Efficacy and Safety of Ticagrelor versus Clopidogrel in Dialysis Patients with Coronary Syndromes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria and Outcomes
2.3. Data Collection and Synthesis
2.4. Quality Assessment
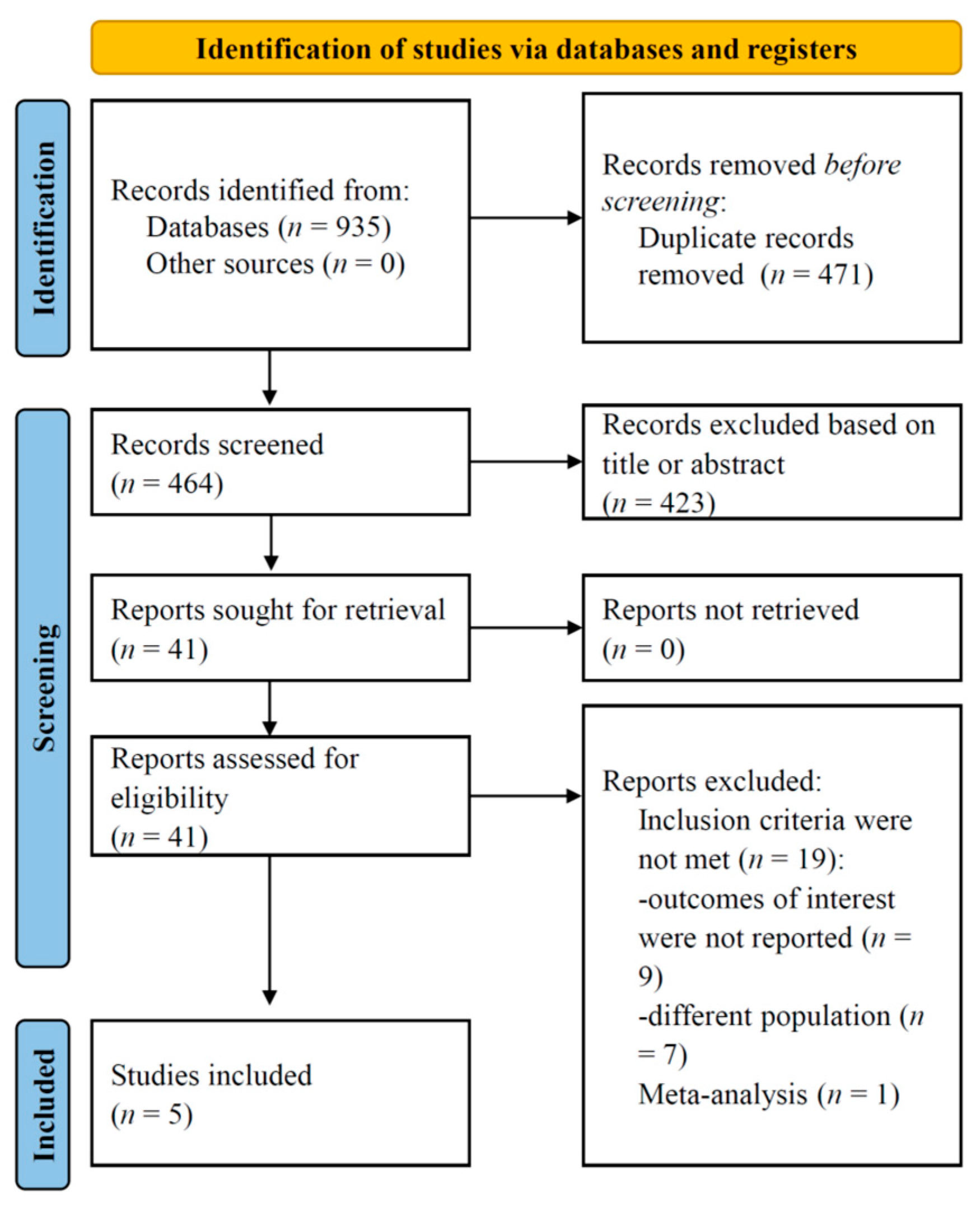
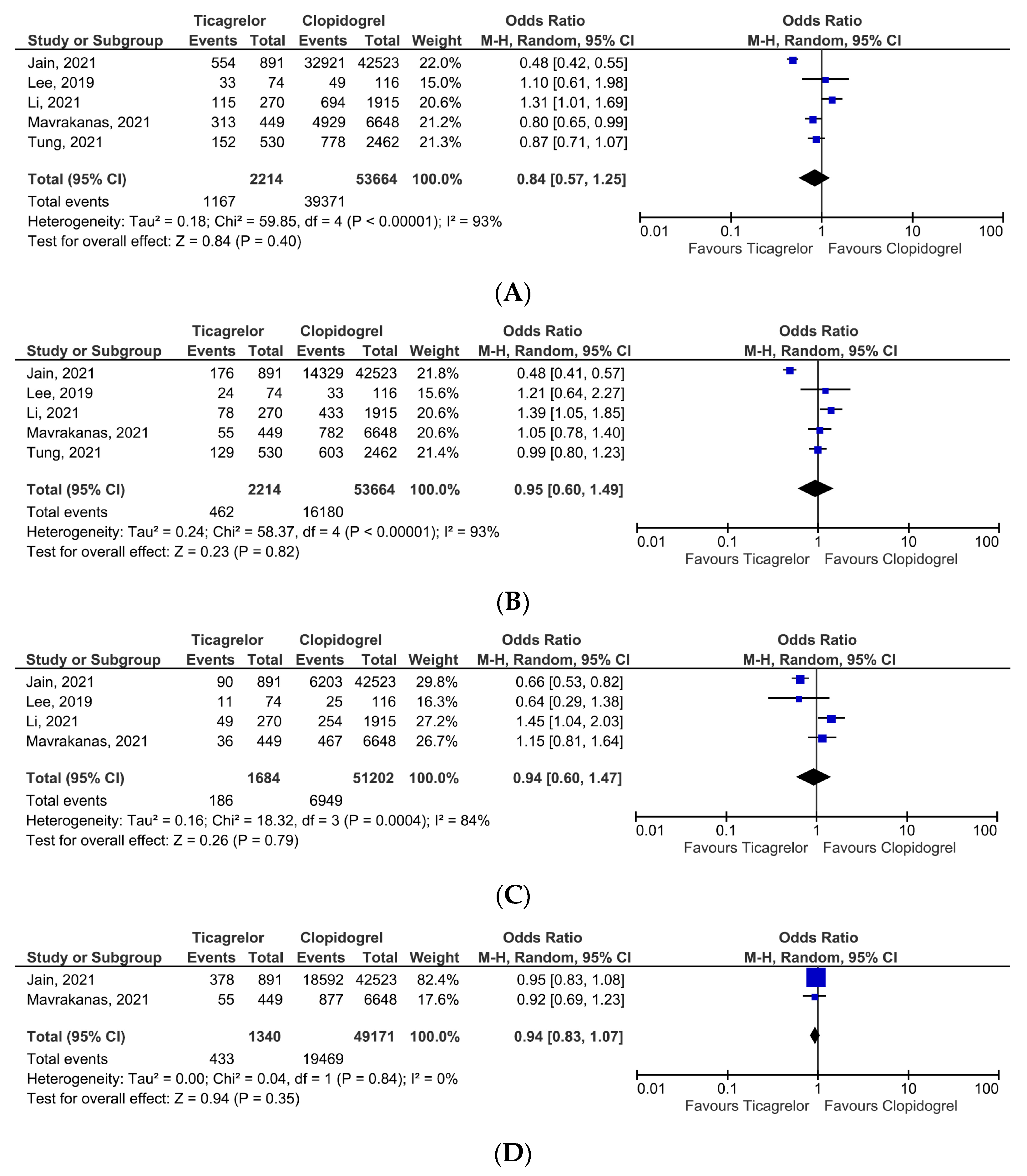
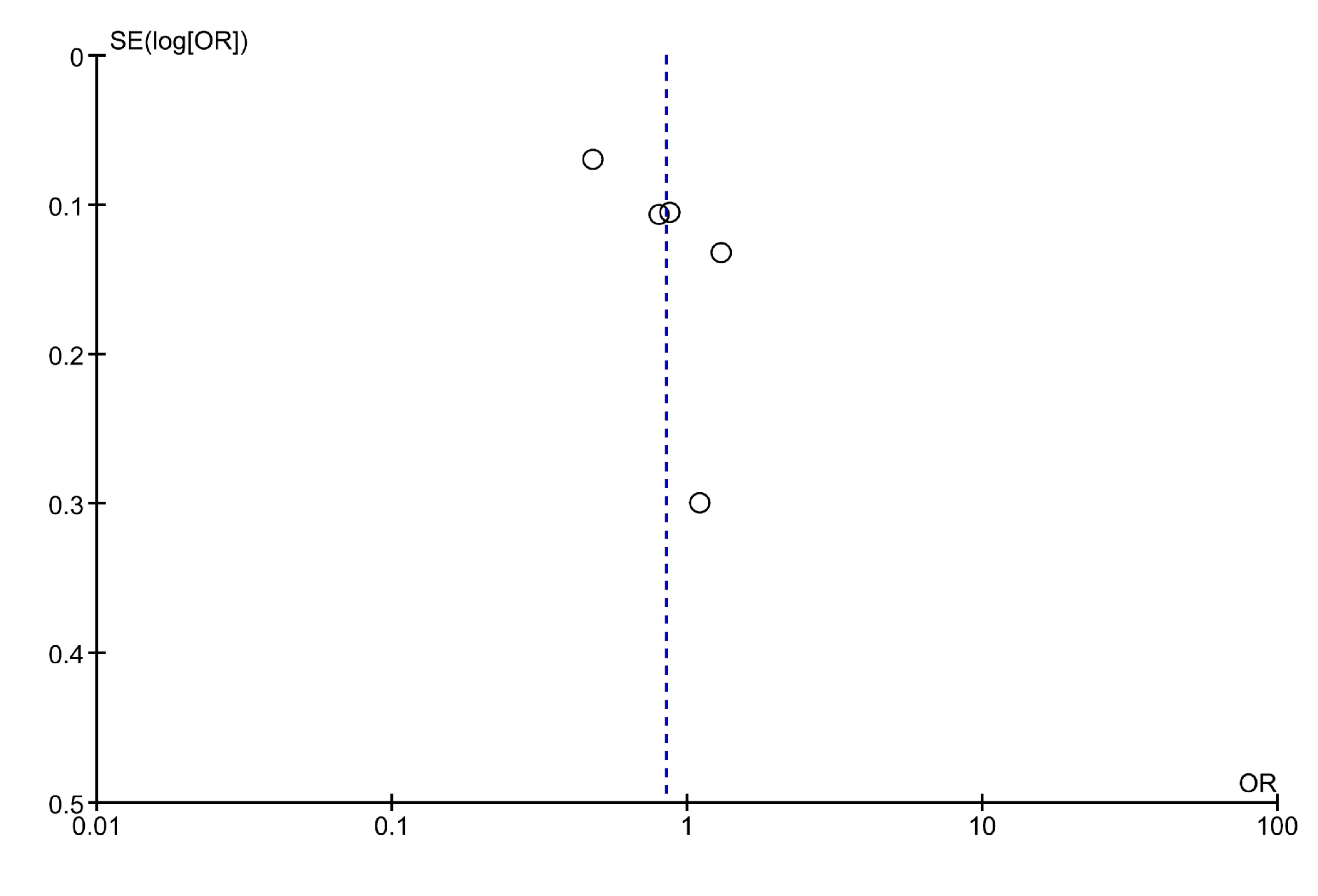
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonello, L.; Angiolillo, D.J.; Aradi, D.; Sibbing, D. P2Y12-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes: Review of the current evidence. Circulation 2018, 138, 1582–1596. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.J.; Kang, J.E.; Kim, M.G.; Geum, M.J.; Kim, S.D.; Rhie, S.J. P2Y12 Antiplatelet Choice for Patients with Chronic Kidney Disease and Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 222. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.; Crea, F.; Goudevenos, J.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Melloni, C.; Cornel, J.H.; Hafley, G.; Neely, M.L.; Clemmensen, P.; Zamoryakhin, D.; Prabhakaran, D.; White, H.D.; Fox, K.A.; Ohman, E.M.; et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: Insights from the TRILOGY ACS Trial. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 443–454. [Google Scholar] [CrossRef]
- Sattar, S.; Ahmed, N.; Akhter, Z.; Aijaz, S.; Lakhani, S.; Malik, R.; Pathan, A. In-Hospital outcomes in acute coronary syndrome patients with concomitant severe chronic kidney disease undergoing percutaneous coronary intervention. Pak. J. Med. Sci. 2019, 35, 291–297. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. New Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- James, S.; Åkerblom, A.; Cannon, C.P.; Emanuelsson, H.; Husted, S.; Katus, H.; Skene, A.; Steg, P.G.; Storey, R.F.; Harrington, R.; et al. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am. Heart J. 2009, 157, 599–605. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. New Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Baber, U.; Dangas, G.; Cohen, D.J.; Gibson, C.M.; Mehta, S.R.; Angiolillo, D.J.; Pocock, S.J.; Krucoff, M.W.; Kastrati, A.; Ohman, E.M.; et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: Rationale and design of the TWILIGHT study. Am. Heart J. 2016, 182, 125–134. [Google Scholar] [CrossRef]
- Chen, Y.; Tu, S.; Chen, Z.; Xia, J.; Chen, B.; Chen, J.; Liang, J.; Liu, X.; Tang, L. Ticagrelor versus Clopidogrel in Patients with Severe Renal Insufficiency Undergoing PCI for Acute Coronary Syndrome. J. Interv. Cardiol. 2022, 2022, 6476777. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Jain, N.; Phadnis, M.A.; Hunt, S.L.; Dai, J.; Shireman, T.I.; Davis, C.L.; Mehta, J.L.; Rasu, R.S.; Hedayati, S.S. Comparative Effectiveness and Safety of Oral P2Y12 Inhibitors in Patients on Chronic Dialysis. Kidney Int. Rep. 2021, 6, 2381–2391. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tsai, T.-H.; Lin, C.-J.; Hsueh, S.-K.; Chung, W.-J.; Cheng, C.-I. Efficacy and Safety of Ticagrelor Compared with Clopidogrel in Patients with End-Stage Renal Disease with Acute Myocardial Infarction. Am. J. Cardiovasc. Drugs 2019, 19, 325–334. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Hwang, S.; Yang, Y.; Hsieh, K. Comparison of effectiveness and safety between ticagrelor and clopidogrel in patients with acute coronary syndrome and on dialysis in Taiwan. Br. J. Clin. Pharmacol. 2021, 88, 145–154. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; Kamal, O.; Charytan, D.M. Prasugrel and Ticagrelor in Patients with Drug-Eluting Stents and Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 757–764. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chang, C.-J.; Liu, J.-R.; Chang, S.-H.; Chan, Y.-H.; Kuo, C.-T.; See, L.-C. Outcomes after ticagrelor versus clopidogrel treatment in end-stage renal disease patients with acute myocardial infarction: A nationwide cohort study. Sci. Rep. 2021, 11, 20826. [Google Scholar] [CrossRef]
- Storey, R.F.; Angiolillo, D.J.; Patil, S.B.; Desai, B.; Ecob, R.; Husted, S.; Emanuelsson, H.; Cannon, C.P.; Becker, R.C.; Wallentin, L. Inhibitory Effects of Ticagrelor Compared with Clopidogrel on Platelet Function in Patients with Acute Coronary Syndromes: The PLATO (PLATelet inhibition and patient Outcomes) PLATELET Substudy. J. Am. Coll. Cardiol. 2010, 56, 1456–1462. [Google Scholar] [CrossRef]
- Matetzky, S.; Shenkman, B.; Guetta, V.; Shechter, M.; Beinart, R.; Goldenberg, I.; Novikov, I.; Pres, H.; Savion, N.; Varon, D.; et al. Clopidogrel Resistance Is Associated with Increased Risk of Recurrent Atherothrombotic Events in Patients with Acute Myocardial Infarction. Circulation 2004, 109, 3171–3175. [Google Scholar] [CrossRef]
- Al-Balushi, S.; Rahhal, A.; Abdelghani, M.; Ashour, M.A.; Al-Mohtasib, Y.; Abid, A.R.; Alsuwaidi, J. Abstract 14255: Effectiveness and Safety of Ticagrelor versus Clopidogrel in Acute Coronary Syndrome: A Meta-Analysis of Real—World Data. Circulation 2022, 146, A14255. [Google Scholar] [CrossRef]




| Author, Year | Design | Patients, No | Age, Median/Mean ± SD | Setting | Outcomes | Follow-Up Period |
|---|---|---|---|---|---|---|
| Jain et al., 2021 [14] | Observational, retrospective (USA) | 42,523 (clopidogrel group) | 64.0 | Patients with ESKD who were prescribed P2Y12 receptor inhibitors. | The primary outcome: all-cause death. The secondary outcomes: cardiovascular death, coronary revascularization and gastrointestinal bleedings. | Median follow-up 52 weeks. |
| 891 (ticagrelor group) | ||||||
| Lee et al., 2019 [15] | Observational, retrospective (Taiwan) | 116 (clopidogrel group) | 67.90 ± 9.18 | Chronic dialysis patient who underwent PCI for acute myocardial infarction (both, STEMI and NSTEMI). | The primary composite outcome: cardiovascular death, recurrence of myocardial infarction, or new stroke. The secondary outcomes: cardiovascular death, all-cause death, recurrence of myocardial infarction, new stroke and any bleeding event. | In-hospital and at 1-year. |
| 74 (ticagrelor group) | 65.19 ± 10.42 | |||||
| Li et al., 2021 [16] | Observational, retrospective (Taiwan) | 1915 (clopidogrel group) | 67.20 ± 11.26 | Patients receiving chronic dialysis who presented with acute coronary syndrome. | The primary efficacy outcome: MACE occurrence (composite of any-cause mortality, recurrent myocardial infarction and stroke). The primary safety outcome: major bleeding events (requiring hospitalization or admission to emergency room). The secondary outcomes: all-cause death, cardiovascular death, recurrent myocardial infarction, stroke, and any bleeding events. | Until outcome occurrence, death, 12 months or the end of the study. |
| 270 (ticagrelor group) | 64.24 ± 11.65 | |||||
| Mavrakanas et al., 2021 [17] | Observational, retrospective (USA) | 6648 (clopidogrel group) | 64 ± 11 | Renal replacement therapy patients (hemodialysis or peritoneal dialysis) who underwent PCI with drug-eluting stent implantation (including for acute coronary syndrome). | The primary composite outcome: cardiovascular mortality, myocardial infarction or stroke. The secondary outcomes: individual components of the primary outcome; composite of cardiovascular mortality, myocardial infarction, stroke or coronary revascularization; all-cause mortality; clinically relevant bleedings or any bleeding requiring hospitalization. | Until death, kidney transplant, loss of coverage or 12 months after stent implantation. |
| 449 (ticagrelor) | 64 ± 12 | |||||
| Tung et al., 2021 [18] | Observational, retrospective (Taiwan) | 2462 (clopidogrel group) | 29.9% patients > 75 years | Patients on chronic hemodialysis admitted with acute myocardial infarction. | The primary composite outcome: all-cause mortality, non-fatal myocardial infarction, or non-fatal stroke. The secondary outcomes: individual components of the primary composite outcome. The safety outcome: BARC type 2, 3, or 5 bleedings. | 9 months, or until outcomes occurrence. |
| 530 (ticagrelor group) | 24.9% patients > 75 years |
| Author, Year | Outcomes | Results (Ticagrelor vs. Clopidogrel) | |
|---|---|---|---|
| Jain et al., 2021 [14] | All-cause death | In propensity matched cohorts: HR 1.05 (95% CI, 0.90–1.24) | |
| Cardiovascular death | In propensity matched cohorts: HR 1.08 (95% CI, 0.86–1.35) | ||
| Coronary revascularization | In propensity matched cohorts: HR 0.99 (95% CI, 0.92–1.06) | ||
| GI bleeding | In propensity matched cohorts: HR 0.82 (95% CI, 0.65–1.03) | ||
| Lee et al., 2019 [15] | The primary composite outcome | The primary composite outcome had similar incidence at 1-year in both treatment groups (free of composite outcome 72.16% vs. 66.06%) | p = 0.424 |
| Cardiovascular death | No statistically significant differences were reported (free of cardiovascular death 83.62% vs. 72.20%) | p = 0.372 | |
| Any-cause death | All-cause death was similar in both treatment arms (free of death 70.24% vs. 64.85%) | p = 0.446 | |
| Recurrent MI | Free of myocardial infarction was similar in both groups (85.47% vs. 81.98%) | p = 0.406 | |
| Stroke | Patients receiving ticagrelor had no stroke reported, while 4 patients from clopidogrel group experienced stroke | p = 0.117 | |
| Bleeding event | Bleeding incidence was similar in both groups (free of bleeding 56.53% vs. 54.42%) | p = 0.664 | |
| Li et al., 2021 [16] | MACE | HR 1.29 (95% CI, 1.16–1.44) | p < 0.0001 |
| Any-cause death | HR 1.65 (95% CI, 1.47–1.86) | p < 0.0001 | |
| Cardiovascular death | HR 1.64 (95% CI, 1.41–1.91) | p < 0.0001 | |
| Recurrent MI | HR 0.93 (95% CI, 0.75–1.16) | p = 0.5063 | |
| Stroke | HR 0.94 (95% CI, 0.75–1.19) | p = 0.6292 | |
| Major bleedings | HR 1.49 (95% CI, 1.34–1.65) | p < 0.0001 | |
| Any bleedings | HR 1.05 (95% CI, 0.95–1.17) | p = 0.3506 | |
| Mavrakanas et al., 2021 [17] | The primary composite outcome | HR 1.00 (95% CI, 0.83–1.20)—main analysis | |
| Cardiovascular death | HR 1.17 (95% CI, 0.75–1.82)—main analysis | ||
| MI | HR 1.04 (95% CI, 0.83–1.31)—main analysis | ||
| Stroke | HR 1.04 (95% CI, 0.82–1.32)—main analysis | ||
| Coronary revascularization | HR 1.19 (95% CI, 0.88–1.62)—main analysis | ||
| Clinically relevant bleeding | HR 1.13 (95% CI, 0.91–1.40)—main analysis | ||
| Tung et al., 2021 [18] | The primary composite outcome | HR 1.16 (95% CI, 0.97–1.39) | p = 0.11 |
| All-cause death | HR 1.17 (95% CI, 0.97–1.42) | p = 0.11 | |
| Non-fatal MI | HR 1.05 (95% CI, 0.66–1.66) | p = 0.84 | |
| Any bleeding | HR 1.25 (95% CI, 0.96–1.63) | p = 0.09 | |
| BARC type 3 or 5 bleeding | HR 0.93 (95% CI, 0.51–1.70) | p = 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlacu, A.; Floria, M.; Brinza, C.; Covic, A. Efficacy and Safety of Ticagrelor versus Clopidogrel in Dialysis Patients with Coronary Syndromes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5011. https://doi.org/10.3390/jcm12155011
Burlacu A, Floria M, Brinza C, Covic A. Efficacy and Safety of Ticagrelor versus Clopidogrel in Dialysis Patients with Coronary Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(15):5011. https://doi.org/10.3390/jcm12155011
Chicago/Turabian StyleBurlacu, Alexandru, Mariana Floria, Crischentian Brinza, and Adrian Covic. 2023. "Efficacy and Safety of Ticagrelor versus Clopidogrel in Dialysis Patients with Coronary Syndromes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 15: 5011. https://doi.org/10.3390/jcm12155011
APA StyleBurlacu, A., Floria, M., Brinza, C., & Covic, A. (2023). Efficacy and Safety of Ticagrelor versus Clopidogrel in Dialysis Patients with Coronary Syndromes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(15), 5011. https://doi.org/10.3390/jcm12155011








