Impact of ILD-Specific Therapies on Perioperative Course in Patients with Progressive Interstitial Lung Disease Undergoing Lung Transplantation
Abstract
1. Introduction
2. Methods
2.1. Study Cohort and Design
2.2. Short- and Long-Term Outcomes
2.3. Statistical Analysis
3. Results
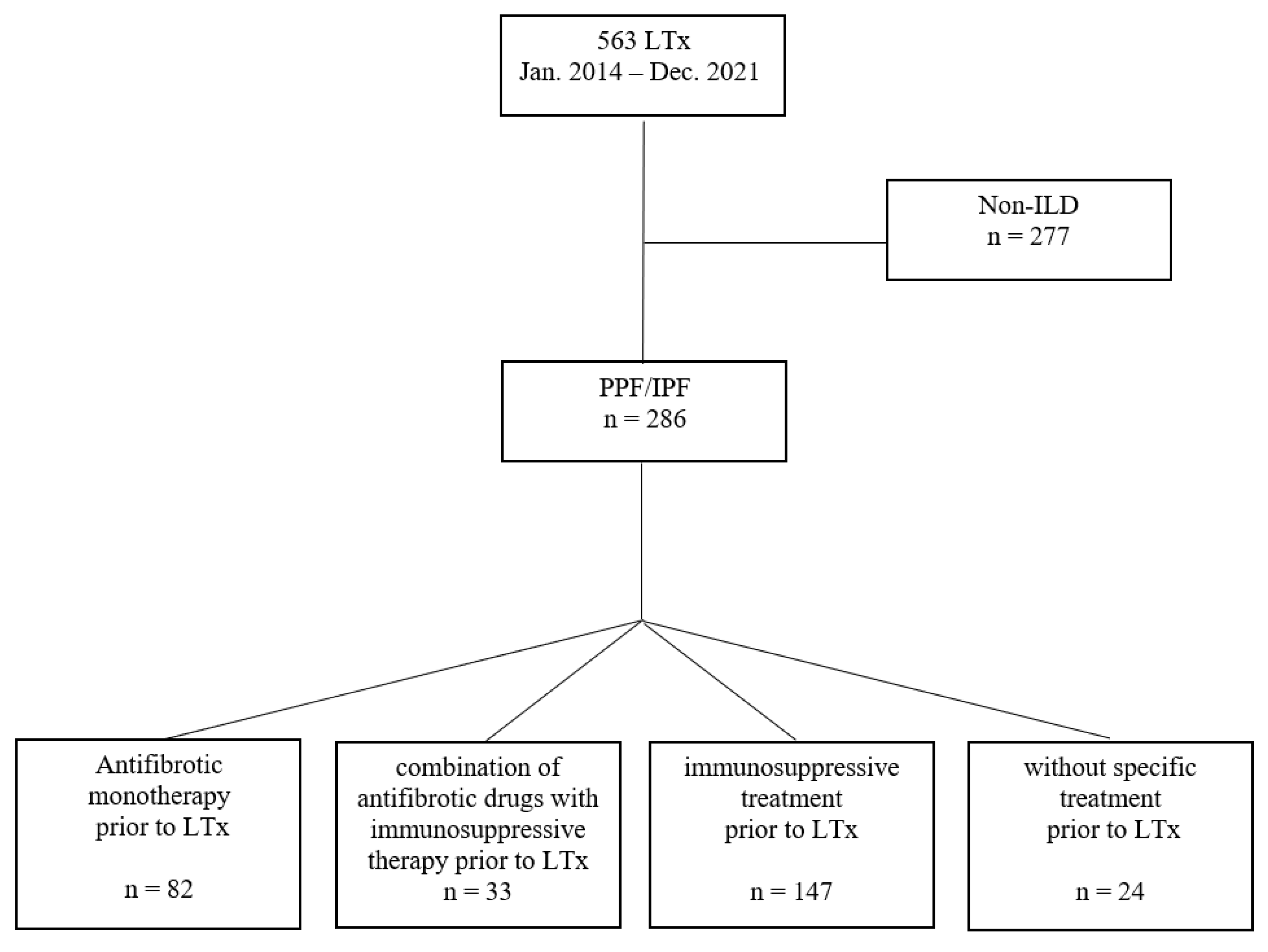
3.1. Study Cohort and Baseline Characteristics
3.2. Perioperative Course, ICU Parameters and Surgery-Related Complications
3.3. Pre-Transplant Treatment and Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AZA | azathioprine |
| BLTx | bilateral lung transplantation |
| BMI | body mass index |
| CTD-ILD | connective tissue disease-related ILD |
| DSA | donor specific antibodies |
| ECMO | extracorporeal membrane oxygenation |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GAP | gender-age-physiology |
| HP | chronic hypersensitivity pneumonitis |
| ICU | intensive care unit |
| IPF | idiopathic pulmonary fibrosis |
| LAS | lung allocation score |
| LTx | lung transplantation |
| MMF | mycophenolate mofetil |
| NSIP | non-specific interstitial pneumonia |
| PAP | pulmonary arterial pressure |
| PGD | primary graft dysfunction |
| PPF | progressive pulmonary fibrosis |
| SD | standard deviation |
| SLTx | single lung transplantation |
| SSc-ILD | systemic sclerosis-associated ILD |
| TBB | transbronchial biopsies |
| uILD | unclassifiable pulmonary fibrosis |
| UIP | usual interstitial pneumonia |
| 6MWD | 6-minute walking distance |
References
- Kolb, M.; Vašáková, M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.S.; Choe, J.; Chae, E.J.; Hwang, H.S.; Kim, Y.G.; Song, J.W. Progressive fibrosing interstitial lung disease: Prevalence and clinical outcome. Respir. Res. 2021, 22, 282. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Griffiths, A.P.; Levvey, B.J.; Pilcher, D.V.; Williams, T.J.; Snell, G.I. Definitions of primary graft dysfunction after lung transplantation: Differences between bilateral and single lung transplantation. J. Thorac. Cardiovasc. Surg. 2006, 132, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Wells, A.U.; Flaherty, K.R.; Brown, K.K.; Inoue, Y.; Devaraj, A.; Richeldi, L.; Moua, T.; Crestani, B.; Wuyts, W.A.; Stowasser, S.; et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir. Med. 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Bos, S.; De Sadeleer, L.J.; Vanstapel, A.; Beeckmans, H.; Sacreas, A.; Yserbyt, J.; Wuyts, W.A.; Vos, R. Antifibrotic drugs in lung transplantation and chronic lung allograft dysfunction: A review. Eur. Respir. Rev. 2021, 30, 210050. [Google Scholar] [CrossRef]
- Lambers, C.; Boehm, P.M.; Lee, S.; Ius, F.; Jaksch, P.; Klepetko, W.; Tudorache, I.; Ristl, R.; Welte, T.; Gottlieb, J. Effect of antifibrotics on short-term outcome after bilateral lung transplantation: A multicentre analysis. Eur. Respir. J. 2018, 51, 1800503. [Google Scholar] [CrossRef]
- Leuschner, G.; Stocker, F.; Veit, T.; Kneidinger, N.; Winter, H.; Schramm, R.; Weig, T.; Matthes, S.; Ceelen, F.; Arnold, P.; et al. Outcome of lung transplantation in idiopathic pulmonary fibrosis with previous anti-fibrotic therapy. J. Heart Lung Transplant. 2017, 37, 268–274. [Google Scholar] [CrossRef]
- Mackintosh, J.A.; Munsif, M.; Ranzenbacher, L.; Thomson, C.; Musk, M.; Snell, G.; Glanville, A.; Chambers, D.C.; Hopkins, P. Risk of anastomotic dehiscence in patients with pulmonary fibrosis transplanted while receiving anti-fibrotics: Experience of the Australian Lung Transplant Collaborative. J. Heart Lung Transplant. 2019, 38, 553–559. [Google Scholar] [CrossRef]
- Veit, T.; Leuschner, G.; Sisic, A.; Ceelen, F.; Munker, D.; Schmitzer, M.; Weig, T.; Michel, S.; Schneider, C.; Meiser, B.; et al. Pirfenidone exerts beneficial effects in patients with IPF undergoing single lung transplantation. Am. J. Transplant. 2019, 19, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- de Boer, W.J.; Mannes, G.P.; van der Bij, W. Preoperative corticosteroids. A contraindication to lung transplantation? Chest 1994, 105, 1908. [Google Scholar] [CrossRef] [PubMed]
- McAnally, K.J.; Valentine, V.G.; LaPlace, S.G.; McFadden, P.M.; Seoane, L.; Taylor, D.E. Effect of pre-transplantation prednisone on survival after lung transplantation. J. Heart Lung Transplant. 2006, 25, 67–74. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar]
- Kondoh, Y.; Makino, S.; Ogura, T.; Suda, T.; Tomioka, H.; Amano, H.; Anraku, M.; Enomoto, N.; Fujii, T.; Fujisawa, T.; et al. 2020 guide for the diagnosis and treatment of interstitial lung disease associated with connective tissue disease. Respir. Investig. 2021, 59, 709–740. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Kneidinger, N.; Winter, H.; Sisic, A.; Preissler, G.; Neurohr, C.; Czerner, S.; Weig, T.; Dolch, M.; Uberfuhr, P.; Schramm, R. Munich lung transplant group: Waiting list during the first 9 months of the lung allocation score era. Thorac. Cardiovasc. Surg. 2014, 62, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005, 24, 1454–1459. [Google Scholar] [CrossRef]
- Kauke, T.; Kneidinger, N.; Martin, B.; Dick, A.; Schneider, C.; Schramm, R.; Meimarakis, G.; Preissler, G.; Eickelberg, O.; von Dossow, V.; et al. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue Antigens 2015, 86, 178–185. [Google Scholar] [CrossRef]
- A Yousem, S.; Berry, G.J.; Cagle, P.T.; Chamberlain, D.; Husain, A.N.; Hruban, R.H.; Marchevsky, A.; Ohori, N.P.; Ritter, J.; Stewart, S.; et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J. Heart Lung Transplant. 1996, 15 (1 Pt 1), 1–15. [Google Scholar]
- Vandermeulen, E.; Verleden, S.E.; Ruttens, D.; Moelants, E.; Mortier, A.; Somers, J.; Bellon, H.; Piloni, D.; Dupont, L.J.; Van Raemdonck, D.E.; et al. BAL neutrophilia in azithromycin-treated lung transplant recipients: Clinical significance. Transpl. Immunol. 2015, 33, 37–44. [Google Scholar] [CrossRef]
- Balestro, E.; Solidoro, P.; Parigi, P.; Boffini, M.; Lucianetti, A.; Rea, F. Safety of nintedanib before lung transplant: An Italian case series. Respirol. Case Rep. 2018, 6, e00312. [Google Scholar] [CrossRef]
- Ito, Y.; Tazaki, G.; Kondo, Y.; Takahashi, G.; Sakamaki, F. Therapeutic effect of nintedanib on acute exacerbation of interstitial lung diseases. Respir. Med. Case Rep. 2019, 26, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Kuo, E.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 2008, 86, 189–195, discussion 196–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.J.; Diamond, J.M. Diamond, Primary Graft Dysfunction (PGD) Following Lung Transplantation. Semin. Respir. Crit. Care Med. 2018, 39, 148–154. [Google Scholar] [CrossRef]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Chen, W.-C.; Chen, N.-J.; Chen, H.-P.; Yu, W.-K.; Su, V.Y.-F.; Chen, H.; Wu, H.-H.; Yang, K.-Y. Nintedanib Reduces Neutrophil Chemotaxis via Activating GRK2 in Bleomycin-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 4735. [Google Scholar] [CrossRef]
- Ubieta, K.; Thomas, M.J.; Wollin, L. The Effect of Nintedanib on T-Cell Activation, Subsets and Functions. Drug. Des. Devel Ther. 2021, 15, 997–1011. [Google Scholar] [CrossRef]
- Lehtonen, S.T.; Veijola, A.; Karvonen, H.; Lappi-Blanco, E.; Sormunen, R.; Korpela, S.; Zagai, U.; Sköld, M.C.; Kaarteenaho, R. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 14. [Google Scholar] [CrossRef]
- Visner, G.A.; Liu, F.; Bizargity, P.; Liu, H.; Liu, K.; Yang, J.; Wang, L.; Hancock, W.W. Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation 2009, 88, 330–338. [Google Scholar] [CrossRef]
- Du, J.; Paz, K.; Flynn, R.; Vulic, A.; Robinson, T.M.; Lineburg, K.E.; Alexander, K.A.; Meng, J.; Roy, S.; Panoskaltsis-Mortari, A.; et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood 2017, 129, 2570–2580. [Google Scholar] [CrossRef]
| All (n = 286) | Antifibrotic Monotherapy (n = 82) | Antifibrotic Treatment + Immunosuppressive Drugs (n = 33) | Immunosuppressive Therapy (n = 147) | w/o Specific Treatment (n = 24) | p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | 56.8 ± 8.8 | 59.8 ± 8.1 | 59.9 ± 6.2 | 55.2 ± 8.2 | 52.7 ± 12.9 | <0.001 |
| Sex (male), n (%) | 181 (63.3) | 65 (79.3) | 21 (63.6) | 79 (53.7) | 16 (66.7) | 0.001 |
| BMI (kg/m2) | 24.3 ± 3.7 | 23.6 ± 3.6 | 23.7 ± 3.5 | 24.9 ± 3.7 | 24.5 ± 3.6 | 0.061 |
| IPF, n (%) | 116 (40.6) | 70 (85.4) | 12 (36.4) | 26 (17.7) | 8 (33.3) | 0.001 |
| Smoker Status | ||||||
| Ex-smoker | 157 (54.9) | 54 (66.7) | 22 (66.7) | 65 (44.5) | 16 (66.7) | 0.003 |
| Pack years | 20.9 ± 18.7 | 22.0 ± 12.5 | 19.2 ± 22.0 | 20.3 ± 20.9 | 21.8 ± 22.4 | 0.924 |
| Lung function | ||||||
| FVC, %predicted | 44.4 ± 16.4 | 46.5 ± 16.4 | 42.0 ± 13.6 | 43.6 ± 15.4 | 44.9 ± 24.6 | 0.492 |
| TLC, %predicted | 54.3 ± 15.7 | 53.9 ± 16.0 | 54.3 ± 15.8 | 54.4 ± 15.1 | 54.7 ± 19.2 | 0.995 |
| DLCO, %predicted a | 21.3 ± 8.1 | 21.4 ± 7.9 | 22.0 ± 9.1 | 20.7 ± 7.2 | 23.6 ± 12.1 | 0.717 |
| 6MWD (m) b | 250.1 ± 124.9 | 245.9 ± 135.1 | 240.9 ± 151.3 | 247.6 ± 112.9 | 202.9 ± 116.9 | 0478 |
| pCO2 (mmHg) | 47.4 ± 12.7 | 46.6 ± 11.5 | 47.0 ± 9.6 | 47.2 ± 13.6 | 52.1 ± 13.5 | 0.297 |
| Pulmonary hypertension, n (%) | 209 (73.6) | 61 (75.3) | 26 (78.8) | 103 (70.5) | 19 (79.2) | 0.688 |
| Mean PAP ± SD mmHg) | 26.8 ± 10.5 | 26.6 ± 9.3 | 26.3 ± 9.4 | 26.7 ± 10.9 | 29.0 ± 13.3 | 0.768 |
| GAP-ILD score | 5.0 ±1.2 | 5.2 ± 1.2 | 5.2 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.2 | 0.055 |
| Lung Allocation Score | 47.6 ± 15.9 | 48.2 ± 15.5 | 45.3 ± 11.8 | 47.3 ± 16.3 | 51.0 ± 19.9 | 0.577 |
| All (n = 286) | Antifibrotic Monotherapy (n = 82) | Antifibrotic Treatment + Immunosuppressive Drugs (n = 33) | Immuno- Suppressive Therapy (n = 147) | w/o Specific Treatment (n = 24) | p-Value | |
|---|---|---|---|---|---|---|
| Preoperative | ||||||
| Mechanical Ventilation, n (%) | 15 (5.2) | 5 (6.1) | 0 (0.0) | 9 (6.1) | 1 (4.2) | 0.591 |
| ECMO, veno-venous, n (%) | 22 (7.7) | 4 (4.9) | 1 (3.1) | 13 (8.9) | 4 (16.7) | 0.199 |
| Cold ischemia time (hours) | ||||||
| SLTx | 7.7 ± 2.4 | 8.0 ± 2.6 | 8.2 ± 3.0 | 7.2 ± 2.0 | 8.1 ± 2.5 | 0.716 |
| BLTx, right side | 7.1 ± 2.0 | 6.6 ± 1.8 | 6.7 ± 2.0 | 7.3 ± 2.1 | 8.2 ± 2.2 | 0.016 |
| BLTx, left side | 7.2 ± 2.3 | 6.6 ± 2.1 | 6.3 ± 2.0 | 7.6 ± 2.3 | 7.6 ± 2.8 | 0.003 |
| Donor age (years) | 46.6 ± 16.4 | 46.0 ± 16.2 | 45.9 ± 15.6 | 47.7 ± 16.4 | 42.4 ± 18.8 | 0.493 |
| Donor BMI (kg/m2) | 25.2 ± 3.6 | 24.8 ± 4.1 | 25.2 ± 3.5 | 25.4 ± 3.6 | 25.0 ± 2.4 | 0.712 |
| Donor TLC (l) | 6.5 ± 1.2 | 6.6 ± 1.1 | 6.6 ± 1.0 | 6.4 ± 1.2 | 6.4 ± 1.3 | 0.557 |
| PaO2 at 100% FiO2 (mmHg) | 443.6 ± 72.8 | 445.8 ± 71.3 | 432.6 ± 96.3 | 442.3 ± 68.7 | 459.0 ± 66.9 | 0.585 |
| Intraoperative | ||||||
| Operation time (hours) BLTx | 5.8 ± 1.2 | 5.5 ± 0.8 | 5.5 ± 0.9 | 5.9 ± 1.3 | 5.7 ± 1.0 | 0.315 |
| Operation time (hours) SLTx | 2.7 ± 0.6 | 2.8 ± 0.8 | 2.3 ± 0.3 | 2.7 ± 0.6 | 2.7 ± 0.2 | 0.554 |
| ECMO, veno-venous, n (%) | 23 (8.1) | 4 (4.9) | 1 (3.1) | 15 (10.3) | 3 (12.5) | 0.301 |
| ECMO, veno-arterial, n (%) | 157 (55.3) | 42 (51.2) | 18 (56.3) | 85 (58.2) | 12 (50.0) | 0.718 |
| Intra-operative blood loss (ml) | 2861.5 ± 2235.0 | 2105.7 ± 1409.7 | 2481.8 ± 1432.5 | 3238.6 ± 2532.2 | 3687.5 ± 2758.9 | <0.001 |
| Surgery-related complications | ||||||
| Anastomotic complications, n (%) * | 15 (5.3) | 6 (7.3) | 0 (0.0) | 8 (5.5) | 1 (4.2) | 0.503 |
| Operative revision, patients, n (%) ** | 68 (23.8) | 15 (18.3) | 5 (15.2) | 40 (27.2) | 8 (33.3) | 0.185 |
| Due to bleeding, n (%) | 47 (16.4) | 7 (8.5) | 5 (15.2) | 29 (19.7) | 6 (25.0) | 0.083 |
| Due to wound dehiscence, hernia, fistula, torsion, n (%) | 23 (8.0) | 8 (9.8) | 0 (0.0) | 13 (8.8) | 2 (8.3) | 0.288 |
| Drainage of effusion, n (%) | 24 (8.4) | 5 (6.1) | 2 (6.1) | 15 (10.2) | 2 (8.3) | 0.745 |
| Pneumothorax, n (%) | 16 (5.6) | 3 (3.7) | 1 (3.0) | 12 (8.2) | 0 (0.0) | 0.343 |
| Postoperative | ||||||
| ECMO therapy | ||||||
| Veno-venous, n (%) | 37 (13.0) | 6 (7.3) | 2 (6.3) | 25 (17.1) | 4 (16.7) | 0.103 |
| Veno-arterial, n (%) | 16 (5.6) | 3 (3.7) | 2 (6.3) | 10 (6.8) | 1 (4.2) | 0.813 |
| Re-intubation rate, n (%) | 26 (9.1) | 7 (8.5) | 2 (6.1) | 17 (11.6) | 0 (0.0) | 0.328 |
| Use of inhaled nitric oxid, n (%) | 153 (53.5) | 48 (58.5) | 14 (42.4) | 77 (52.4) | 14 (58.3) | 0.436 |
| Mechanical ventilation (hours) a | 73.0 (7.0–1128.0) | 59.1 (9.2–1128.0) | 57.4 (10.0–814.0) | 98.0 (10.3–792.0) | 69.3 (7.0–808.8) | 0.019 |
| PGD T72, grade | 1.3 ± 1.0 | 0.9 ± 0.9 | 0.8 ± 0.1 | 1.5 ± 1.1 | 1.7 ± 1.0 | <0.001 |
| T72 Grade 3, n (%) | 51 (18.4) | 6 (7.5) | 1 (3.0) | 37 (26.4) | 7 (29.2) | <0.001 |
| Length of ICU stay (days) | 22.1 ± 34.6 | 18.1 ± 42.0 | 14.0 ± 14.7 | 25.6 ± 34.0 | 25.6 ± 26.4 | 0.196 |
| All Patients (n = 286) | Grade of PGD | |||
|---|---|---|---|---|
| UR | MLR | |||
| p-Value | Coeff. r | p-Value | Coeff. Beta | |
| Gender (m = 1) | 0.042 | 0.123 | 0.033 | 0.275 |
| IPF (1) vs. Non-IPF (0) | 0.017 | −0.143 | 0.205 | 0.198 |
| CTD-ILD | 0.887 | 0.009 | 0.160 | −0.285 |
| Age for GAP ILD | 0.001 | −0.203 | 0.232 | −0.009 |
| SLTX (0) vs. BLTX (1) | 0.088 | 0.103 | 0.952 | −0.006 |
| Use of ECMO before LTX | <0.001 | 0.222 | 0.077 | 0.703 |
| BMI | 0.072 | 0.123 | 0.022 | 0.038 |
| LAS Score | 0.003 | 0.178 | 0.945 | <0.001 |
| Time from diagnosis to LTX (months) | 0.043 | 0.122 | 0.149 | 0.012 |
| Intraoperative blood loss (ml) | <0.001 | 0.282 | 0.003 | 0.181 |
| Antifibrotics * | <0.001 | −0.237 | 0.011 | −0.532 |
| Antifibrotics and immunosuppression before LTX | 0.012 | −0.151 | 0.025 | −0.519 |
| Steroid (Prednisolone > 5 mg) before LTX | 0.036 | 0.126 | 0.246 | −0.240 |
| Combined Immunosuppression before LTX ** | 0.024 | 0.135 | 0.182 | 0.255 |
| w/o specific treatment before LTX | 0.039 | 0.124 | 0.837 | −0.049 |
| All (n = 286) | Antifibrotic Monotherapy (n = 82) | Antifibrotic Treatment + Immunosuppressive Drugs (n = 33) | Immuno- Suppressive Therapy (n = 147) | w/o Specific Treatment (n = 24) | p-Value | |
|---|---|---|---|---|---|---|
| Episode of acute cellular rejection (≥A1) | ||||||
| Within 30 days after LTx, n (%) a | 34 (13.5) | 6 (7.7) | 5 (15.6) | 17 (13.8) | 6 (31.6) | 0.057 |
| Within 1 year after LTx, n (%) | 46 (19.4) | 13 (21.0) | 5 (21.7) | 22 (16.9) | 6 (28.6) | 0.549 |
| De novo donor specific HLA-antibodies (DSA) | ||||||
| Within 30 days after LTx, n (%) b; (class I/class II/class I + II, n) | 63 (23.2) (26/25/12) | 10 (12.5) (5/4/1) | 5 (15.2) (3/1/1) | 40 (29.6) (16/15/9) | 8 (34.8) (2/5/1) | 0.009 0.732 |
| Within 1 year after LTx, n (%) c; (class I/class II/class I + II, n) | 77 (31.0) (31/35/11) | 14 (21.5) (5/9/0) | 5 (26.3) (3/1/1) | 50 (35.2) (21/20/9) | 8 (34.8) (2/5/1) | 0.249 0.498 |
| Bronchoalveolar Lavage (BAL) | ||||||
| Neutrophilia within 3 months after LTx d (%) | 8.4 ± 14.9 | 6.9 ± 8.9 | 7.2 ± 13.0 | 10.1 ± 18.3 | 4.7 ± 7.9 | 0.523 |
| Neutrophilia > 15%, n (%) | 18 (15.3) | 2 (10.0) | 2 (20.0) | 13 (17.1) | 1 (8.3) | 0.801 |
| Neutrophilia within 6 months after LTx (%) | 5.1 ± 10.5 | 4.6 ± 5.8 | 5.4 ± 12.3 | 4.8 ± 10.1 | 7.5 ± 19.3 | 0.806 |
| 30-days mortality, n (%) | 6 (2.1) | 1 (1.2) | 0 (0.0) | 5 (3.4) | 0 (0.0) | 0.701 |
| ICU-mortality, n (%) | 8 (2.8) | 2 (2.4) | 0 (0.0) | 6 (4.1) | 0 (0.0) | 0.749 |
| 1-year mortality, n (%) | 24 (4.2) | 6 (7.3) | 1 (3.0) | 16 (10.9) | 1 (4.2) | 0.516 |
| All Patients n = 286 | ||||
|---|---|---|---|---|
| p-Value | Coeff. ß | Odd Ratio | CI | |
| Gender (m = 1) | 0.174 | 0.431 | 1.538 | 0.827–2.861 |
| IPF (1) vs. Non-IPF (0) | 0.003 | 1.148 | 3.152 | 1.463–6.790 |
| CTD-ILD | 0.774 | 0.168 | 1.183 | 0.375–3.732 |
| Age (years) | 0.004 | 0.070 | 1.072 | 1.022–1.124 |
| SLTX (0) vs. BLTX (1) | 0.859 | 0.038 | 0.859 | 0.685–1.573 |
| Use of ECMO before LTX | 0.081 | −1.375 | 0.253 | 0.060–1.068 |
| Mean PAP (mmHg) | 0.222 | −0.018 | 0.982 | 0.954–1.011 |
| BMI | 0.054 | 0.081 | 1.084 | 0.998–1.177 |
| LAS Score | 0.004 | 0.031 | 1.032 | 1.010–1.054 |
| Time from diagnosis to LTX (months) | 0.103 | 0.023 | 1.023 | 0.995–1.052 |
| Intraoperative blood loss (ml) | 0.006 | <0.001 | 1.000 | 1.000–1.000 |
| Antifibrotics * | 0.178 | −0.673 | 0.510 | 0.192–1.358 |
| Antifibrotics and immunosuppression before LTX | 0.181 | −0.974 | 0.388 | 0.097–1.556 |
| Steroid (Prednisolone > 5 mg) before LTX | 0.597 | 0.246 | 1.278 | 0.514–3.178 |
| Combined Immunosuppression before LTX ** | 0.448 | −0.331 | 0.718 | 0.306–1.686 |
| w/o specific treatment before LTX | 0.884 | 0.078 | 1.081 | 0.378–3.091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munker, D.; Arnold, P.; Leuschner, G.; Irlbeck, M.; Michel, S.; Kauke, T.; Meiser, B.; Behr, J.; Kneidinger, N.; Veit, T. Impact of ILD-Specific Therapies on Perioperative Course in Patients with Progressive Interstitial Lung Disease Undergoing Lung Transplantation. J. Clin. Med. 2023, 12, 4996. https://doi.org/10.3390/jcm12154996
Munker D, Arnold P, Leuschner G, Irlbeck M, Michel S, Kauke T, Meiser B, Behr J, Kneidinger N, Veit T. Impact of ILD-Specific Therapies on Perioperative Course in Patients with Progressive Interstitial Lung Disease Undergoing Lung Transplantation. Journal of Clinical Medicine. 2023; 12(15):4996. https://doi.org/10.3390/jcm12154996
Chicago/Turabian StyleMunker, Dieter, Paola Arnold, Gabriela Leuschner, Michael Irlbeck, Sebastian Michel, Teresa Kauke, Bruno Meiser, Jürgen Behr, Nikolaus Kneidinger, and Tobias Veit. 2023. "Impact of ILD-Specific Therapies on Perioperative Course in Patients with Progressive Interstitial Lung Disease Undergoing Lung Transplantation" Journal of Clinical Medicine 12, no. 15: 4996. https://doi.org/10.3390/jcm12154996
APA StyleMunker, D., Arnold, P., Leuschner, G., Irlbeck, M., Michel, S., Kauke, T., Meiser, B., Behr, J., Kneidinger, N., & Veit, T. (2023). Impact of ILD-Specific Therapies on Perioperative Course in Patients with Progressive Interstitial Lung Disease Undergoing Lung Transplantation. Journal of Clinical Medicine, 12(15), 4996. https://doi.org/10.3390/jcm12154996






