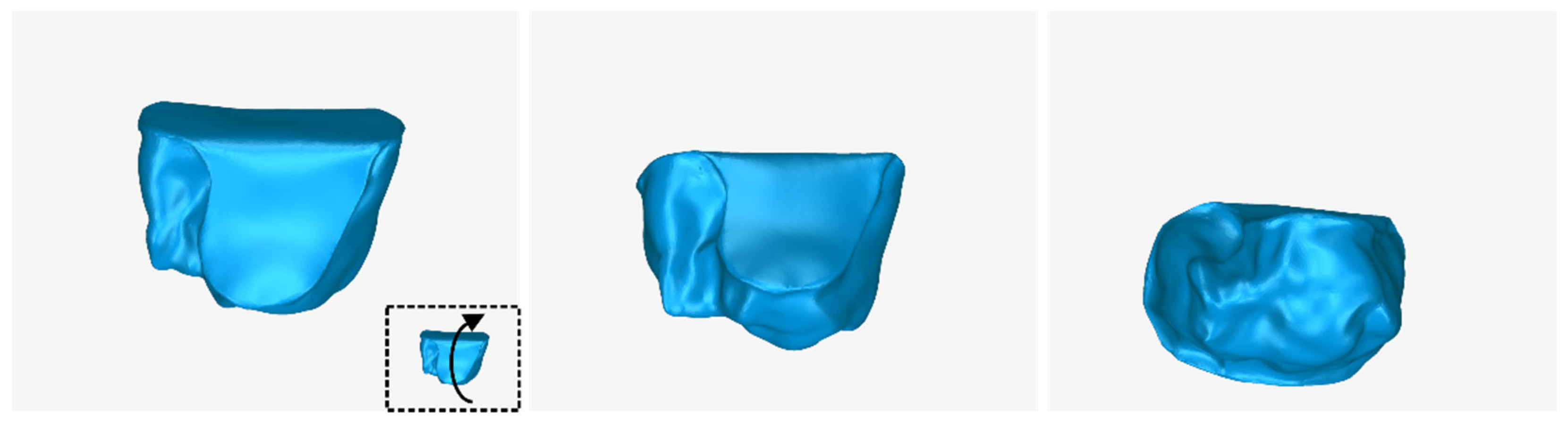
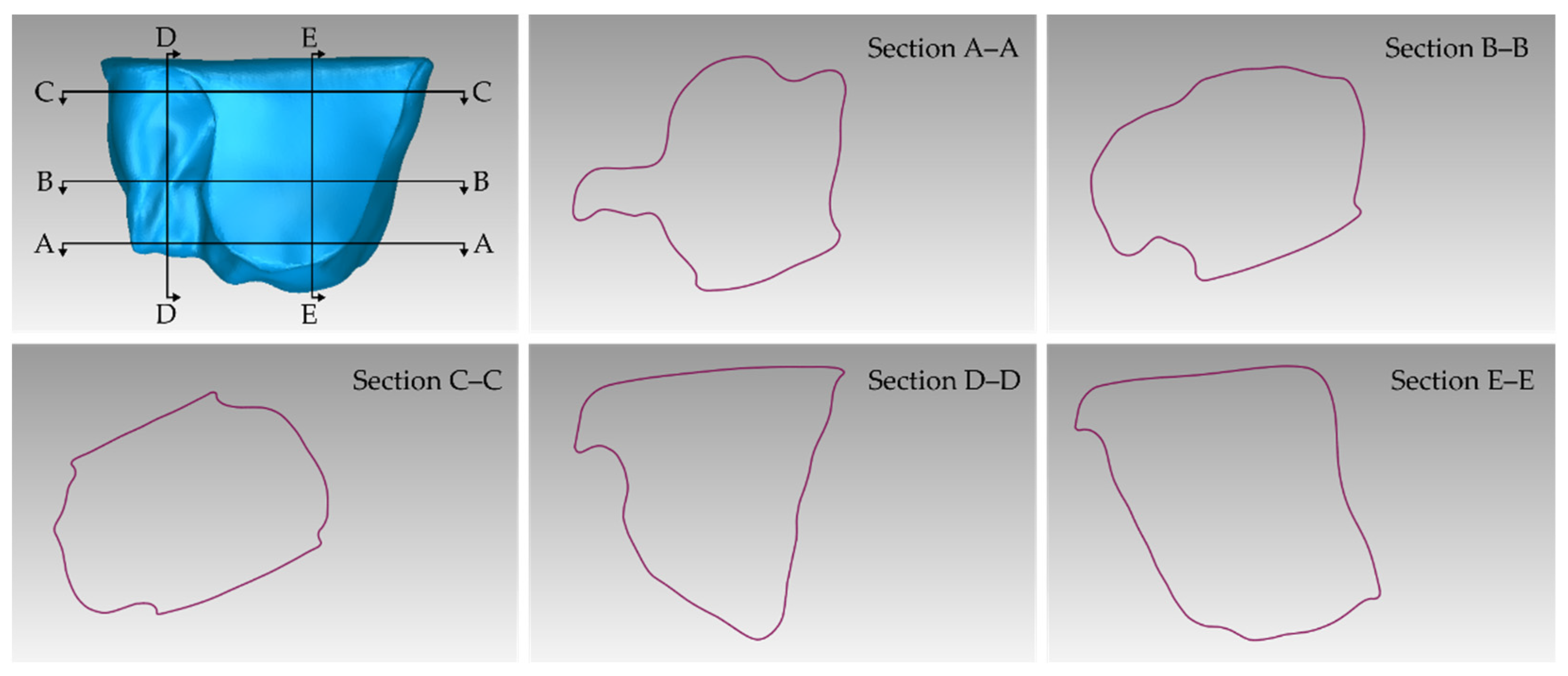
3.1. 3D Model of Hard Tissues in the Area of the Periodontal Defects
- The 3D teeth model.
- The 3D alveolar bone model.
- The 3D model of teeth and alveolar bone.
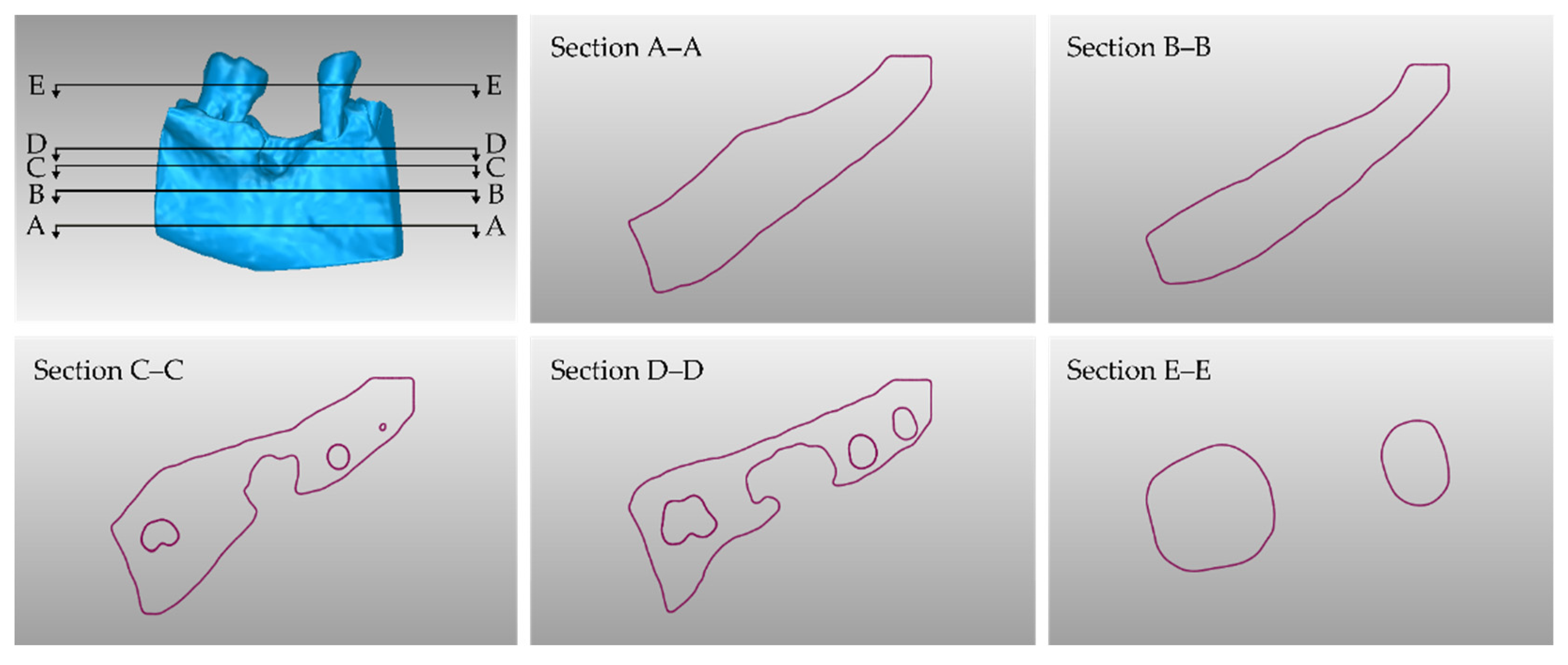
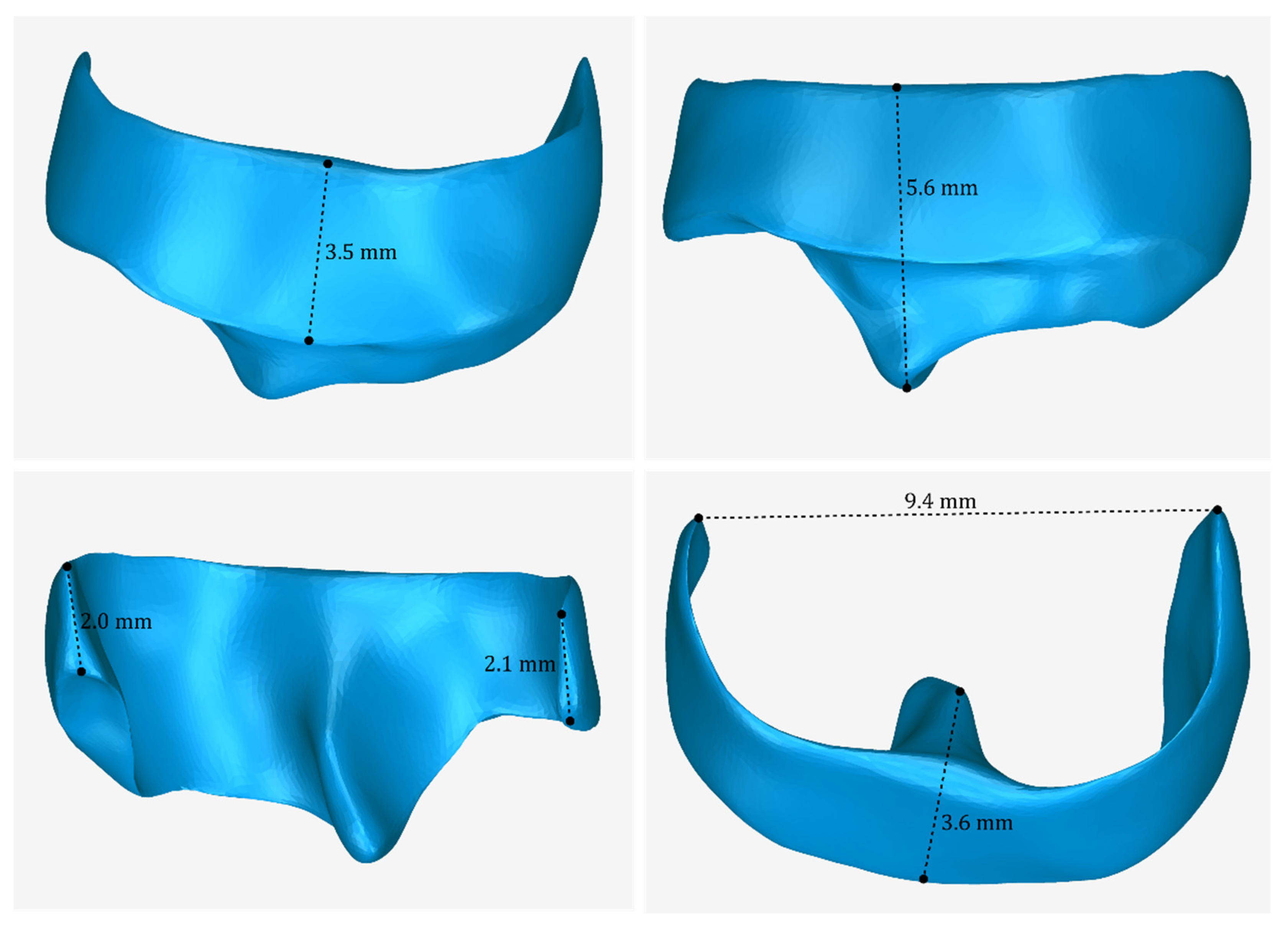
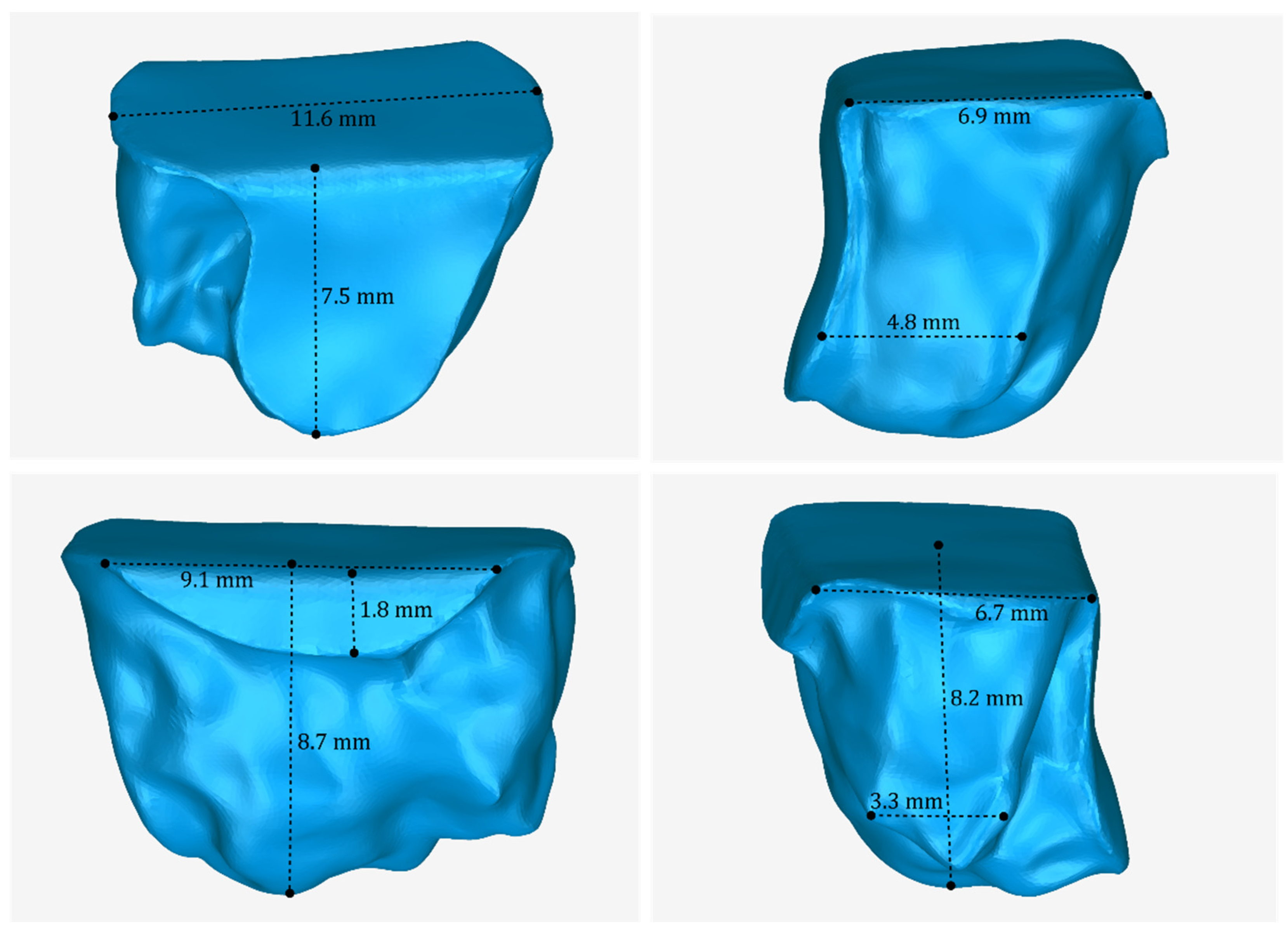
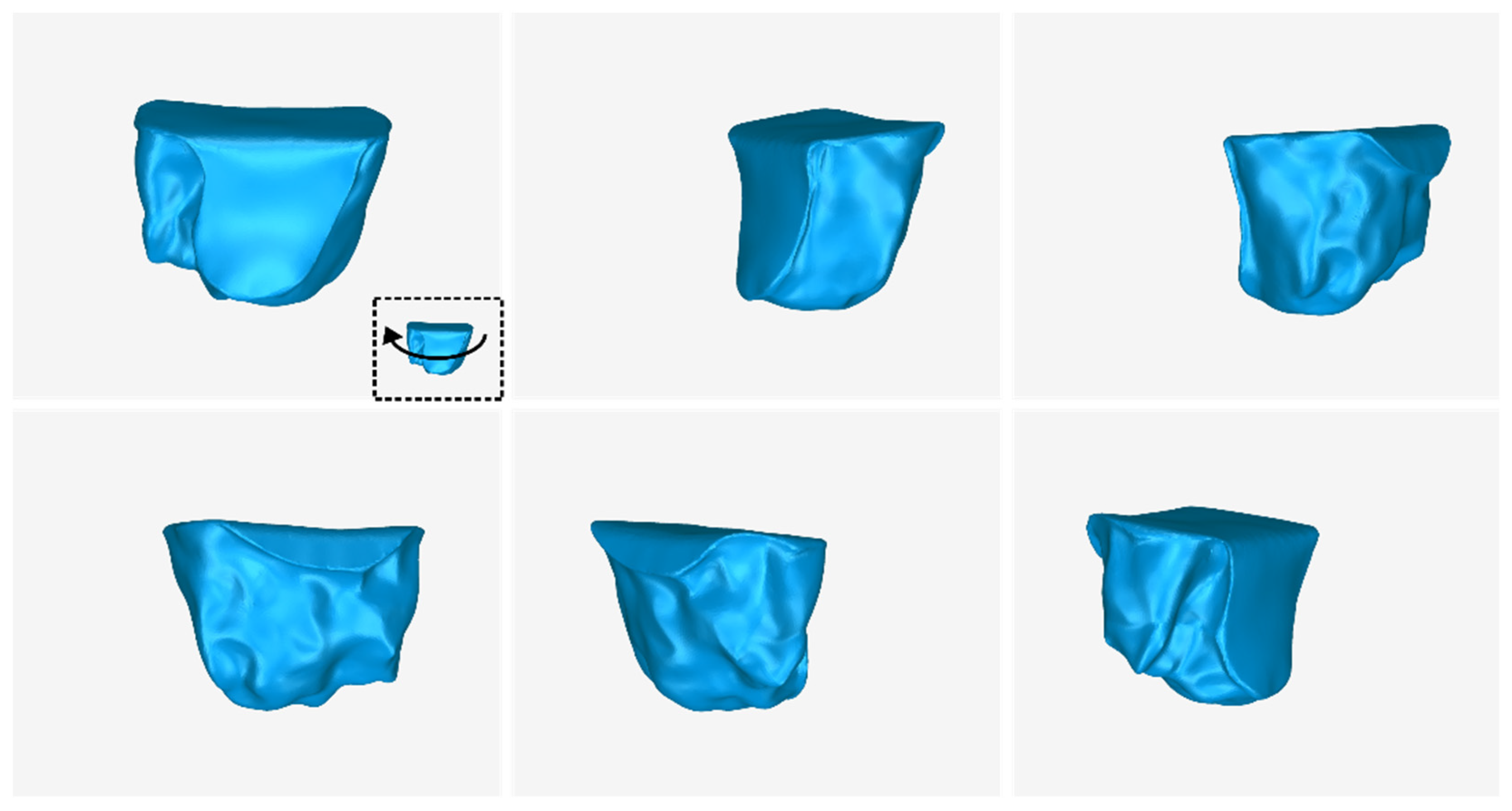
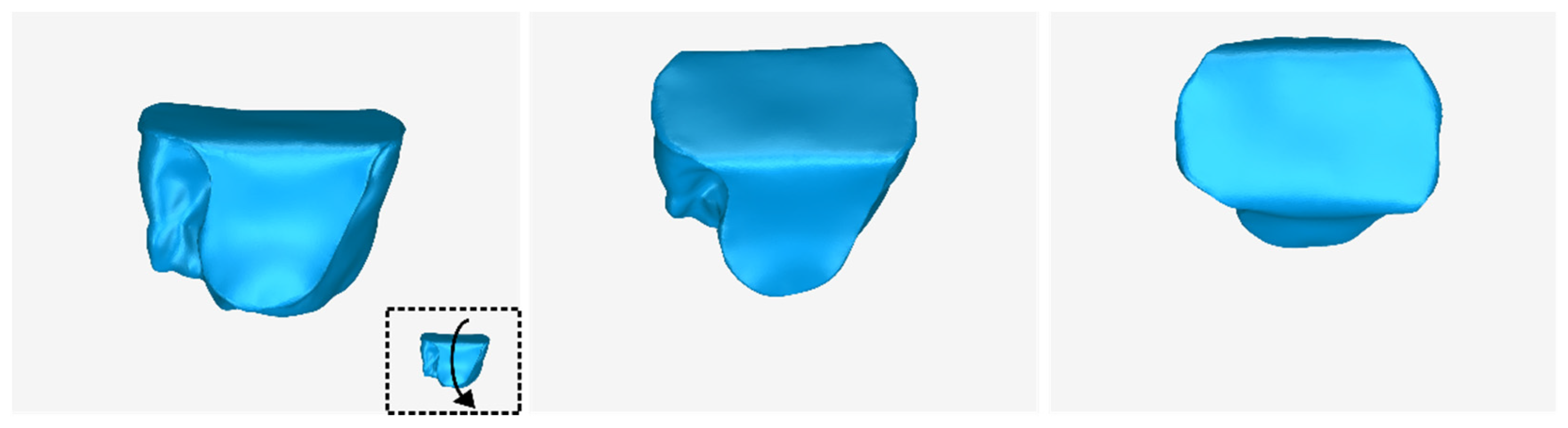
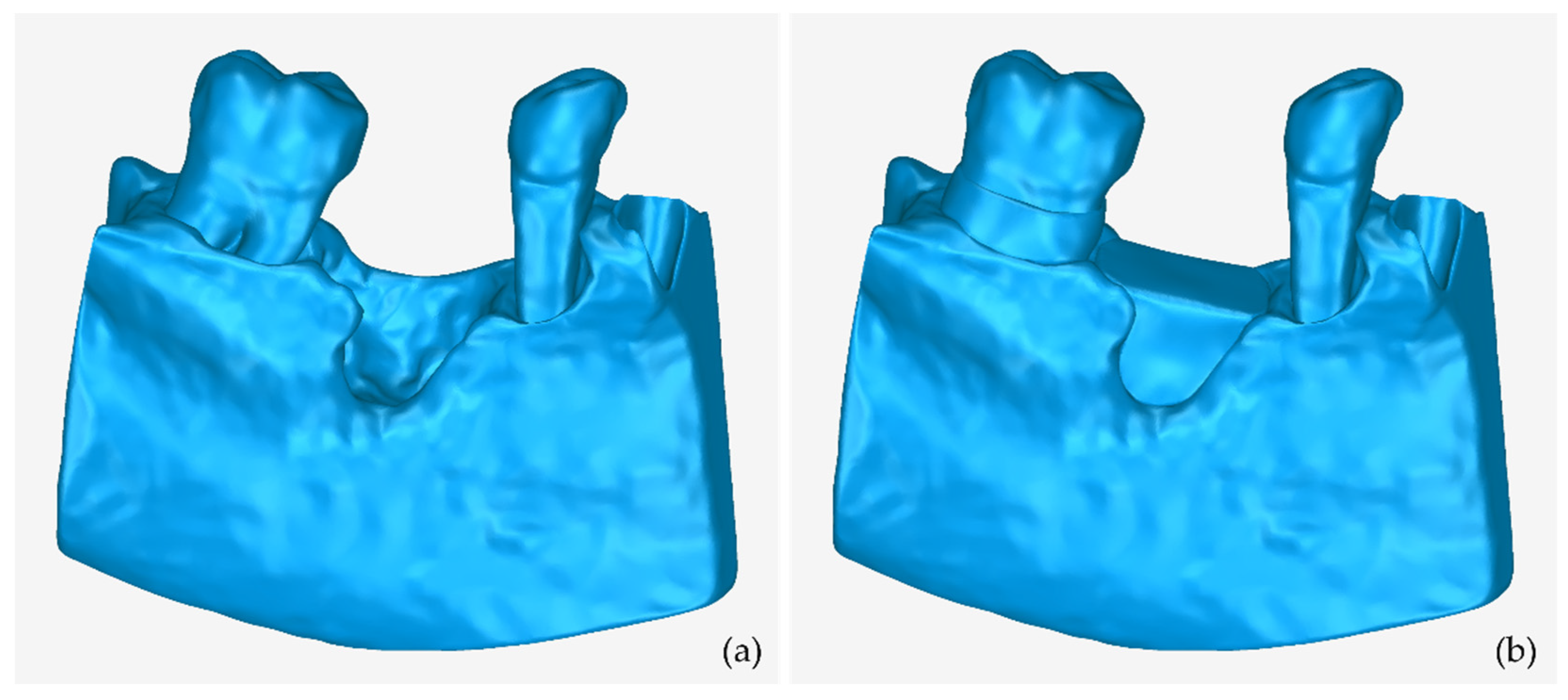
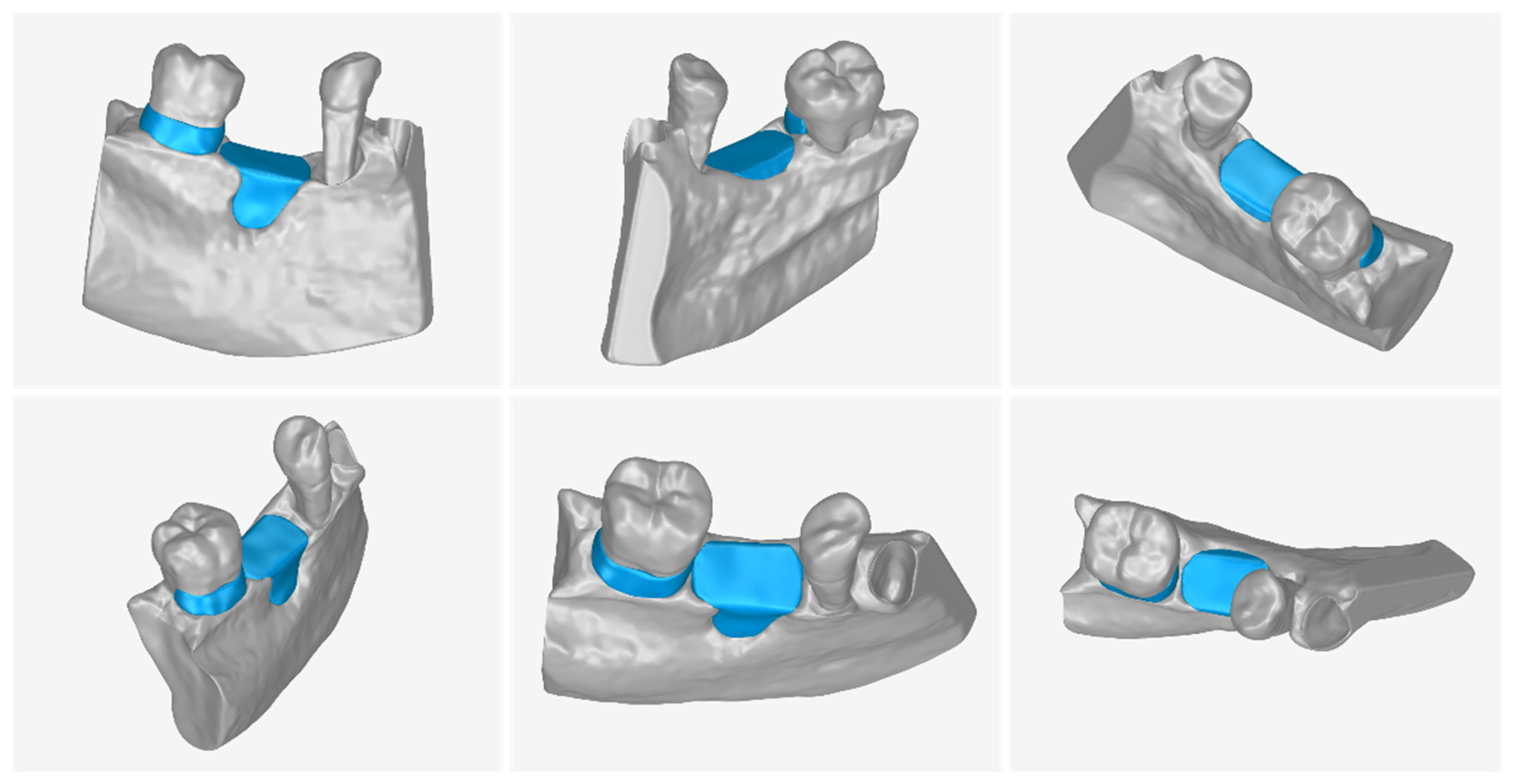
3.2. 3D Models of Scaffolds
4. Discussion
- The evaluation of the methodology proposed in our previous research work [49] for generating a 3D model of the hard tissues of a patient suffering from periodontitis, using the patient’s CBCT scan.
- The establishment of two workflows for designing two types of 3D periodontal scaffolds for personalized treatment against periodontitis.
- The evaluation of the aforementioned workflows for the generation of a periodontal defect customized block graft and an extraction socket preservation customized graft.
- The further investigation, development, and application of tomographic image segmentation algorithms, in order to propose more automated 3D modeling methodologies using CBCT data.
- The comparison of Fused Deposition Modeling (FDM) and Selective Laser Sintering (SLS) as suitable 3D printing techniques for the designed bone grafts.
- The use of new biodegradable materials for 3D printing of the scaffolds, which will also act as carriers of drugs in patients.
- The clinical trials of surgical placement of the 3D-printed bone grafts in patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, e5340616. [Google Scholar] [CrossRef] [PubMed]
- Verykokou, S.; Ioannidis, C. An Overview on Image-Based and Scanner-Based 3D Modeling Technologies. Sensors 2023, 23, 596. [Google Scholar] [CrossRef] [PubMed]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D Printed Scaffolds for Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Bahraminasab, M. Challenges on Optimization of 3D-Printed Bone Scaffolds. BioMed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef]
- Kanwar, S.; Vijayavenkataraman, S. Design of 3D Printed Scaffolds for Bone Tissue Engineering: A Review. Bioprinting 2021, 24, e00167. [Google Scholar] [CrossRef]
- Yadav, L.R.; Chandran, S.V.; Lavanya, K.; Selvamurugan, N. Chitosan-Based 3D-Printed Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Ansari, M.A.A.; Golebiowska, A.A.; Dash, M.; Kumar, P.; Jain, P.K.; Nukavarapu, S.P.; Ramakrishna, S.; Nanda, H.S. Engineering Biomaterials to 3D-Print Scaffolds for Bone Regeneration: Practical and Theoretical Consideration. Biomater. Sci. 2022, 10, 2789–2816. [Google Scholar] [CrossRef]
- Karanth, D.; Song, K.; Martin, M.L.; Meyer, D.R.; Dolce, C.; Huang, Y.; Holliday, L.S. Towards Resorbable 3D-printed Scaffolds for Craniofacial Bone Regeneration. Orthod. Craniofacial Res. 2023, online. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D Printed Bone Scaffolds: A Review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-Printed Bioceramic Scaffolds: From Bone Tissue Engineering to Tumor Therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, e9950131. [Google Scholar] [CrossRef]
- Habib, A.A.I.; Sheikh, N.A. 3D Printing Review in Numerous Applications for Dentistry. J. Inst. Eng. India Ser. C 2022, 103, 991–1000. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-Implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, e1239842. [Google Scholar] [CrossRef]
- Louvrier, A.; Marty, P.; Barrabé, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Meyer, C. How Useful Is 3D Printing in Maxillofacial Surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Yu, N.; Nguyen, T.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized Scaffolding Technologies for Alveolar Bone Regenerative Medicine. Orthod. Craniofac Res. 2019, 22 (Suppl. S1), 69–75. [Google Scholar] [CrossRef]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Huang, M.F.; Alfi, D.; Alfi, J.; Huang, A.T. The Use of Patient-Specific Implants in Oral and Maxillofacial Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Mathur, V.P.; Satapathy, B.K. Biodegradable and Biocompatible 3D Constructs for Dental Applications: Manufacturing Options and Perspectives. Ann. Biomed. Eng. 2021, 49, 2030–2056. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, P.; Ehsani, A.; Khademi, A.; Asefnejad, A.; Shahriari, S.; Soleimani, M.; Ghadiri Nejad, M.; Saber-Samandari, S.; Khandan, A. Application of 3D Bioprinters for Dental Pulp Regeneration and Tissue Engineering (Porous Architecture). Transp. Porous Med. 2022, 142, 265–293. [Google Scholar] [CrossRef]
- Sikdar, R.; Bag, A.; Shirolkar, S.; Gayen, K.; Sarkar, S.; Roychowdhury, S. 3D Printing: Its Application in Pediatric Dental Practice. Acta Sci. Dent. Sci. 2022, 6, 103–111. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D Bioprinting of Tissues/Organs for Regenerative Medicine and in-Vitro Models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and Characterization of 3D-Printed Bone-like β-Tricalcium Phosphate/Polycaprolactone Scaffolds for Dental Tissue Engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D Printed Hybrid Bone Constructs of PCL and Dental Pulp Stem Cells Loaded GelMA. J. Biomed. Mater. Res. 2021, 109, 2425–2437. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Liu, J.; Wu, M.; Yang, J.; Zhu, Y.; Lu, W.W.; Ruan, C. 3D-Printed Pre-Tapped-Hole Scaffolds Facilitate One-Step Surgery of Predictable Alveolar Bone Augmentation and Simultaneous Dental Implantation. Compos. Part B Eng. 2022, 229, 109461. [Google Scholar] [CrossRef]
- Staples, R.J.; Ivanovski, S.; Vaquette, C. Fibre Guiding Scaffolds for Periodontal Tissue Engineering. J. Periodont Res. 2020, 55, 331–341. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chiu, Y.-C.; Lee, A.K.-X.; Lin, Y.-A.; Lin, P.-Y.; Shie, M.-Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chao, P.-H.G.; Tai, W.-C.; Chang, P.-C. 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef]
- Lee, U.-L.; Yun, S.; Cao, H.-L.; Ahn, G.; Shim, J.-H.; Woo, S.-H.; Choung, P.-H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.-J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(ε-Caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Gao, J.; Zhang, X.; Chen, Y.; Li, R.; Shen, J. 3D Printed Scaffold for Repairing Bone Defects in Apical Periodontitis. BMC Oral Health 2022, 22, 327. [Google Scholar] [CrossRef]
- Yao, Y.; Raymond, J.E.; Kauffmann, F.; Maekawa, S.; Sugai, J.V.; Lahann, J.; Giannobile, W.V. Multicompartmental Scaffolds for Coordinated Periodontal Tissue Engineering. J. Dent. Res. 2022, 101, 1457–1466. [Google Scholar] [CrossRef]
- Alhroob, K.H.; Alsabbagh, M.M.; Alsabbagh, A.Y. Effect of the Use of a Surgical Guide on Heat Generation during Implant Placement: A Comparative in Vitro Study. Dent. Med. Probl. 2021, 58, 55–59. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Malysa, A.; Mikulewicz, M. Comparison of the Compression and Tensile Modulus of Two Chosen Resins Used in Dentistry for 3D Printing. Materials 2022, 15, 8956. [Google Scholar] [CrossRef]
- AlJehani, Y.A. Diagnostic Applications of Cone-Beam CT for Periodontal Diseases. Int. J. Dent. 2014, 2014, 865079. [Google Scholar] [CrossRef]
- Bagis, N.; Kolsuz, M.E.; Kursun, S.; Orhan, K. Comparison of Intraoral Radiography and Cone-Beam Computed Tomography for the Detection of Periodontal Defects: An in Vitro Study. BMC Oral Health 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Cetmili, H.; Tassoker, M.; Sener, S. Comparison of Cone-Beam Computed Tomography with Bitewing Radiography for Detection of Periodontal Bone Loss and Assessment of Effects of Different Voxel Resolutions: An in Vitro Study. Oral Radiol. 2019, 35, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Ge, Z.; Zhao, H.; Miao, L.; Pan, Y. The Dimension and Morphology of Alveolar Bone at Maxillary Anterior Teeth in Periodontitis: A Retrospective Analysis-Using CBCT. Int. J. Oral Sci. 2020, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Rinne, C.A.; Dagassan-Berndt, D.C.; Connert, T.; Müller-Gerbl, M.; Weiger, R.; Walter, C. Impact of CBCT Image Quality on the Confidence of Furcation Measurements. J. Clin. Periodontol. 2020, 47, 816–824. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-Printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Korn, P.; Ahlfeld, T.; Lahmeyer, F.; Kilian, D.; Sembdner, P.; Stelzer, R.; Pradel, W.; Franke, A.; Rauner, M.; Range, U.; et al. 3D Printing of Bone Grafts for Cleft Alveolar Osteoplasty—In Vivo Evaluation in a Preclinical Model. Front. Bioeng. Biotechnol. 2020, 8, 217. [Google Scholar] [CrossRef]
- Anderson, M.; Dubey, N.; Bogie, K.; Cao, C.; Li, J.; Lerchbacker, J.; Mendonça, G.; Kauffmann, F.; Bottino, M.C.; Kaigler, D. Three-Dimensional Printing of Clinical Scale and Personalized Calcium Phosphate Scaffolds for Alveolar Bone Reconstruction. Dent. Mater. 2022, 38, 529–539. [Google Scholar] [CrossRef]
- Schulz, M.C.; Holtzhausen, S.; Nies, B.; Heinemann, S.; Muallah, D.; Kroschwald, L.; Paetzold-Byhain, K.; Lauer, G.; Sembdner, P. Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration. J. Pers. Med. 2023, 13, 464. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Angelopoulos, C. Evaluation of 3D Modeling Workflows Using Dental CBCT Data for Periodontal Regenerative Treatment. J. Pers. Med. 2022, 12, 1355. [Google Scholar] [CrossRef]
- 3D Slicer Image Computing Platform. Available online: https://www.slicer.org/ (accessed on 9 May 2023).
- Geomagic Wrap. Available online: https://www.artec3d.com/3d-software/geomagic-wrap (accessed on 9 May 2023).
- Zhu, L.; Kolesov, I.; Gao, Y.; Kikinis, R.; Tannenbaum, A. An Effective Interactive Medical Image Segmentation Method Using Fast GrowCut. In Proceedings of the 17th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), Cambridge, MA, USA, 14–18 September 2014. [Google Scholar]
- Kapourani, A.; Koromili, M.; Verykokou, S.; Angelopoulos, C.; Ioannidis, C.; Valkanioti, V.; Chatzitheodoridou, M.; Barmpalexis, P. Comparison of FDM and SLS printing of periodontal scaffolds using 3D models derived from CBCT scans. In Proceedings of the 4th European Conference on Pharmaceutics, Marseille, France, 20–21 March 2023; Available online: http://users.ntua.gr/sveryk/Papers/Poster-Kapourani-et-al-2023.pdf/ (accessed on 9 May 2023).
- 3D-BioPerioDontis. Available online: https://3dperiodontis.gr/ (accessed on 9 May 2023).








| 3D Model | Number of Triangles | Size (MB) |
|---|---|---|
| Teeth (before processing) | 156,852 | 7.7 |
| Alveolar bone (before processing) | 608,144 | 26.7 |
| Teeth and alveolar bone (after processing) | 509,518 | 24.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verykokou, S.; Ioannidis, C.; Angelopoulos, C. CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. J. Clin. Med. 2023, 12, 5023. https://doi.org/10.3390/jcm12155023
Verykokou S, Ioannidis C, Angelopoulos C. CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. Journal of Clinical Medicine. 2023; 12(15):5023. https://doi.org/10.3390/jcm12155023
Chicago/Turabian StyleVerykokou, Styliani, Charalabos Ioannidis, and Christos Angelopoulos. 2023. "CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration" Journal of Clinical Medicine 12, no. 15: 5023. https://doi.org/10.3390/jcm12155023
APA StyleVerykokou, S., Ioannidis, C., & Angelopoulos, C. (2023). CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. Journal of Clinical Medicine, 12(15), 5023. https://doi.org/10.3390/jcm12155023








