Characterizing Sjögren-Associated Fatigue: A Distinct Phenotype from ME/CFS
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Questionnaires for Symptom Scoring
2.3. Hand Grip Strength Measurement
2.4. Determination of Autoantibody Levels and Laboratory Blood Data
2.5. Statistical Analysis
3. Results
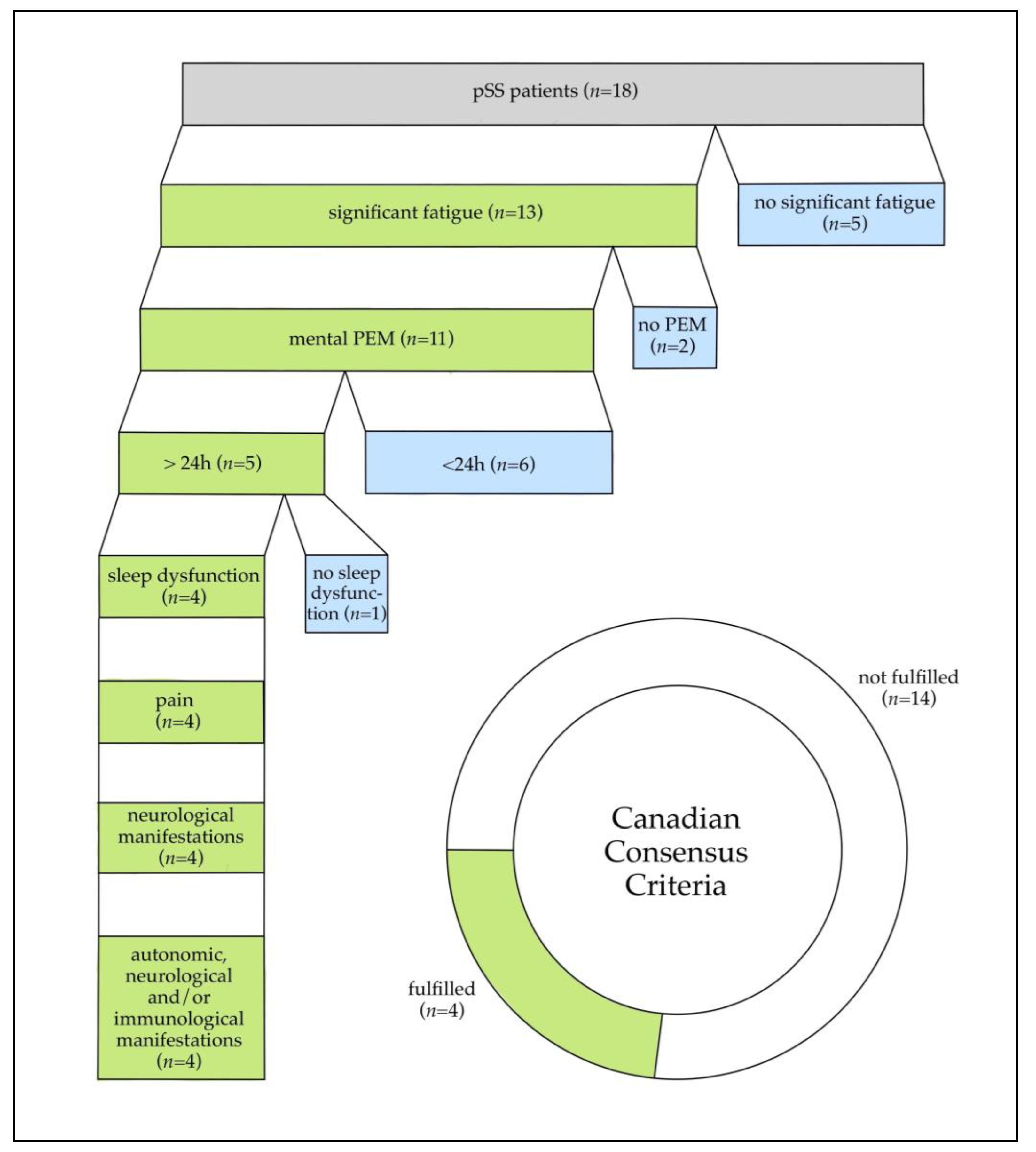
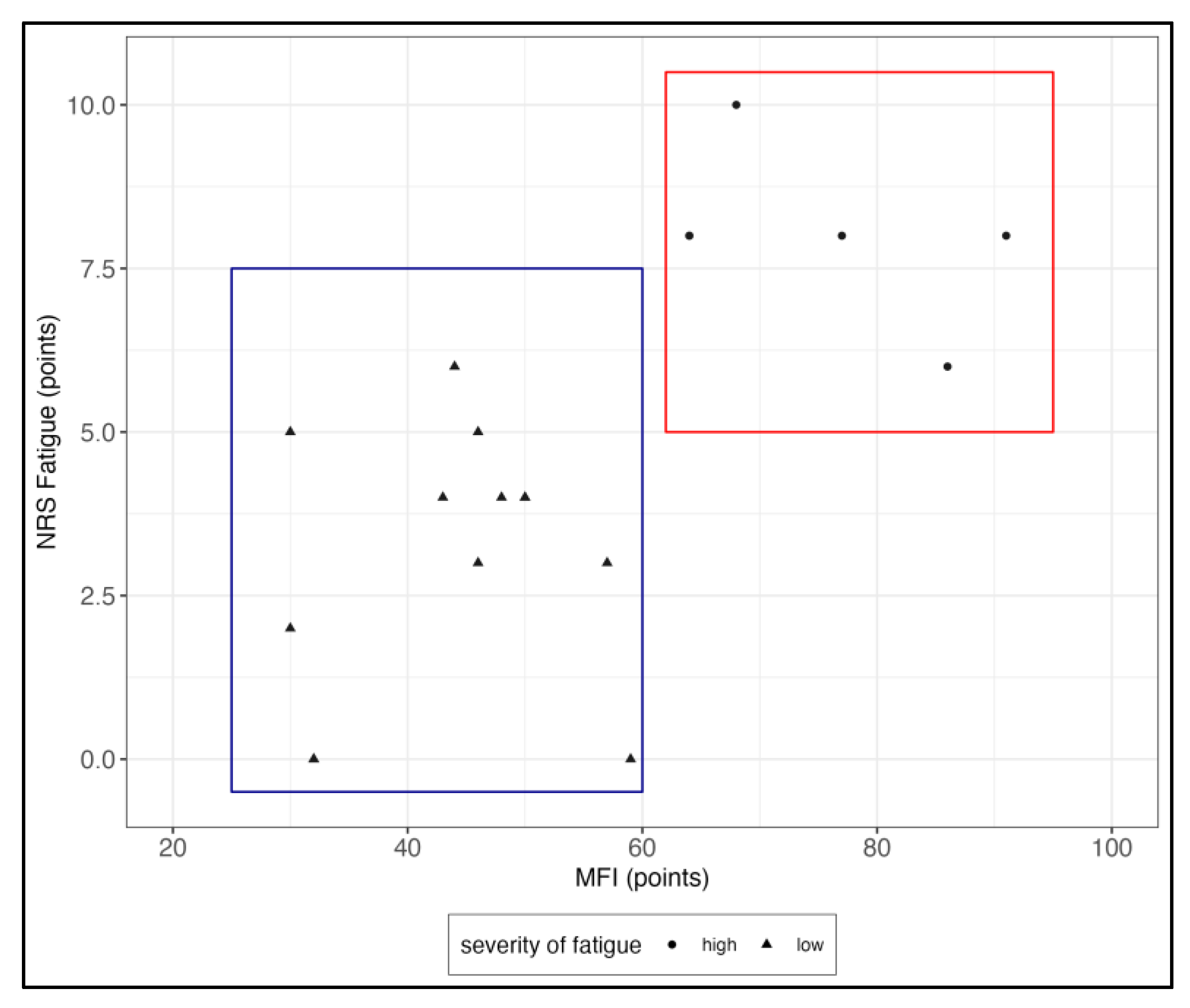
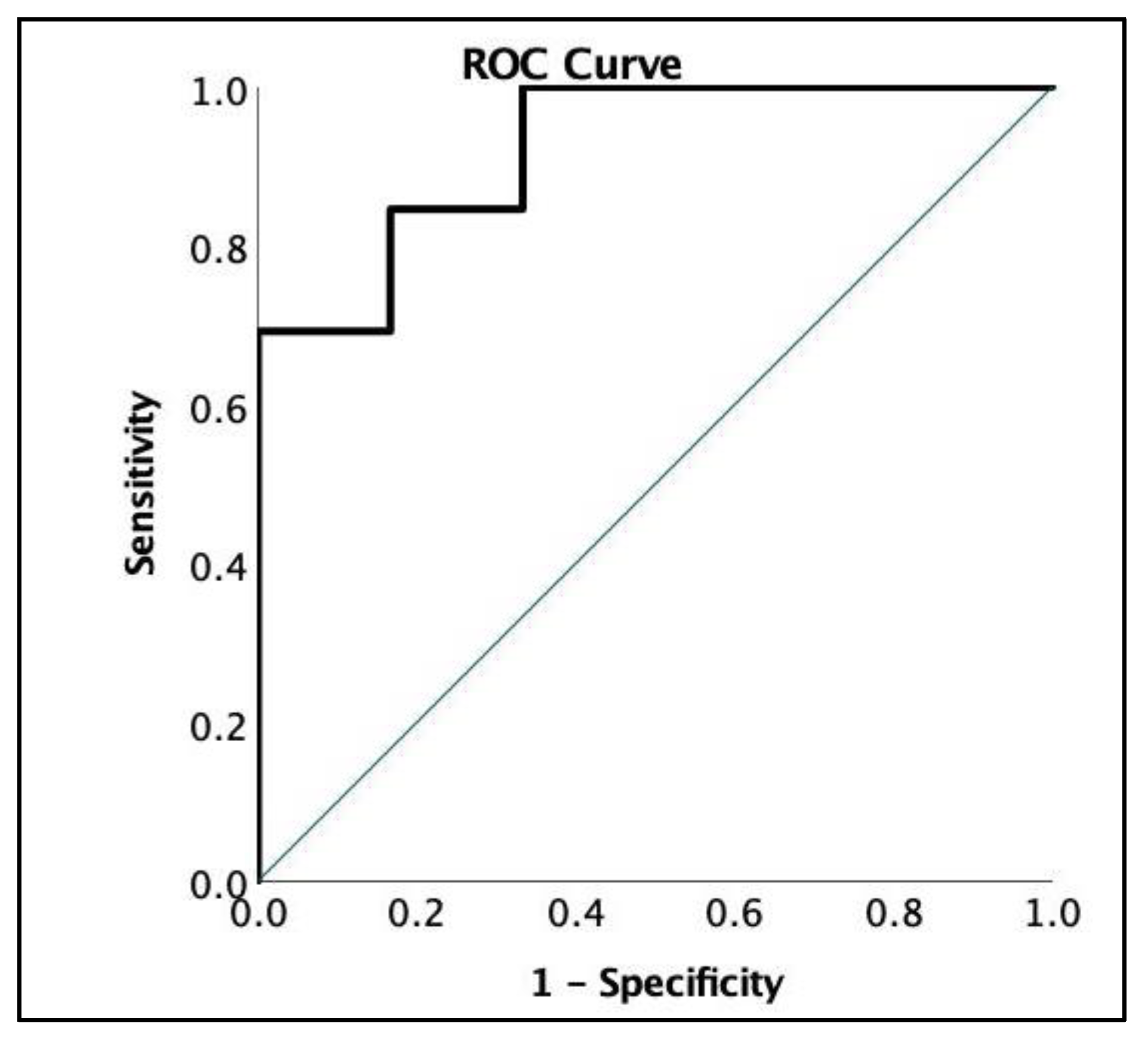
3.1. Patient Characteristics and Symptom Presentation
3.2. Hand Grip Strength
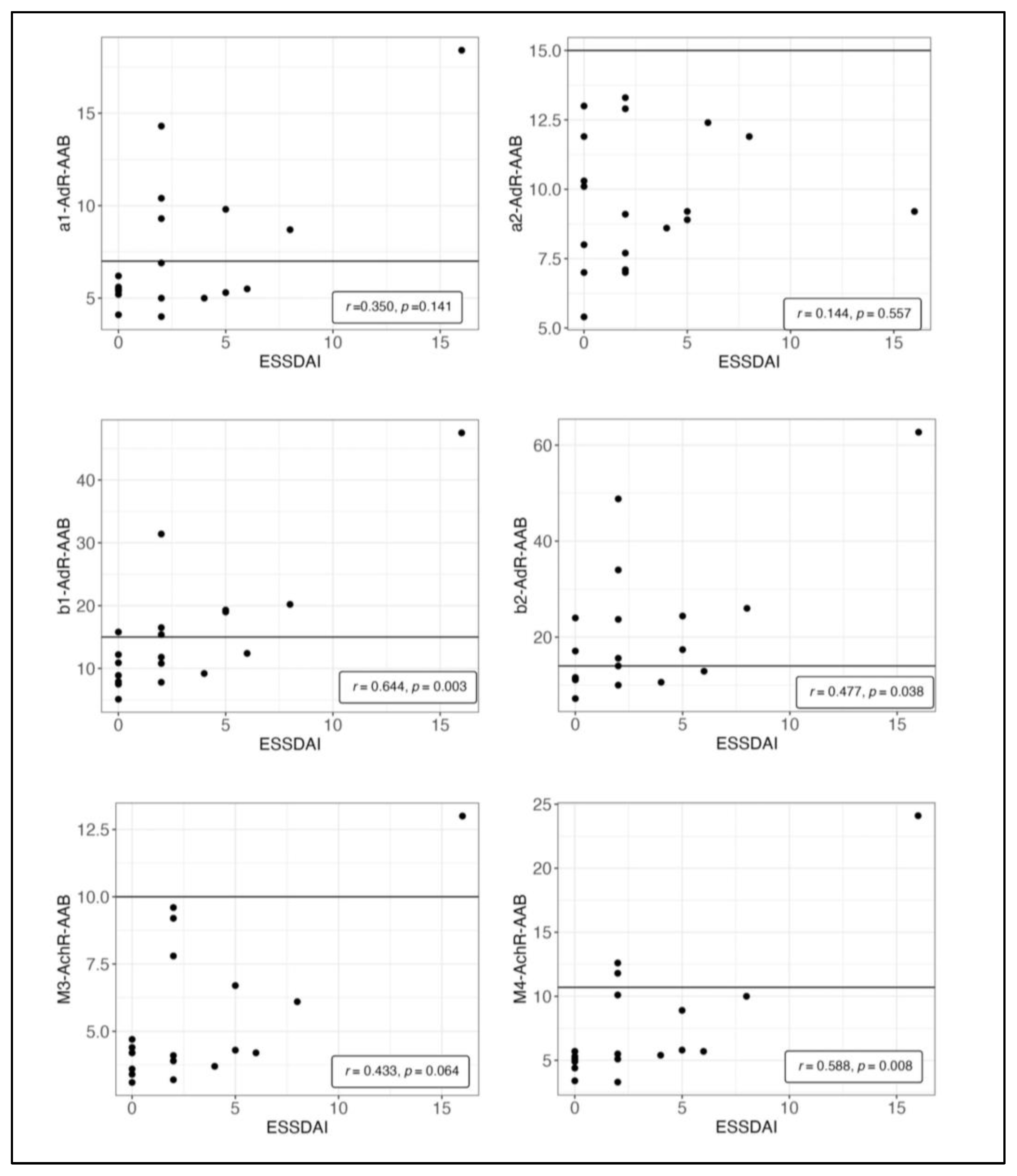
3.3. Autoantibodies
4. Discussion
4.1. Fatigue Severity
4.2. Exertional Intolerance and PEM
4.3. Severity of Other Symptoms
4.4. Autoantibody Correlations
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjogren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dorner, T.; Gottenberg, J.E.; et al. Early diagnosis of primary Sjogren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjogren syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.F.; Bowman, S.J. Primary Sjogren’s syndrome: Too dry and too tired. Rheumatology 2010, 49, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.T.; Lendrem, D.W.; Ng, W.F.; Hackett, K.L.; Valim, V. Managing fatigue in patients with primary Sjogren’s syndrome: Challenges and solutions. Open Access Rheumatol. 2019, 11, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Nishikai, M.; Akiya, K.; Tojo, T.; Onoda, N.; Tani, M.; Shimizu, K. ‘Seronegative’ Sjogren’s syndrome manifested as a subset of chronic fatigue syndrome. Br. J. Rheumatol. 1996, 35, 471–474. [Google Scholar] [CrossRef]
- Sirois, D.A.; Natelson, B. Clinicopathological findings consistent with primary Sjogren’s syndrome in a subset of patients diagnosed with chronic fatigue syndrome: Preliminary observations. J. Rheumatol. 2001, 28, 126–131. [Google Scholar] [PubMed]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C.; on behalf of the European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chonic. Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Alunno, A.; Carubbi, F.; Bartoloni, E.; Cipriani, P.; Giacomelli, R.; Gerli, R. The kaleidoscope of neurological manifestations in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S118), 192–198. [Google Scholar]
- Cafaro, G.; Bursi, R.; Chatzis, L.G.; Fulvio, G.; Ferro, F.; Bartoloni, E.; Baldini, C. One year in review 2021: Sjogren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S133), 3–13. [Google Scholar] [CrossRef]
- Jakel, B.; Kedor, C.; Grabowski, P.; Wittke, K.; Thiel, S.; Scherbakov, N.; Doehner, W.; Scheibenbogen, C.; Freitag, H. Hand grip strength and fatigability: Correlation with clinical parameters and diagnostic suitability in ME/CFS. J. Transl. Med. 2021, 19, 159. [Google Scholar] [CrossRef]
- Goldblatt, J.; James, O.F.; Jones, D.E. Grip strength and subjective fatigue in patients with primary biliary cirrhosis. JAMA 2001, 285, 2196–2197. [Google Scholar] [CrossRef]
- Strandkvist, V.; Andersson, M.; Backman, H.; Larsson, A.; Stridsman, C.; Lindberg, A. Hand grip strength is associated with fatigue among men with COPD: Epidemiological data from northern Sweden. Physiother. Theory Prac. 2020, 36, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset Patterns and Course of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatr. 2019, 7, 12. [Google Scholar] [CrossRef]
- Bartoloni, E.; Alunno, A.; Gerli, R. The dark side of Sjogren’s syndrome: The possible pathogenic role of infections. Curr. Opin. Rheumatol. 2019, 31, 505–511. [Google Scholar] [CrossRef]
- Stefanski, A.L.; Tomiak, C.; Pleyer, U.; Dietrich, T.; Burmester, G.R.; Dorner, T. The Diagnosis and Treatment of Sjogren’s Syndrome. Dtsch. Arztebl. Int. 2017, 114, 354–361. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; Lopez-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef] [PubMed]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, O.; et al. Antibodies to beta adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Freitag, H.; Szklarski, M.; Lorenz, S.; Sotzny, F.; Bauer, S.; Philippe, A.; Kedor, C.; Grabowski, P.; Lange, T.; Riemekasten, G.; et al. Autoantibodies to Vasoregulative G-Protein-Coupled Receptors Correlate with Symptom Severity, Autonomic Dysfunction and Disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2021, 10, 3675. [Google Scholar] [CrossRef]
- Bynke, A.; Julin, P.; Gottfries, C.G.; Heidecke, H.; Scheibenbogen, C.; Bergquist, J. Autoantibodies to beta-adrenergic and muscarinic cholinergic receptors in Myalgic Encephalomyelitis (ME) patients—A validation study in plasma and cerebrospinal fluid from two Swedish cohorts. Brain Behav. Immun. Health 2020, 7, 100107. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Sato, W.; Kimura, Y.; Matsuda, H.; Ota, M.; Maikusa, N.; Suzuki, F.; Amano, K.; Shin, I.; Yamamura, T.; et al. Altered Structural Brain Networks Related to Adrenergic/Muscarinic Receptor Autoantibodies in Chronic Fatigue Syndrome. J. Neuroimaging 2020, 30, 822–827. [Google Scholar] [CrossRef]
- Tolle, M.; Freitag, H.; Antelmann, M.; Hartwig, J.; Schuchardt, M.; van der Giet, M.; Eckardt, K.U.; Grabowski, P.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption. J. Clin. Med. 2020, 9, 2443. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to remove ss2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS ONE 2018, 13, e0193672. [Google Scholar] [CrossRef]
- Davies, K.; Ng, W.F. Autonomic Nervous System Dysfunction in Primary Sjogren’s Syndrome. Front. Immunol. 2021, 12, 702505. [Google Scholar] [CrossRef]
- Newton, J.L.; Frith, J.; Powell, D.; Hackett, K.; Wilton, K.; Bowman, S.; Price, E.; Pease, C.; Andrews, J.; Emery, P.; et al. Autonomic symptoms are common and are associated with overall symptom burden and disease activity in primary Sjogren’s syndrome. Ann. Rheum. Dis. 2012, 71, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, A.; Kurien, B.T.; Scofield, R.H. Autoantibodies in Sjogren’s Syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Dachverband Osteologie e.V. Prophylaxe, Diagnostik und Therapie der Osteoporose bei Postmenopausalen Frauen und Männern—Leitlinie des Dachverbands der Deutschsprachigen Wissenschaftlichen Osteologischen Gesellschaften e.V.; AWMF online Dachverband Osteologie e.V.: Essen, Germany, 2017. [Google Scholar]
- Seror, R.; Theander, E.; Brun, J.G.; Ramos-Casals, M.; Valim, V.; Dorner, T.; Bootsma, H.; Tzioufas, A.; Solans-Laque, R.; Mandl, T.; et al. Validation of EULAR primary Sjogren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann. Rheum. Dis. 2015, 74, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, S.; Dures, E.; Almeida, C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res. 2011, 63 (Suppl. S11), S263–S286. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef]
- Koh, J.H.; Kwok, S.K.; Lee, J.; Son, C.N.; Kim, J.M.; Kim, H.O.; Park, S.H.; Sung, Y.K.; Choe, J.Y.; Lee, S.S.; et al. Pain, xerostomia, and younger age are major determinants of fatigue in Korean patients with primary Sjogren’s syndrome: A cohort study. Scand J. Rheumatol. 2017, 46, 49–55. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Hsu, C.W.; Lu, M.C.; Koo, M. Increased risks of psychiatric disorders in patients with primary Sjogren’s syndrome-a secondary cohort analysis of nationwide, population-based health claim data. Clin. Rheumatol. 2019, 38, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Priori, R.; Minniti, A.; Antonazzo, B.; Fusconi, M.; Valesini, G.; Curcio, G. Sleep quality in patients with primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2016, 34, 373–379. [Google Scholar]
- Cai, F.Z.; Lester, S.; Lu, T.; Keen, H.; Boundy, K.; Proudman, S.M.; Tonkin, A.; Rischmueller, M. Mild autonomic dysfunction in primary Sjogren’s syndrome: A controlled study. Arthritis Res. Ther. 2008, 10, R31. [Google Scholar] [CrossRef]
- Cui, Y.; Xia, L.; Li, L.; Zhao, Q.; Chen, S.; Gu, Z. Anxiety and depression in primary Sjogren’s syndrome: A cross-sectional study. BMC Psychiatry 2018, 18, 131. [Google Scholar] [CrossRef]
- Segal, B.; Thomas, W.; Rogers, T.; Leon, J.M.; Hughes, P.; Patel, D.; Patel, K.; Novitzke, J.; Rohrer, M.; Gopalakrishnan, R.; et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjogren’s syndrome. Arthritis Rheum. 2008, 59, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.; Sterin-Borda, L.; Camusso, J.J.; Arana, R.; Hubscher, O.; Borda, E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjogren’s syndrome. Clin. Exp. Immunol. 1996, 104, 454–459. [Google Scholar] [CrossRef]
- Nardi, N.; Brito-Zeron, P.; Ramos-Casals, M.; Aguilo, S.; Cervera, R.; Ingelmo, M.; Font, J. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjogren’s syndrome: Prevalence and clinical significance in 335 patients. Clin. Rheumatol. 2006, 25, 341–346. [Google Scholar] [CrossRef] [PubMed]
| Whole Cohort (n = 19, Median with IQR) | High Fatigue (n = 6, Median with IQR) | Low Fatigue (n = 13, Median with IQR) | High vs. Low | |
|---|---|---|---|---|
| Sex (f/m) | 18/1 | 6/0 | 12/1 | p = 0.497 |
| Age (years) | 63 (52–68) | 68.5 (52–77) | 60 (54–67) | p = 0.253 |
| Disease Duration (years) | 7 (3–10) | 7.5 (2.5–10.5) | 7 (3.5–10.5) | p = 0.792 |
| pSS Indices | ||||
| ESSDAI | 2 (0–5) | 3.5 (1.5–8.5) | 2 (0–3) | p = 0.131 |
| ESSPRI | 4 (3.33–6) | 7 (5.9–8) | 4 (3.3–4.3) | p < 0.001 ** |
| NRS Pain | 3 (0–5) | 6 (3–7.25) | 3 (0–4) | p = 0.045 * |
| NRS Dryness | 6 (5–9) | 8.5 (5–9.25) | 6 (4.5–8.5) | p = 0.266 |
| NRS Fatigue | 4 (3–8) | 8 (7.5–8.5) | 4 (1–4) | p < 0.001 ** |
| MFI | 50 (43–64) | 72.5 (64–87.25) | 46 (32–50) | p < 0.001 ** |
| General Fatigue | 12 (11–15) | 15.5 (14.25–16.5) | 12 (7.5–13.5) | p = 0.004 ** |
| Physical Fatigue | 10 (7–14) | 15.5 (14–18.5) | 8 (6.5–11) | p < 0.001 ** |
| Reduced Activity | 11 (7–14) | 16.5 (13.5–18.5) | 9 (6.5–11.5) | p < 0.001 ** |
| Reduced Motivation | 8 (6–13) | 13 (8.75–15.5) | 7 (5–8.5) | p = 0.005 ** |
| Mental Fatigue | 8 (6–15) | 15 (11–19.25) | 7 (4–12.5) | p = 0.010 * |
| HGS (kg) | ||||
| Fmean 1 | 20.18 (15.18–25.27) | 11.73 (6.5–17.8) | 24.75 (19.24–27.95) | p = 0.004 ** |
| Fmean 2 | 19.30 (15.03–25.35) | 11.36 (5.22–15.62) | 23.27 (18.37–28.24) | p = 0.002 ** |
| Fmax 1 | 25.70 (19.20–30.70) | 16.55 (8.25–21.6) | 27.10 (21.65–31.85) | p = 0.023 * |
| Fmax 2 | 21.20 (18.50–28.00) | 13.8 (8.25–21.6) | 25.3 (20.95–30.8) | p = 0.008 ** |
| Fatigue Ratio 1 | 1.18 (1.09–1.36) | 1.41 (1.2–1.75) | 1.14 (1.09–1.22) | p = 0.011 * |
| Fatigue Ratio 2 | 1.11 (1.07–1.35) | 1.37 (1.28–1.75) | 1.08 (1.06–1.23) | p = 0.005 ** |
| Recovery Ratio | 0.98 (0.79–1.35) | 0.85 (0.76–1.15) | 0.99 (0.91–1.05) | p = 0.430 |
| HADS | 10 (8–15) | 16.5 (11.25–24.75) | 9 (5.5–11) | p = 0.015 * |
| Depression | 4 (1–8) | 8 (3.3–12-25) | 3 (1–4.5) | p = 0.021 * |
| Anxiety | 7 (5–9) | 9 (6.25–14.25) | 6 (3.5–7.5) | p = 0.084 |
| PSQI | 8 (6–13) | 13.5 (9–16.25) | 7 (4–11) | p = 0.020 * |
| COMPASS 31 | 18.22 (13.47–39.12) | 43.27 (24.01–54.59) | 16.46 (13.33–20.79) | p = 0.062 |
| IPAQ | ||||
| MET-Minutes/Week (1) | 2919 (1278–6238) | 1159 (0–6692) | 3519 (1586–5687) | p = 0.223 |
| Autoantibodies (U/mL) | ||||
| M3 | 4.2 (3.7–6.7) | 5.45 (3.93–10.15) | 4.2 (3.65–5.4) | p = 0.380 |
| M4 | 5.7 (5.1–10) | 7.3 (5.05–14.87) | 5.5 (4.75–7.9) | p = 0.356 |
| α1 | 5.5 (5.2–9.3) | 7.65 (5.3–12.4) | 5.5 (5.1–7.8) | p = 0.272 |
| α2 | 9.2 (7.7–11.9) | 9.65 (8.45–12.63) | 9.1 (7.35–11.9) | p = 0.404 |
| β1 | 12.2 (8.9–19) | 14.45 (10.83–26.35) | 10.9 (8.35–17.4) | p = 0.254 |
| β2 | 15.6 (11.2–24.4) | 20 (12.48–41.18) | 14 (10.85–23.85) | p = 0.219 |
| CCC (Fulfilled/Not Fulfilled) | (4/14) | (2/3) | (2/11) | p = 0.274 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, L.; Kedor, C.; Buttgereit, F.; Heidecke, H.; Schaumburg, D.; Scheibenbogen, C. Characterizing Sjögren-Associated Fatigue: A Distinct Phenotype from ME/CFS. J. Clin. Med. 2023, 12, 4994. https://doi.org/10.3390/jcm12154994
Kim L, Kedor C, Buttgereit F, Heidecke H, Schaumburg D, Scheibenbogen C. Characterizing Sjögren-Associated Fatigue: A Distinct Phenotype from ME/CFS. Journal of Clinical Medicine. 2023; 12(15):4994. https://doi.org/10.3390/jcm12154994
Chicago/Turabian StyleKim, Laura, Claudia Kedor, Frank Buttgereit, Harald Heidecke, Desiree Schaumburg, and Carmen Scheibenbogen. 2023. "Characterizing Sjögren-Associated Fatigue: A Distinct Phenotype from ME/CFS" Journal of Clinical Medicine 12, no. 15: 4994. https://doi.org/10.3390/jcm12154994
APA StyleKim, L., Kedor, C., Buttgereit, F., Heidecke, H., Schaumburg, D., & Scheibenbogen, C. (2023). Characterizing Sjögren-Associated Fatigue: A Distinct Phenotype from ME/CFS. Journal of Clinical Medicine, 12(15), 4994. https://doi.org/10.3390/jcm12154994






