Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease?
Abstract
1. Introduction
2. Microvascular Remodeling in Hypertension
2.1. Pathophysiology of Microvascular Remodeling
2.2. Role of the the Immune System in Microvascular Remodeling: Interaction with Hormonal Signals, the Sympathetic Nervous System, and PVAT
2.3. Prognostic Role of Microvascular Structural Alterations
2.4. Possible Prevention/Regression of Microvascular Remodeling
3. Interrelationship between Microvascular and Macrovascular Remodeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 14 June 2023).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, E.; Rizzoni, D. Microvascular structure as a prognostically relevant endpoint. J. Hypertens. 2017, 35, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Agabiti-Rosei, C.; De Ciuceis, C. State of the art review: Vascular remodeling in hypertension. Am. J. Hypertens. 2023, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, G.; Agabiti Rosei, C.; Lemoli, M.; Rossini, C.; Muiesan, M.L.; Rizzoni, D.; De Ciuceis, C. Organ damage in hypertension: Role of the microcirculation. Front. Cardiovasc. Med. 2023; submitted. [Google Scholar]
- Mulvany, M.J.; Aalkjaer, C. Structure and function of small arteries. Physiol. Rev. 1990, 70, 921–971. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am. J. Hypertens. 2004, 17 Pt 1, 1192–1200. [Google Scholar] [CrossRef]
- Rizzoni, D.; De Ciuceis, C.; Szczepaniak, P.; Paradis, P.; Schiffrin, E.L.; Guzik, T.J. Immune system and microvascular remodeling in humans. Hypertension 2022, 79, 691–705. [Google Scholar] [CrossRef]
- Schiffrin, E.L. How structure, mechanics, and function of the vasculature contribute to BP elevation in hypertension. Can. J. Cardiol. 2020, 36, 648–658. [Google Scholar] [CrossRef]
- Folkow, B. Physiological aspects of primary hypertension. Physiol. Rev. 1982, 62, 347–504. [Google Scholar] [CrossRef]
- Bohlen, H.G. Localization of vascular resistance changes during hypertension. Hypertension 1986, 8, 181–183. [Google Scholar] [CrossRef]
- Borders, J.L.; Granger, H.J. Power dissipation as a measure of peripheral resistance in vascular networks. Hypertension 1986, 8, 184–191. [Google Scholar] [CrossRef]
- Christensen, K.L.; Mulvany, M.J. Location of resistance arteries. J. Vasc. Res. 2001, 38, 1–12. [Google Scholar] [CrossRef]
- Rizzoni, D.; De Ciuceis, C.; Porteri, E.; Paiardi, S.; Boari, G.E.; Mortini, P.; Cornali, C.; Cenzato, M.; Rodella, L.F.; Borsani, E.; et al. Altered structure of small cerebral arteries in patients with essential hypertension. J. Hypertens. 2009, 27, 838–845. [Google Scholar] [CrossRef]
- Heagerty, A.M.; Aalkjaaer, C.; Bund, S.J.; Korsgaard, N.; Mulvany, M.J. Small artery structure in hypertension. Dual process of remodeling and growth. Hypertension 1993, 21, 391–397. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Baumbach, G.L.; Aalkjaer, C.; Heagerty, A.M.; Korsgaard, N.; Schiffrin, E.L.; Heistad, D.D. Vascular remodeling. Hypertension 1996, 28, 505–506. [Google Scholar]
- Rizzoni, D.; Porteri, E.; Castellano, M.; Bettoni, G.; Muiesan, M.L.; Muiesan, P.; Giulini, S.M.; Agabiti Rosei, E. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996, 28, 785–790. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Guelfi, D.; Muiesan, M.L.; Valentini, U.; Cimino, A.; Girelli, A.; Rodella, L.; Bianchi, R.; Sleiman, I.; et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin dependent diabetes mellitus. Circulation 2001, 103, 1238–1244. [Google Scholar] [CrossRef]
- Schofield, I.; Malik, R.; Izzard, A.; Austin, C.; Heagerty, A.M. Vascular structural and functional changes in type 2 diabetes mellitus. Evidence for the role of abnormal myogenic responsiveness and dyslipidemia. Circulation 2002, 106, 3037–3043. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Scopelliti, F.; Dell’Oro, R.; Fattori, L.; Quarti-Trevano, F.; Brambilla, G.; Schiffrin, E.L.; Mancia, G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity 2010, 18, 92–98. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Porteri, E.; Rizzoni, D.; Corbellini, C.; La Boria, E.; Boari, G.E.; Pilu, A.; Mittempergher, F.; Di Betta, E.; Casella, C.; et al. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension 2011, 58, 29–36. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Brambilla, G.; Facchetti, R.; Bolla, G.; Mozzi, E.; Mancia, G. Impact of the metabolic syndrome on subcutaneous microcirculation in obese patients. J. Hypertens. 2010, 28, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; De Ciuceis, C.; Rodella, L.F.; Paiardi, S.; Rizzardi, N.; Platto, C.; Boari, G.E.; Pilu, A.; Tiberio, G.A.; et al. Hypertrophic remodeling of subcutaneous small resistance arteries in patients with Cushing’s syndrome. Clin. Endocrinol. Metab. 2009, 94, 5010–5018. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Giustina, A.; De Ciuceis, C.; Sleiman, I.; Boari, G.E.; Castellano, M.; Muiesan, M.L.; Bonadonna, S.; Burattin, A.; et al. Acromegalic patients show the presence of hypertrophic remodeling of subcutaneous small resistance arteries. Hypertension 2004, 43, 561–565. [Google Scholar] [CrossRef] [PubMed]
- De Ciuceis, C.; Rossini, C.; Porteri, E.; La Boria, E.; Corbellini, C.; Mittempergher, F.; Di Betta, E.; Petroboni, B.; Sarkar, A.; Agabiti-Rosei, C.; et al. Circulating endothelial progenitor cells, microvascular density and fibrosis in obesity before and after bariatric surgery. Blood Press. 2013, 22, 165–172. [Google Scholar] [CrossRef]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Heagerty, A.M.; Petersen, K.K.; Swales, J.D.; Mulvany, M.J. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation—contraction coupling in isolated resistance vessels from essential hypertensives. Circ. Res. 1987, 61, 181–186. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Hayoz, D. How to assess vascular remodelling in small and medium-sized muscular arteries in humans. J. Hypertens. 1997, 15, 571–584. [Google Scholar] [CrossRef]
- Virdis, A.; Savoia, C.; Grassi, G.; Lembo, G.; Vecchione, C.; Seravalle, G.; Taddei, S.; Volpe, M.; Aganiti Rosei, E.; Rizzoni, D. Evaluation of microvascular structure in humans: A ‘state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J. Hypertens. 2014, 32, 2120–2129. [Google Scholar] [CrossRef]
- Rizzoni, D.; Mengozzi, A.; Masi, S.; Agabiti Rosei, C.; De Ciuceis, C.; Virdis, A. New noninvasive methods to evaluate microvascular structure and function. Hypertension 2022, 79, 874–886. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Agabiti Rosei, C.; Caletti, S.; Trapletti, V.; Coschignano, M.A.; Tiberio, G.A.M.; Duse, S.; Docchio, F.; Pasinetti, S.; Zambonardi, F.; et al. Comparison between invasive and noninvasive techniques of evaluation of microvascular structural alterations. J. Hypertens. 2018, 36, 1154–1163. [Google Scholar] [CrossRef]
- Harazny, J.M.; Ritt, M.; Baleanu, D.; Ott, C.; Heckmann, J.; Schlaich, M.P.; Michelson, G.; Schmieder, R.E. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension 2007, 50, 623–829. [Google Scholar] [CrossRef]
- Ritt, M.; Harazny, J.M.; Ott, C.; Schlaich, M.P.; Schneider, M.P.; Michelson, G.; Schmieder, R.E. Analysis of retinal arteriolar structure in never-treated patients with essential hypertension. J. Hypertens. 2008, 26, 1427–1434. [Google Scholar] [CrossRef]
- Koch, E.; Rosenbaum, D.; Brolly, A.; Sahel, J.A.; Chaumet-Riffaud, P.; Girerd, X.; Rossant, F.; Paques, M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: Relationship with BP and focal vascular changes. J. Hypertens. 2014, 32, 890–898. [Google Scholar] [CrossRef]
- Heagerty, A.M.; Heerkens, E.H.; Izzard, A.S. Small artery structure and function in hypertension. J. Cell. Mol. Med. 2010, 14, 1037–1043. [Google Scholar] [CrossRef]
- Heerkens, E.H.; Izzard, A.S.; Heagerty, A.M. Integrins, vascular remodeling, and hypertension. Hypertension 2007, 49, 1–4. [Google Scholar] [CrossRef]
- Bakker, E.N.; Buus, C.L.; Spaan, J.A.; Perree, J.; Ganga, A.; Rolf, T.M.; Sorop, O.; Bramsen, L.H.; Mulvany, M.J.; Vanbavel, E. Small artery remodeling depends on tissue-type transglutaminase. Circ. Res. 2005, 96, 119–126. [Google Scholar] [CrossRef]
- Rizzoni, D.; Paiardi, S.; Rodella, L.; Porteri, E.; De Ciuceis, C.; Rezzani, R.; Boari, G.E.; Zani, F.; Miclini, M.; Tiberio, G.A.; et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 2006, 91, 2638–2642. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Szczepaniak, P.; Vidler, F.; Maffia, P.; Graham, G.J.; Guzik, T.J. Role of inflammatory chemokines in hypertension. Pharmacol. Ther. 2021, 223, 107799. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Touyz, R.M. From bedside to bench to bedside: Role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am. J. Physio. Circ. Physiol. 2004, 287, H435–H446. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Montezano, A.C. Angiotensin-(1–7) and vascular function. Hypertension 2018, 71, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, C.; Paini, A.; De Ciuceis, C.; Withers, S.; Greenstein, A.; Heagerty, A.M.; Rizzoni, D. Modulation of vascular reactivity by perivascular adipose tissue (PVAT). Curr. Hypertens. Rep. 2018, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Meyer, M.R.; Fredette, N.C.; Barton, M.; Prossnitz, E.R. Regulation of vascular smooth muscle tone by adipose derived contracting factor. PLoS ONE 2013, 8, e79245. [Google Scholar] [CrossRef]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef]
- Harrison, D.G.; Vinh, A.; Lob, H.; Madhur, M.S. Role of the adaptive immune system in hypertension. Curr. Opin. Pharmacol. 2010, 10, 203–207. [Google Scholar] [CrossRef]
- Shao, J.; Nangaku, M.; Miyata, T.; Inagi, R.; Yamada, K.; Kurokawa, K.; Fujita, T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 2003, 42, 31–38. [Google Scholar] [CrossRef]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef]
- Barhoumi, T.; Kasal, D.A.; Li, M.W.; Shbat, L.; Laurant, P.; Neves, M.F.; Paradis, P.; Schiffrin, E.L. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 2011, 57, 469–476. [Google Scholar] [CrossRef]
- Viel, E.C.; Lemarié, C.A.; Benkirane, K.; Paradis, P.; Schiffrin, E.L. Immune regulation and vascular inflammation in genetic hypertension. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H938–H944. [Google Scholar] [CrossRef]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Carnevale, D.; Pallante, F.; Fardella, V.; Fardella, S.; Iacobucci, R.; Federici, M.; Cifelli, G.; De Lucia, M.; Lembo, G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 2014, 41, 737–752. [Google Scholar] [CrossRef]
- Lori, A.; Perrotta, M.; Lembo, G.; Carnevale, D. The spleen: A hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2017, 18, 1216. [Google Scholar] [CrossRef]
- Marvar, P.J.; Thabet, S.R.; Guzik, T.J.; Lob, H.E.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ. Res. 2010, 107, 263–270. [Google Scholar] [CrossRef]
- Lob, H.E.; Marvar, P.J.; Guzik, T.J.; Sharma, S.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 2010, 55, 277–283. [Google Scholar] [CrossRef]
- Case, A.J.; Zimmerman, M.C. Redox-regulated suppression of splenic T-lymphocyte activation in a model of sympathoexcitation. Hypertension 2015, 65, 916–923. [Google Scholar] [CrossRef]
- Jones, B.H.; Standridge, M.K.; Moustaid, N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 1997, 138, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Georgiopoulos, G.; Chiriacò, M.; Grassi, G.; Seravalle, G.; Savoia, C.; Volpe, M.; Taddei, S.; Rizzoni, D.; Virdis, A. The importance of endothelial dysfunction in resistance artery remodelling and cardiovascular risk. Cardiovasc. Res. 2020, 116, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Masi SRizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; Lanza, G.A.; et al. Assessment and pathophysiology of microvascular disease: Recent progress and clinical implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.; de Ciuceis, C.; Dell’oro, R.; Georgiopoulos, G.; Lazaridis, A.; Nosalski, R.; Pavlidis, G.; Tual-Chalot, S.; Agabiti-Rosei, C.; Anyfanti, P.; et al. The importance of microvascular inflammation in ageing and age-related diseases: A position paper from the ESH working group on small arteries, section of microvascular inflammation. J. Hypertens. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Schiffrin, E.L. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J. Hypertens. 2001, 19, 921–930. [Google Scholar] [CrossRef]
- Lever, A.F. Slow pressor mechanisms in hypertension: A role for hypertrophy of resistance vessels? J. Hypertens. 1986, 4, 515–524. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Reactivity of small blood vessel in hypertension: Relation with structural changes. Hypertension 1992, 19 (Suppl. SII), II1–II9. [Google Scholar]
- Rizzoni, D.; Agabiti-Rosei, C.; Agabiti-Rosei, E. Hemodynamic consequences of changes in microvascular structure. Am. J. Hypertens. 2017, 30, 939–946. [Google Scholar] [CrossRef]
- Rizzoni, D.; Palombo, C.; Porteri, E.; Muiesan, M.L.; Kozàkovà, M.; La Canna, G.; Nardi, M.; Guelfi, D.; Salvetti, M.; Morizzo, C.; et al. Relationships between coronary vasodilator capacity and small artery remodeling in hypertensive patients. J. Hypertens. 2003, 21, 625–632. [Google Scholar] [CrossRef]
- Agabiti Rosei, E.; Rizzoni, D.; Castellano, M.; Porteri, E.; Zulli, R.; Muiesan, M.L.; Bettoni, G.; Salvetti, M.; Muiesan, P.; Giulini, S.M. Media: Lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. J. Hypertens. 1995, 13, 341–347. [Google Scholar]
- Muiesan, M.L.; Rizzoni, D.; Salvetti, M.; Porteri, E.; Monteduro, C.; Guelfi, D.; Castellano, M.; Garavelli, G.; Agabiti-Rosei, E. Structural changes in small resistance arteries and left ventricular geometry in patients with primary and secondary hypertension. J. Hypertens. 2002, 20, 1439–1444. [Google Scholar] [CrossRef]
- Rizzoni, D.; Muiesan, M.L.; Porteri, E.; Salvetti, M.; Castellano, M.; Bettoni, G.; Tiberio, G.; Giulini, S.M.; Monteduro, C.; Garavelli, G.; et al. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J. Am. Coll. Cardiol. 1998, 32, 985–992. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Boari, G.E.M.; De Ciuceis, C.; Sleiman, I.; Muiesan, M.L.; Castellano, M.; Miclini, M.; Agabiti-Rosei, E. Prognostic significance of small artery structure in hypertension. Circulation 2003, 108, 2230–2235. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Porteri, E.; Rizzoni, D.; Rizzardi, N.; Paiardi, S.; Boari, G.E.M.; Miclini, M.; Zani, F.; Muiesan, M.L.; Donato, F.; et al. Structural alterations of subcutaneous small arteries may predict major cardiovascular events in hypertensive patients. Am. J. Hypertens. 2007, 20, 846–852. [Google Scholar] [CrossRef]
- Mathiassen, O.N.; Buus, N.H.; Sihm, I.; Thybo, N.K.; Mørn, B.; Schroeder, A.P.; Thygesen, K.; Aalkjaer, C.; Lederballe, O.; Mulvany, M.J.; et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens. 2007, 25, 1021–1026. [Google Scholar] [CrossRef]
- Izzard, A.S.; Rizzoni, D.; Agabiti-Rosei, E.; Heagerty, A.M. Small artery structure and hypertension: Adaptive changes and target organ damage. J. Hypertens. 2005, 23, 247–250. [Google Scholar] [CrossRef]
- Heagerty, A.M. Predicting hypertension complications from small artery structure. J. Hypertens. 2007, 25, 939–940. [Google Scholar] [CrossRef]
- Ritt, M.; Harazny, J.M.; Ott, C.; Schneider, M.P.; Schlaich, M.P.; Michelson, G.; Schmieder, R.E. Wall-to-lumen ratio of retinal arterioles is related with urinary albumin excretion and altered vascular reactivity to infusion of the nitric oxide synthase inhibitor N-monomethyl-L-arginine. J. Hypertens. 2009, 27, 2201–2218. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Mattina, A.; Kock, E.; Rossant, F.; Gallo, A.; Kachenoura, N.; Paques, M.; Redheuil, A.; Gired, X. Effects of age, BP and antihypertensive treatment on retinal arterioles remodeling assessed by adaptive optics. J. Hpertens. 2016, 34, 1115–1122. [Google Scholar] [CrossRef]
- Meixner, E.; Michelson, G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1985–1995. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Agabiti-Rosei, C.; Malerba, P.; Rossini, C.; Chiarini, G.; Brami, V.; Famà, F.; Gaggero, A.; Nardin, M.; Lemoli, M.; et al. Prognostic significance of the wall to lumen ratio of retinal arterioles evaluated by adaptive optics in human hypertension. Eur. J. Intern. Med. 2023, 41, e63. [Google Scholar] [CrossRef]
- Kopeva, K.; Grakova, E.; Maltseva, A.; Mochula, A.; Gusakova, A.; Smorgon, A.; Zavadovsky, K. Coronary microvascular dysfunction: Features and prognostic value. J. Clin. Med. 2023, 12, 2964. [Google Scholar] [CrossRef] [PubMed]
- Agabiti-Rosei, E.; Heagerty, A.M.; Rizzoni, D. Effects of antihypertensive treatment on small artery remodelling. J. Hypertens. 2009, 27, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Touyz, R.M.; Endemann, D.H.; Pu, Q.; Ko, E.A.; De Ciuceis, C.; Schiffrin, E.L. Angiotensin receptor blocker added to previous antihypertensive agents on arteries of diabetic hypertensive patients. Hypertension 2006, 48, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; De Ciuceis, C.; Sleiman, I.; Rodella, L.; Rezzani, R.; Paiardi, S.; Bianchi, R.; Ruggeri, G.; Boari, G.E.M.; et al. Effects of treatment with candesartan or enalapril on subcutaneous small resistance artery structure in hypertensive patients with NIDDM. Hypertension 2005, 45, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Agabiti Rosei, E. Small artery remodeling in diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 587–592. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Savoia, C.; Arrabito, E.; Porteri, E.; Mazza, M.; Rossini, C.; Duse, S.; Semeraro, F.; Agabiti Rosei, C.; Alonzo, A.; et al. Effects of a long-term treatment with aliskiren or ramipril on structural alterations of subcutaneous small-resistance arteries of diabetic hypertensive patients. Hypertension 2014, 64, 717–724. [Google Scholar] [CrossRef][Green Version]
- Jumar, A.; Ott, C.; Kistner, I.; Friedrich, S.; Schmidt, S.; Harazny, J.M.; Schmieder, R.E. Effect of aliskiren on vascular remodelling in small retinal circulation. J. Hypertens. 2015, 33, 2491–2499. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Salvetti, M.; Rossini, C.; Muiesan, M.L.; Paini, A.; Duse, S.; La Boria, E.; Semeraro, F.; Cancarini, A.; Agabiti Rosei, C.; et al. Effect of antihypertensive treatment on microvascular structure, central BP and oxidative stress in patients with mild essential hypertension. J. Hypertens. 2014, 32, 565–574. [Google Scholar] [CrossRef]
- Buus, N.H.; Mathiassen, O.N.; Fenger-Grøn, M.; Præstholm, M.N.; Sihm, I.; Thybo, N.K.; Schroeder, A.P.; Thygesen, K.; Aalkjær, C.; Pedersen, O.L.; et al. Small artery structure during antihypertensive therapy is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens. 2013, 31, 791–797. [Google Scholar] [CrossRef]
- Buus, N.H.; Bøttcher, M.; Jørgensen, C.G.; Christensen, K.L.; Thygesen, K.; Nielsen, T.T.; Mulvany, M.J. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension 2004, 44, 465–470. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bolognesi, M.; Rizzoni, D.; Seccia, T.M.; Piva, A.; Porteri, E.; Tiberio, G.A.; Giulini, S.M.; Agabiti-Rosei, E.; Pessina, A.C. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008, 51, 1366–1371. [Google Scholar] [CrossRef]
- Debbabi, H.; Uzan, L.; Mourad, J.J.; Safar, M.; Levy, B.I.; Tibirica`, E. Increased skin capillary density in treated essential hypertensive patients. Am. J. Hypertens. 2006, 19, 477–483. [Google Scholar] [CrossRef]
- Antonios, T.F. Microvascular rarefaction in hypertension--reversal or over-correction by treatment? Am. J. Hypertens. 2006, 19, 484–485. [Google Scholar] [CrossRef]
- Laurent, S.; Agabiti-Rosei, C.; Bruno, R.M.; Rizzoni, D. Microcirculation and macrocirculation in hypertension: A dangerous cross-link? Hypertension 2022, 79, 479–490. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial stiffness and hypertension in the elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef]
- Rizzoni, D.; Rizzoni, M.; Nardin, M.; Chiarini, G.; Agabiti-Rosei, C.; Aggiusti, C.; Paini, A.; Salvetti, M.; Muiesan, M.L. Vascular aging and disease of the small vessels. High. Blood Press. Cardiovasc. Prev. 2019, 26, 183–189. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]
- Laurent, S.; Agabiti-Rosei, E. The cross-talk between the macro- and the microcirculation. In Early Vascular Aging (EVA): New Directions in Cardiovascular Protection; Nilsson, P., Olsen, M.H., Laurent, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 105–118. [Google Scholar]
- Sasaki, R.; Yamano, S.; Yamamoto, Y.; Minami, S.; Yamamoto, J.; Nakashima, T.; Takaoka, M.; Hashimoto, T. Vascular remodeling of the carotid artery in patients with untreated essential hypertension increases with age. Hypertens. Res. 2002, 25, 373–379. [Google Scholar] [CrossRef][Green Version]
- Vasan, R.S.; Pan, S.; Xanthakis, V.; Beiser, A.; Larson, M.G.; Seshadri, S.; Mitchell, G.F. Arterial Stiffness and long-term risk of health outcomes: The Framingham Heart Study. Hypertension 2022, 79, 1045–1056. [Google Scholar] [CrossRef]
- Laurent, S.; Briet, M.; Boutouyrie, P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension 2009, 54, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Mulè, G.; Cottone, S.; Vadalà, A.; Volpe, V.; Mezzatesta, G.; Mongiovì, R.; Piazza, G.; Nardi, E.; Andronico, G.; Cerasola, G. Relationship between albumin excretion rate and aortic stiffness in untreated essential hypertensive patients. J. Intern. Med. 2004, 256, 22–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muiesan, M.L.; Salvetti, M.; Rizzoni, D.; Paini, A.; Agabiti-Rosei, C.; Aggiusti, C.; Bertacchini, F.; Stassaldi, D.; Gavazzi, A.; Porteri, E.; et al. Pulsatile hemodynamics and microcirculation: Evidence for a close relationship in hypertensive patients. Hypertension 2013, 61, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Raff, U.; Harazny, J.M.; Michelson, G.; Schmieder, R.E. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension 2013, 61, 1340–1345. [Google Scholar] [CrossRef]
- Salvetti, M.; Agabiti Rosei, C.; Paini, A.; Aggiusti, C.; Cancarini, A.; Duse, S.; Semeraro, F.; Rizzoni, D.; Agabiti Rosei, E.; Muiesan, M.L. Relationship of wall-to-lumen ratio of retinal arterioles with clinic and 24-hour BP. Hypertension 2014, 63, 1110–1115. [Google Scholar] [CrossRef]
- Rizzoni, D.; Muiesan, M.L.; Porteri, E.; De Ciuceis, C.; Boari, G.E.; Salvetti, M.; Paini, A.; Agabiti Rosei, E. Vascular remodeling, macro- and microvessels: Therapeutic implications. Blood Press. 2009, 18, 242–246. [Google Scholar] [CrossRef]
- Laurent, S.; Rizzoni, D. Targeting central BP through the micro-and macrocirculation cross-talk. In Early Vascular Aging (EVA): New Directions in Cardiovascular Protection; Nilsson, P., Olsen, M.H., Laurent, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 297–306. [Google Scholar]
- Mancia Chairperson, G.; Kreutz Co-Chair, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J. Hypertens. 2023; ahead of print. [Google Scholar]
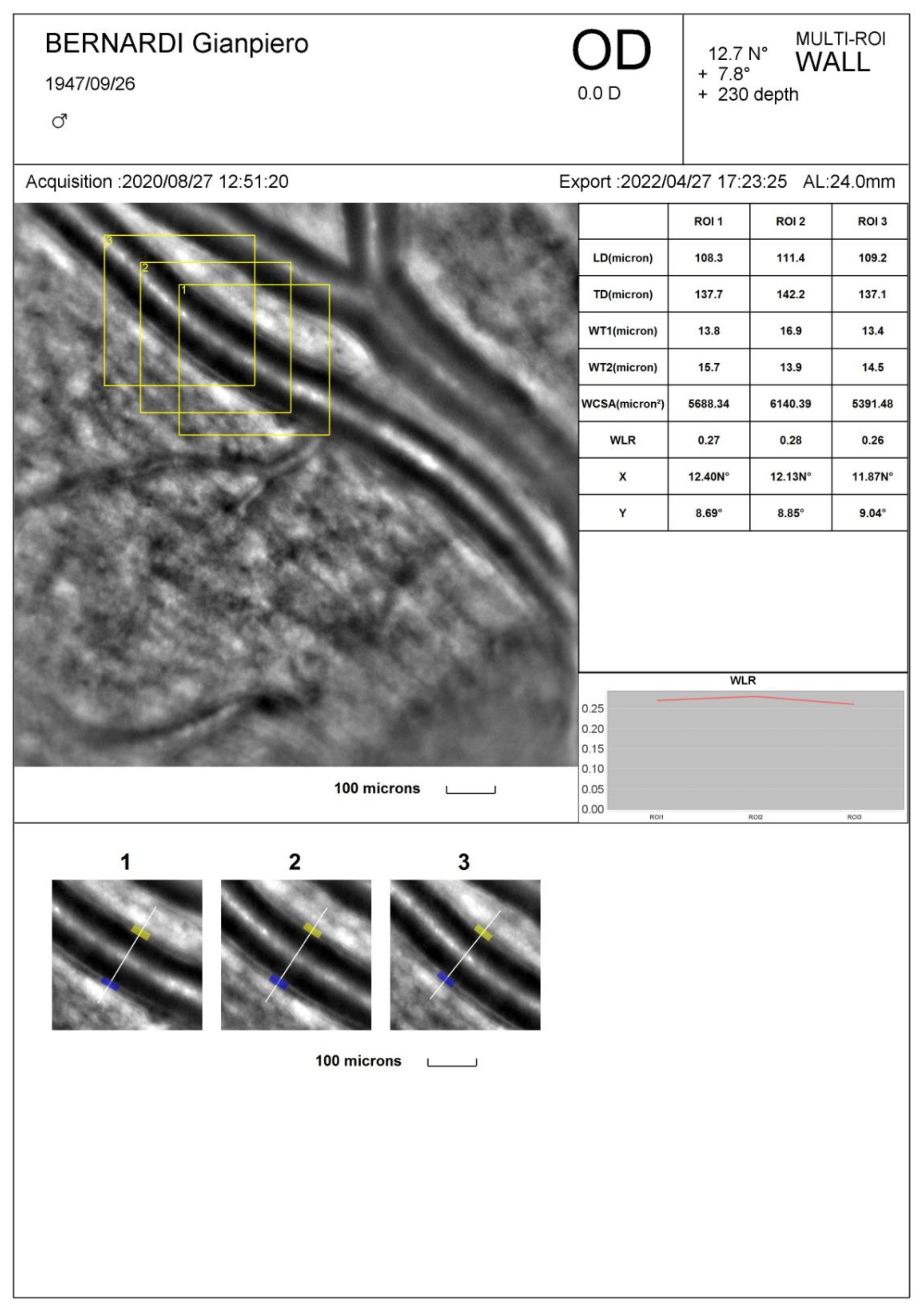
| Technique | Cost ($) | Advantages | Disadvantages | Indications/Perspectives |
|---|---|---|---|---|
| Forearm plethysmography | 1500–2000 | Relatively inexpensive | Locally invasive, needs experience | Research purposes, not for extensive clinical use |
| Intravital microscopy/Glycocalyx | 3000–5000 | Non-invasive | No prognostic data | Research purposes, not for extensive clinical use, though adoption in specific settings (i.e., critical care) is being proposed |
| Nailfold videocapillaroscopy | 10,000–20,000 | Non-invasive | No prognostic data | Research purposes in the cardiovascular setting, commonly used in rheumatology. Possible future demonstration of prognostic usefulness of such an approach might extend its clinical application to patients at elevated cardiovascular risk. |
| SLDF (Heidelberg Retina Flowmeter) | 30,000–40,000 | Some prognostic data available/possibility to assess endothelial function | No more in the market | Research purposes; potential for an extensive future clinical application in the cardiovascular field if technically developed by the producer |
| Adaptive optics cameras | 130,000–160,000 | Reliable/possibility to assess endothelial function | Cost, no prognostic data | Commonly used in ophthalmology for specific clinical purposes. Potential for an extensive future clinical application in the cardiovascular field |
| Dynamic Vessel Analyzer (AV ratio) | 5000–6000 | Some prognostic data, relatively easy to perform | Cost, some limitations in reliability | Commonly used in ophthalmology for specific purposes |
| OCTA | 20,000–70,000 | Useful vascular information on choroidal microvessels in hypertensive patients, plenty of data provided. | Imaging artefacts, high acquisition costs, role in the cardiovascular field still under evaluation | Commonly used in ophthalmology for specific purposes |
| Techniques for the evaluation of topological changes in the retinal vascular architecture or fractal dimensions | 30,000–50,000 | Some prognostic data (fractal dimensions) | Not standardized | Only research purposes |
| EndoPAT | 2500–4000 | Easy to perform, cost | Limited reliability | Only research purposes |
| Laser Doppler Flowmetry (skin) | 1500–20,000 | Easy to perform, cost | Limited reliability | Only research purposes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzoni, D.; Agabiti-Rosei, C.; Boari, G.E.M.; Muiesan, M.L.; De Ciuceis, C. Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease? J. Clin. Med. 2023, 12, 4892. https://doi.org/10.3390/jcm12154892
Rizzoni D, Agabiti-Rosei C, Boari GEM, Muiesan ML, De Ciuceis C. Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease? Journal of Clinical Medicine. 2023; 12(15):4892. https://doi.org/10.3390/jcm12154892
Chicago/Turabian StyleRizzoni, Damiano, Claudia Agabiti-Rosei, Gianluca E. M. Boari, Maria Lorenza Muiesan, and Carolina De Ciuceis. 2023. "Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease?" Journal of Clinical Medicine 12, no. 15: 4892. https://doi.org/10.3390/jcm12154892
APA StyleRizzoni, D., Agabiti-Rosei, C., Boari, G. E. M., Muiesan, M. L., & De Ciuceis, C. (2023). Microcirculation in Hypertension: A Therapeutic Target to Prevent Cardiovascular Disease? Journal of Clinical Medicine, 12(15), 4892. https://doi.org/10.3390/jcm12154892






